Phase Equilibria in the Ni-V-Ta Ternary System
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
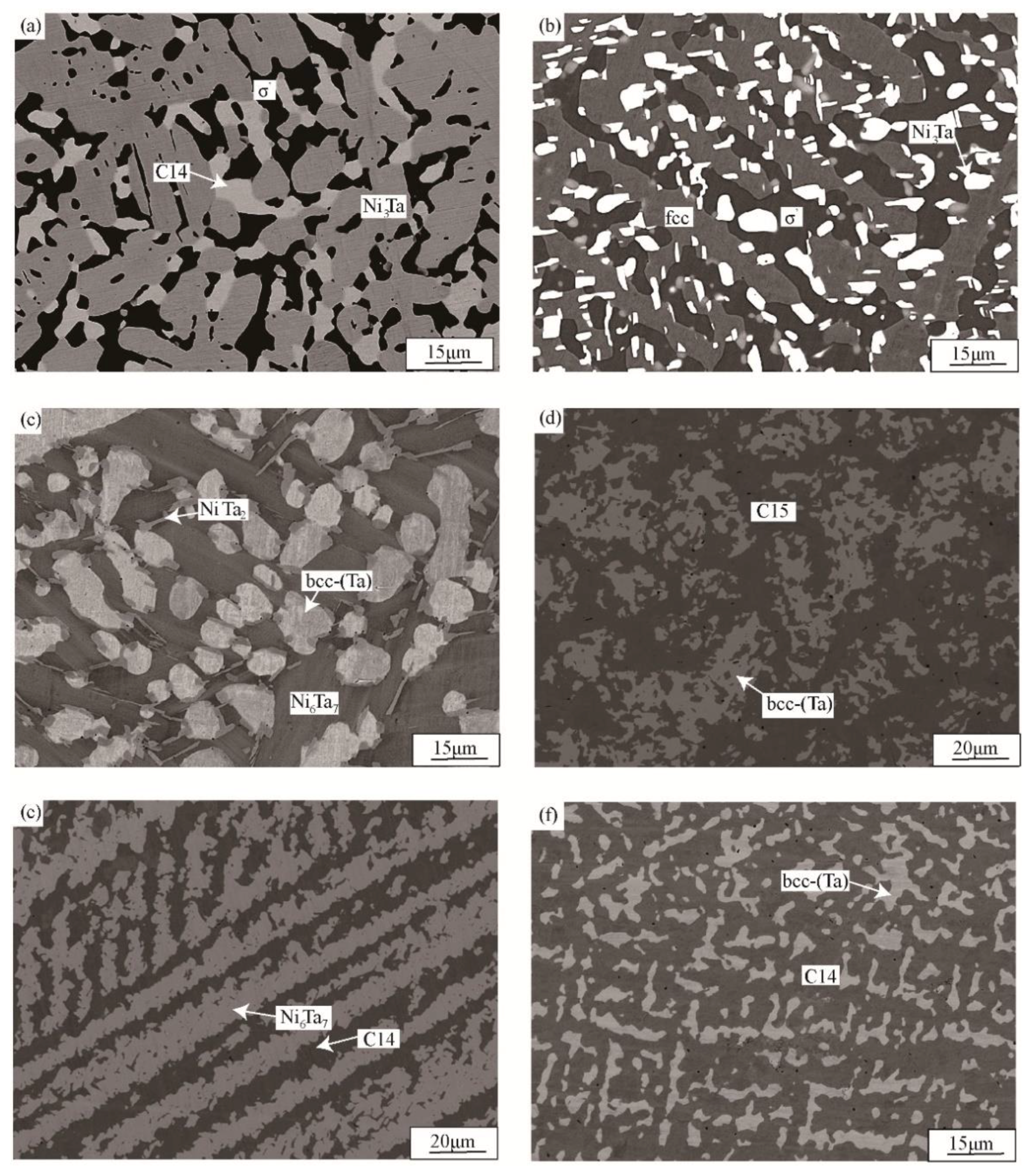
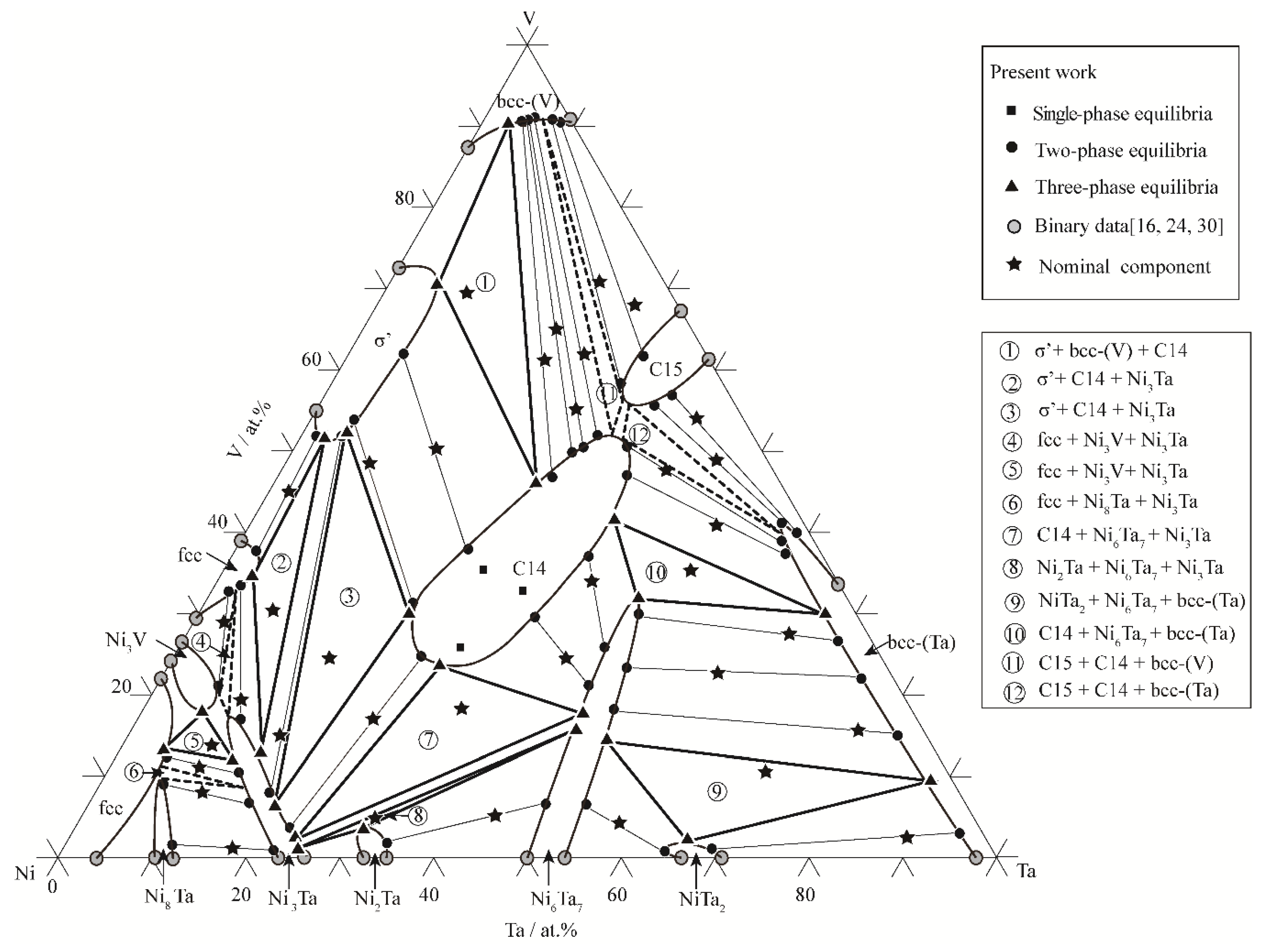
3.1. Phase Equilibria at 1000 °C
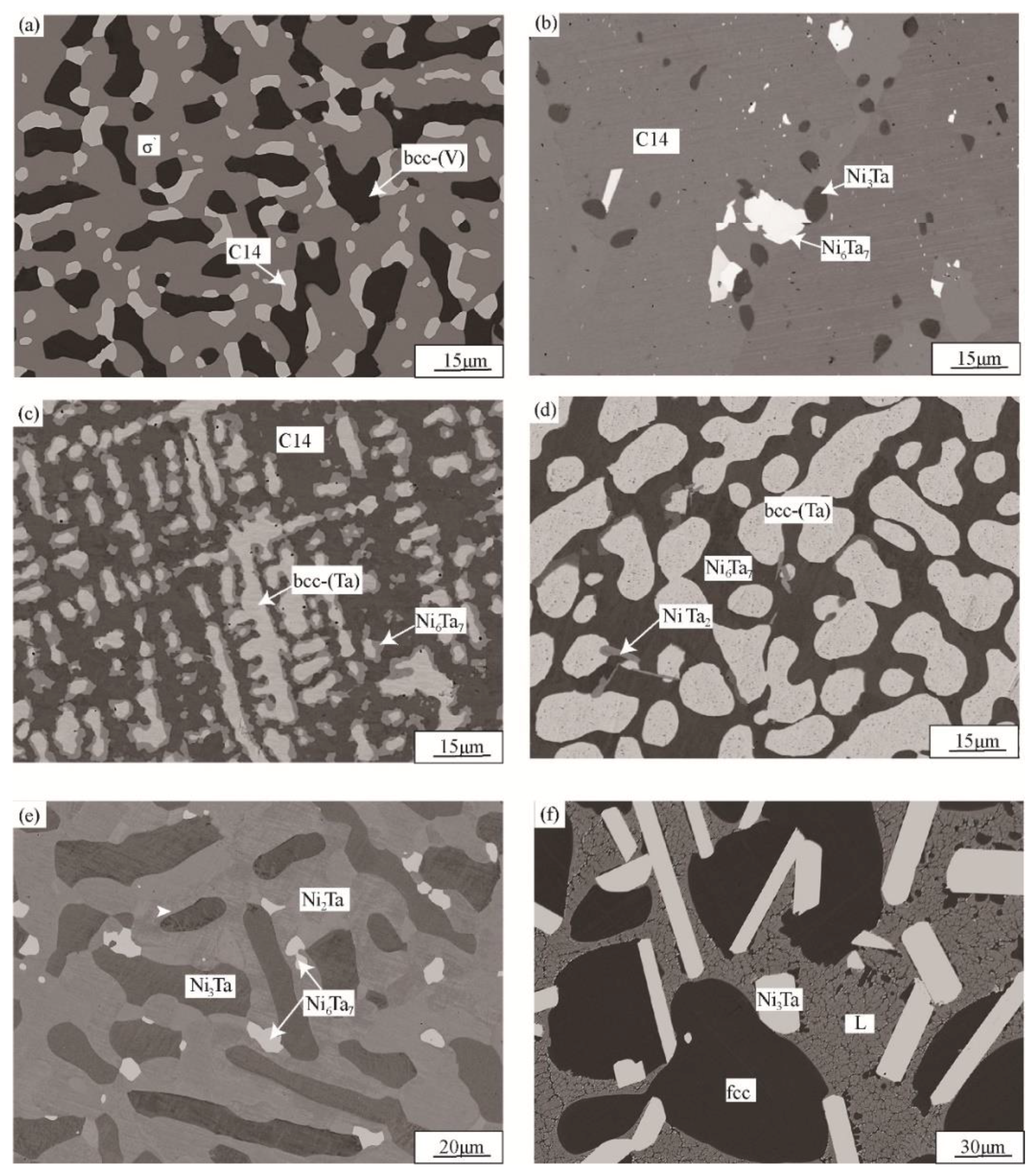
3.2. Phase Equilibria at 1200 °C
3.3. Liquid Region
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, Microstructure and Properties. J. Propul. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, G.; Lee, T.L.; Gorley, M.J.; Wang, Y.; Xiao, C.; Li, Z. The effects of Ta on the stress rupture properties and microstructural stability of a novel Ni-base superalloy for land-based high temperature applications. Mater. Des. 2014, 61, 61–69. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, Z.; Noebe, R.D.; Seidman, D.N. The effects of refractory elements on Ni-excesses and Ni-depletions at γ(f.c.c.)/γ′(L12) interfaces in model Ni-based superalloys: Atom-probe tomographic experiments and first-principles calculations. Acta Mater. 2016, 121, 288–298. [Google Scholar] [CrossRef]
- Yang, S.W. Effect of Ti and Ta on the oxidation of a complex superalloy. Oxida. Met. 1981, 15, 375–397. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, S.M.; Yoo, Y.S.; Jeong, H.W.; Jang, H. Effects of Al and Ta on the high temperature oxidation of Ni-based superalloys. Corros. Sci. 2015, 90, 305–312. [Google Scholar] [CrossRef]
- Shibuya, S.; Kaneno, Y.; Yoshida, M.; Takasugi, T. Dual multi-phase intermetallic alloys composed of geometrically close packed Ni3X (X: Al, Ti and V) type structures—II. Mechanical properties. Acta Mater. 2006, 54, 861–870. [Google Scholar] [CrossRef]
