Al-1.5Fe-xLa Alloys for Lithium-Ion Battery Package
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
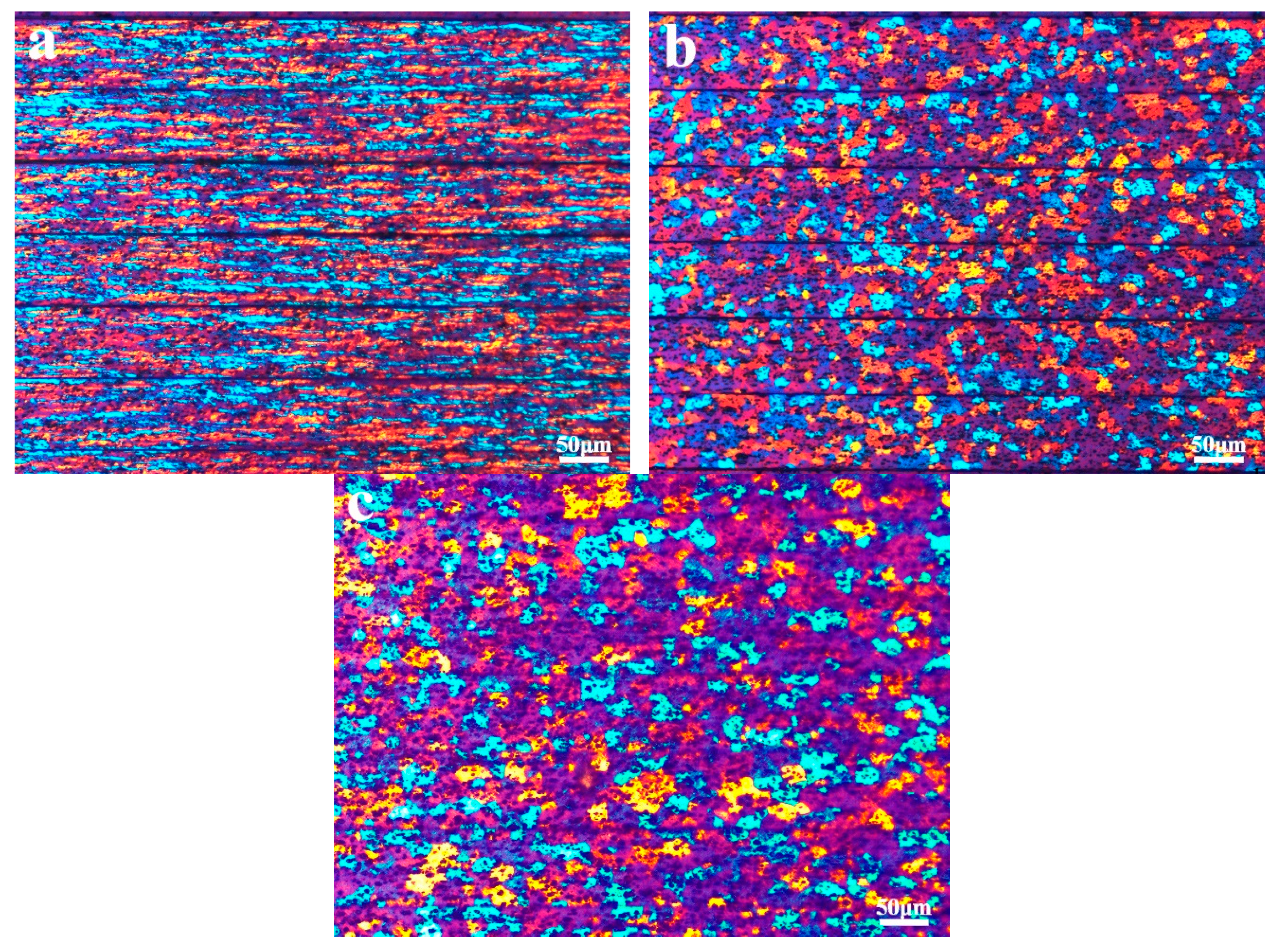
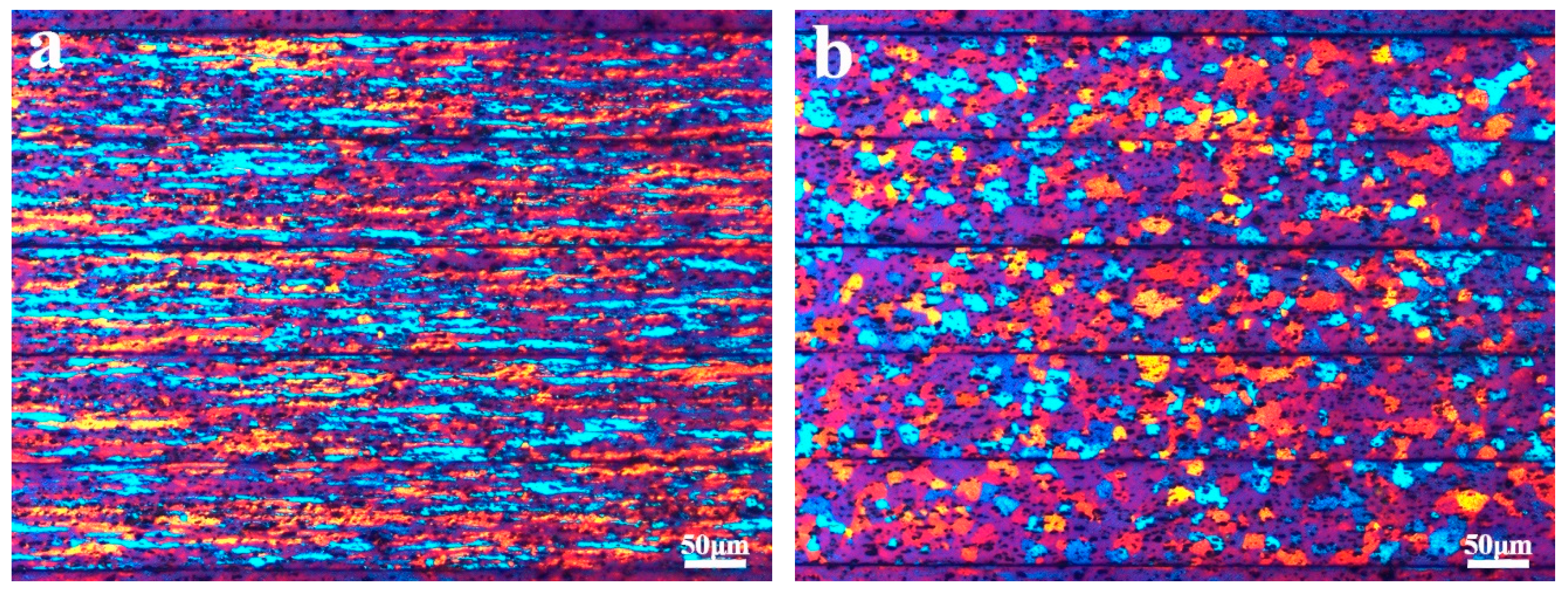