- Soga, W.; Kaneno, Y.; Yoshida, M.; Takasugi, T. Microstructure and mechanical property in dual two-phase intermetallic alloys composed of geometrically close-packed Ni3X (X: Al and V) containing Nb. Mater. Sci. Eng. A 2008, 473, 180–188. [Google Scholar] [CrossRef]
- Kawahara, K.; Kaneno, Y.; Kakitsuji, A.; Takasugi, T. Microstructural factors affecting hardness property of dual two-phase intermetallic alloys based on Ni3Al-Ni3V pseudo-binary alloy system. Intermetallics 2009, 17, 938–944. [Google Scholar] [CrossRef]
- Moreen, H.A.; Taggart, R.; Polonis, D.H. Ni8X phases in the systems Ni-V, Ni-V-Nb, and Ni-V-Ta. J. Mater. Sci. 1971, 6, 1425–1432. [Google Scholar] [CrossRef]
- Gupta, K.L. The Ni-Ta-V (Nickel-Tantalum-Vanadium) System. In Phase Diagrams of Ternary Nickel Alloys, Part 2, 1st ed.; Indian Institute of Metals ASM International: Bidhannagar, India, 1990; pp. 208–213. ISBN 8185307113. [Google Scholar]
- Kripyakevich, P.I.; Pylaeva, E.N. Crystal structure of the compound Ta2Ni. J. Struct. Chem. 1962, 3, 35–37. [Google Scholar] [CrossRef]
- Giessen, B.C.; Grant, N.J. The crystal structure of TaNi3 and its change on cold working. Acta Metall. 1967, 15, 871–877. [Google Scholar] [CrossRef]
- Larson, J.M.; Taggart, R.; Polonis, D.H. Ni8Ta in Nickel-rich Ni−Ta alloys. Metall. Mater. Trans. B 1970, 1, 485–489. [Google Scholar] [CrossRef]
- Saburi, T.; Nakamura, M.; Nenno, S. Crystal structure and twin lamellae of Ni3Ta. J. Less-Common Met. 1975, 41, 135–139. [Google Scholar] [CrossRef]
- Nash, P.; West, D.R.F. Ni–Al and Ni–Ta phase diagrams. Met. Sci. 1983, 17, 99–100. [Google Scholar] [CrossRef]
- Nash, A.; Nash, P. The Ni−Ta system. J. Phase Equilib. 1984, 5, 444–445. [Google Scholar] [CrossRef]
- Kaufman, L. Coupled thermochemical and phase diagram data for tantalum based binary alloys. Calphad 1991, 15, 243–259. [Google Scholar] [CrossRef]
- Ansara, I.; Selleby, M. Thermodynamic analysis of the Ni-Ta system. Calphad 1994, 18, 99–107. [Google Scholar] [CrossRef]
- Cui, Y.; Jin, Z. Experimental study and reassessment of the Ni-Ta binary system. Z. Metallkd. 1999, 90, 233–241. [Google Scholar]
- Pan, X.; Jin, Z. Experimental determination and Re-optimization of Ni-Ta binary system. Trans. Nonferrous Met. Soc. China 2002, 12, 748–753. [Google Scholar] [CrossRef]
- Zhou, S.H.; Wang, Y.; Chen, L.Q.; Liu, Z.K.; Napolitano, R.E. Solution-based thermodynamic modeling of the Ni-Ta and Ni-Mo-Ta systems using first-principle calculations. Calphad 2009, 33, 631–641. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, B.; Ma, Y.; Melnik, R.; Liu, X. First-principles studies of Ni-Ta intermetallic compounds. J. Solid State Chem. 2012, 187, 211–218. [Google Scholar] [CrossRef]
- Kosorukova, T.; Firstov, G.; Noel, H.; Ivanchenko, V. Crystal structure changes in the Ni3Ta intermetallic compound. Chem. Met. Alloys 2013, 6, 196–199. [Google Scholar]
- Smith, J.F.; Carlson, O.N.; Nash, P.G. The Ni−V (Nickel-Vanadium) system. J. Phase Equilib. 1982, 3, 342–348. [Google Scholar] [CrossRef]
- Stevens, E.R.; Carlson, O.N. V-Ni System. Metall. Mater. Trans. B 1970, 1, 1267–1271. [Google Scholar] [CrossRef]
- Watson, A.; Hayes, F.H. Some experiences modelling the sigma phase in the Ni-V system. J. Alloys Compd. 2001, 320, 199–206. [Google Scholar] [CrossRef]
- Kabanova, E.G.; Kuznetsov, V.N.; Zhmurko, G.P. Thermodynamic modeling of phase equilibria in the Ni-V system. Russ. J. Phys. Chem. A 2010, 84, 755–759. [Google Scholar] [CrossRef]
- Savicky, E.M.; Efimov, J.V. Superconducting metallic compounds and their alloys. Monatsh. Chem. 1972, 103, 270–287. [Google Scholar] [CrossRef]
- Smith, J.F.; Carlson, O.N. The Ta-V (Tantalum-Vanadium) system. J. Phase Equilib. 1983, 4, 284–289. [Google Scholar] [CrossRef]
- Danon, C.A.; Servant, C. A thermodynamic evaluation of the Ta-V system. J. Alloys Compd. 2004, 366, 191–200. [Google Scholar] [CrossRef]
- Okamoto, H. Ta-V (Tantalum-Vanadium). J. Phase Equilib. Diffus. 2005, 26, 298–299. [Google Scholar] [CrossRef]
- Pavlů, J.; Vřešt’ál, J.; Chen, X.Q.; Rogl, P. Thermodynamic modeling of Laves phases in the Ta-V system: Reassessment using first-principles results. Calphad 2011, 35, 103–108. [Google Scholar] [CrossRef]




| System | Phase | Pearson Symbol | Prototye | Space Group | Strukturbericht | References |
|---|---|---|---|---|---|---|
| Ni-Ta | fcc | cF4 | Cu | Fm-3m | A1 | [16] |
| Ni8Ta | tI36 | Ni8Nb | I4/mmm | [16] | ||
| Ni3Ta | tI8 | TiAl3 | I4/mmm | D022 | [16] | |
| mP16 | NbPt3 | P21/m | [16] | |||
| oP8 | Cu3Ti | Pmmn | D0a | [16] | ||
| Ni2Ta | tI6 | MoSi2 | I4/mmm | C11b | [16] | |
| Ni6Ta7 | hR39 | Fe7W6 | R-3mh | D85 | [16] | |
| NiTa2 | tI12 | Al2Cu | I4/mcm | C16 | [16] | |
| bcc-(Ta) | cI2 | W | Im-3m | A2 | [16] | |
| Ni-V | fcc | cF4 | Cu | Fm-3m | A1 | [24] |
| Ni8V | tI18 | Ni8Nb | I4/mmm | [24] | ||
| Ni2V | oI6 | MoPt2 | Immm | [24] | ||
| Ni3V | tI8 | Al3Ti | I4/mmm | D022 | [24] | |
| σ′ | tP30 | σ | P42/mnm | [24] | ||
| NiV3 | cP8 | Cr3Si | Pm3n | [24] | ||
| bcc-(V) | cI2 | W | Im-3m | A2 | [24] | |
| V-Ta | bcc-(V, Ta) | cI2 | W | Im-3m | A2 | [30] |
| C14 | hP12 | MgZn2 | P63/mmc | C14 | [30] | |
| C15 | cF4 | MgCu2 | Fd-3m | C15 | [30] |
| Nominal Alloys (at. %) | Phase Equilibrium | Composition (at. %) | |||||
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | ||||
| V | Ta | V | Ta | V | Ta | ||
| Ni24V67Ta9 | bcc-(V)/σ′/C14 | 90 | 3.1 | 70.6 | 5.2 | 47.1 | 27.1 |
| Ni13V65Ta22 | bcc-(V)/C14 | 90.5 | 5.3 | 49.8 | 30.1 | ||
| Ni41V49Ta10 | σ′/C14 | 54.2 | 4.4 | 31.8 | 21.8 | ||
| Ni9V41Ta50 | bcc-(Ta)/C14 | 37 | 58.5 | 47.3 | 36.2 | ||
| Ni61V30Ta9 | fcc/Ni3Ta/σ′ | 36.4 | 3.2 | 13.2 | 15 | 51.6 | 2.7 |
| Ni59V24Ta17 | σ′/C14/Ni3Ta | 53 | 4.7 | 31 | 22.1 | 6.8 | 19.8 |
| Ni48V18Ta34 | Ni6Ta7/C14/Ni3Ta | 17.9 | 47 | 23.5 | 29 | 2.5 | 24.1 |
| Ni77V14Ta9 | fcc/Ni3V/Ni3Ta | 13.1 | 4.6 | 17 | 6.7 | 12 | 12.4 |
| Ni19V11Ta70 | bcc-(Ta)/Ni6Ta7/NiTa2 | 9.6 | 88.3 | 14.5 | 51.5 | 2.2 | 65.9 |
| Ni17V61Ta22 | bcc-(V)/C14 | 90.3 | 4.8 | 46.5 | 29.5 | ||
| Ni52V45Ta3 | fcc/σ′ | 37.9 | 2.8 | 51.8 | 2.7 | ||
| Ni26V34Ta40 | Ni6Ta7/C14 | 26.1 | 45 | 37.3 | 37.9 | ||
| Ni68V15Ta17 | Ni3Ta/σ′ | 8.1 | 18.6 | 52.5 | 3.8 | ||
| Ni64V5Ta31 | Ni2Ta/Ni3Ta/Ni6Ta7 | 3.4 | 30.6 | 1.1 | 25.2 | 15.8 | 47.5 |
| Ni82V7Ta11 | Ni8Ta/Ni3Ta | 8.9 | 7.1 | 6.5 | 17 | ||
| Ni18V23Ta59 | Ni6Ta7/bcc-(Ta) | 23.1 | 49 | 22 | 74.9 | ||
| Ni8V28Ta64 | Ni6Ta7/bcc-(Ta) | 30 | 46.9 | 26.6 | 70 | ||
| Ni5V49Ta46 | C15/bcc-(Ta) | 55.5 | 35.3 | 41.1 | 56.3 | ||
| Ni6V71Ta23 | C15/bcc-(V) | 58.4 | 30.5 | 90.7 | 7.5 | ||
| Ni35V50Ta15 | σ′/C14 | 62.5 | 5.9 | 37.5 | 25 | ||
| Ni71V19Ta10 | fcc/Ni3Ta | 33.7 | 2.5 | 17.1 | 11 | ||
| Ni79V10Ta11 | fcc/Ni3Ta | 12.2 | 5.3 | 10.2 | 14.3 | ||
| Ni50V6Ta44 | Ni2Ta/Ni6Ta7 | 2.1 | 34 | 6.7 | 48.8 | ||
| Ni39V4Ta57 | NiTa2/Ni6Ta7 | 0.6 | 64.1 | 6.5 | 52.9 | ||
| Ni9V2Ta89 | NiTa2/bcc-(Ta) | 1 | 69.1 | 3.1 | 94.5 | ||
| Ni7V13Ta80 | Ni6Ta7/bcc-(Ta) | 18 | 50.2 | 15.1 | 81.9 | ||
| Ni59V17Ta24 | Ni3Ta/C14 | 3.5 | 22.6 | 25.2 | 26.4 | ||
| Ni37V35Ta28 | C14 | 35 | 28 | ||||
| Ni34V33Ta33 | C14 | 33 | 33 | ||||
| Ni44V26Ta30 | C14 | 26 | 30 | ||||
| Ni4V68Ta28 | C15/bcc-(V) | 61.2 | 31.5 | 90.1 | 8.5 | ||
| Ni5V54Ta41 | C15/bcc-(Ta) | 56.5 | 37 | 39.8 | 48.9 | ||
| Ni13V46Ta41 | bcc-(Ta)/C14 | 39.1 | 57.8 | 51 | 35 | ||
| Ni14V36Ta50 | Ni6Ta7/C14/bcc-(Ta) | 31.6 | 45.2 | 44.1 | 36.4 | 28.7 | 66.4 |
| Ni18V55Ta27 | C14/bcc-(V) | 50.2 | 31 | 90.2 | 5.4 | ||
| Ni81V1Ta18 | Ni8Ta/Ni3Ta | 1.5 | 11.5 | 0.7 | 22.5 | ||
| Ni12V62Ta26 | C14/bcc-(V) | 52 | 31.3 | 91.3 | 4.8 | ||
| Ni33V25Ta42 | Ni6Ta7/C14 | 21.4 | 45.9 | 29.8 | 36.1 | ||
| Alloys (at. %) | Phase Equilibrium | Composition (at. %) | |||||
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | ||||
| V | Ta | V | Ta | V | Ta | ||
| Ni24V67Ta9 | bcc-(V)/σ′/C14 | 85.1 | 4.1 | 66.2 | 6.5 | 43.6 | 24.6 |
| Ni13V65Ta22 | bcc-(V)/C14 | 88.8 | 7.5 | 49.5 | 29 | ||
| Ni41V49Ta10 * | σ′/L/C14 | 55.6 | 5.5 | 42.2 | 11.1 | 34.5 | 22.2 |
| Ni9V41Ta50 | bcc-(Ta)/C14 | 33.2 | 66.4 | 45.6 | 37.3 | ||
| Ni61V30Ta9 * | fcc/Ni3Ta/L | 34.2 | 4 | 9.1 | 27.5 | 37.3 | 11.8 |
| Ni75V18Ta7 | fcc/Ni3Ta | 20.9 | 6.5 | 16.8 | 9.1 | ||
| Ni59V24Ta17 * | Ni3Ta/L/C14 | 6.4 | 20.2 | 37.5 | 12.2 | 26.3 | 23.7 |
| Ni48V18Ta34 | Ni3Ta/Ni6Ta7/C14 | 2.3 | 23.1 | 18.6 | 46 | 21 | 28.6 |
| Ni77V14Ta9 | fcc/Ni3Ta | 15.1 | 6.2 | 10.8 | 14.1 | ||
| Ni19V11Ta70 | bcc-(Ta)/Ni6Ta7/NiTa2 | 9.3 | 89.1 | 12.5 | 52.7 | 0.9 | 66.1 |
| Ni17V61Ta22 | bcc-(V)/C14 | 88.3 | 6.6 | 48.3 | 28.1 | ||
| Ni52V45Ta3 * | fcc/σ′/L | 41.6 | 2 | 53.8 | 3 | 45.9 | 5 |
| Ni26V34Ta40 | Ni6Ta7/C14 | 28.3 | 44.9 | 38 | 36.8 | ||
| Ni68V15Ta17 * | Ni3Ta/L | 7.9 | 18.7 | 37.3 | 12 | ||
| Ni64V5Ta31 | Ni2Ta/Ni3Ta/Ni6Ta7 | 3.8 | 29.9 | 1.7 | 24.5 | 16 | 46.5 |
| Ni82V7Ta11 | Ni8Ta/Ni3Ta | 8 | 7.9 | 5.5 | 18.2 | ||
| Ni18V23Ta59 | Ni6Ta7/bcc-(Ta) | 23.8 | 50 | 20.9 | 78.9 | ||
| Ni8V28Ta64 | Ni6Ta7/bcc-(Ta) | 31.5 | 47.5 | 24.8 | 75 | ||
| Ni5V49Ta46 | C14/bcc-(Ta) | 55 | 36.2 | 44.3 | 55.6 | ||
| Ni6V71Ta23 | C14/bcc-(V) | 56.5 | 30.6 | 89.8 | 9.5 | ||
| Ni35V50Ta15 | σ′/C14 | 60.5 | 7.2 | 39.4 | 23.1 | ||
| Ni71V19Ta10 | fcc/Ni3Ta | 26.8 | 5.1 | 14.7 | 12.1 | ||
| Ni79V10Ta11 | fcc/Ni3Ta | 10.8 | 7.5 | 6.6 | 17.2 | ||
| Ni50V6Ta44 | Ni2Ta/Ni6Ta7 | 2.1 | 32.5 | 8.1 | 48.5 | ||
| Ni39V4Ta57 | NiTa2/Ni6Ta7 | 0.6 | 64.8 | 5.7 | 53.5 | ||
| Ni9V2Ta89 | NiTa2/bcc-(Ta) | 0.6 | 66.9 | 2.6 | 97.1 | ||
| Ni7V13Ta80 | Ni6Ta7/bcc-(Ta) | 18 | 51.5 | 12.4 | 87.5 | ||
| Ni59V17Ta24 | Ni3Ta/C14 | 4.7 | 21.5 | 24.4 | 25.1 | ||
| Ni37V35Ta28 | C14 | 35 | 28 | ||||
| Ni34V33Ta33 | C14 | 33 | 33 | ||||
| Ni44V26Ta30 | C14 | 26 | 30 | ||||
| Ni3V57Ta40 | C14/C15 | 47 | 35.9 | 46.8 | 42.1 | ||
| Ni13V46Ta41 | C14/bcc-(Ta) | 48.8 | 36.8 | 41.5 | 58 | ||
| Ni14V36Ta50 | Ni6Ta7/C14/bcc-(Ta) | 34 | 44.5 | 40.9 | 37 | 30.1 | 68.8 |
| Ni18V55Ta27 | C14/bcc-(V) | 49.6 | 30.5 | 89.1 | 8.2 | ||
| Ni81V1Ta18 | Ni8Ta/Ni3Ta | 1.5 | 12.1 | 0.9 | 22.1 | ||
| Ni12V62Ta26 | C14/bcc-(V) | 54.1 | 30.4 | 89.3 | 9.1 | ||
| Ni33V25Ta42 | Ni6Ta7/C14 | 22.5 | 45 | 30.2 | 34.7 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liang, Y.; Yang, S.; Zhang, J.; Huang, Y.; Han, J.; Lu, Y.; Liu, X. Phase Equilibria in the Ni-V-Ta Ternary System. Metals 2018, 8, 774. https://doi.org/10.3390/met8100774
Wang C, Liang Y, Yang S, Zhang J, Huang Y, Han J, Lu Y, Liu X. Phase Equilibria in the Ni-V-Ta Ternary System. Metals. 2018; 8(10):774. https://doi.org/10.3390/met8100774
Chicago/Turabian StyleWang, Cuiping, Yuhui Liang, Shuiyuan Yang, Jinbin Zhang, Yixiong Huang, Jiajia Han, Yong Lu, and Xingjun Liu. 2018. "Phase Equilibria in the Ni-V-Ta Ternary System" Metals 8, no. 10: 774. https://doi.org/10.3390/met8100774
APA StyleWang, C., Liang, Y., Yang, S., Zhang, J., Huang, Y., Han, J., Lu, Y., & Liu, X. (2018). Phase Equilibria in the Ni-V-Ta Ternary System. Metals, 8(10), 774. https://doi.org/10.3390/met8100774






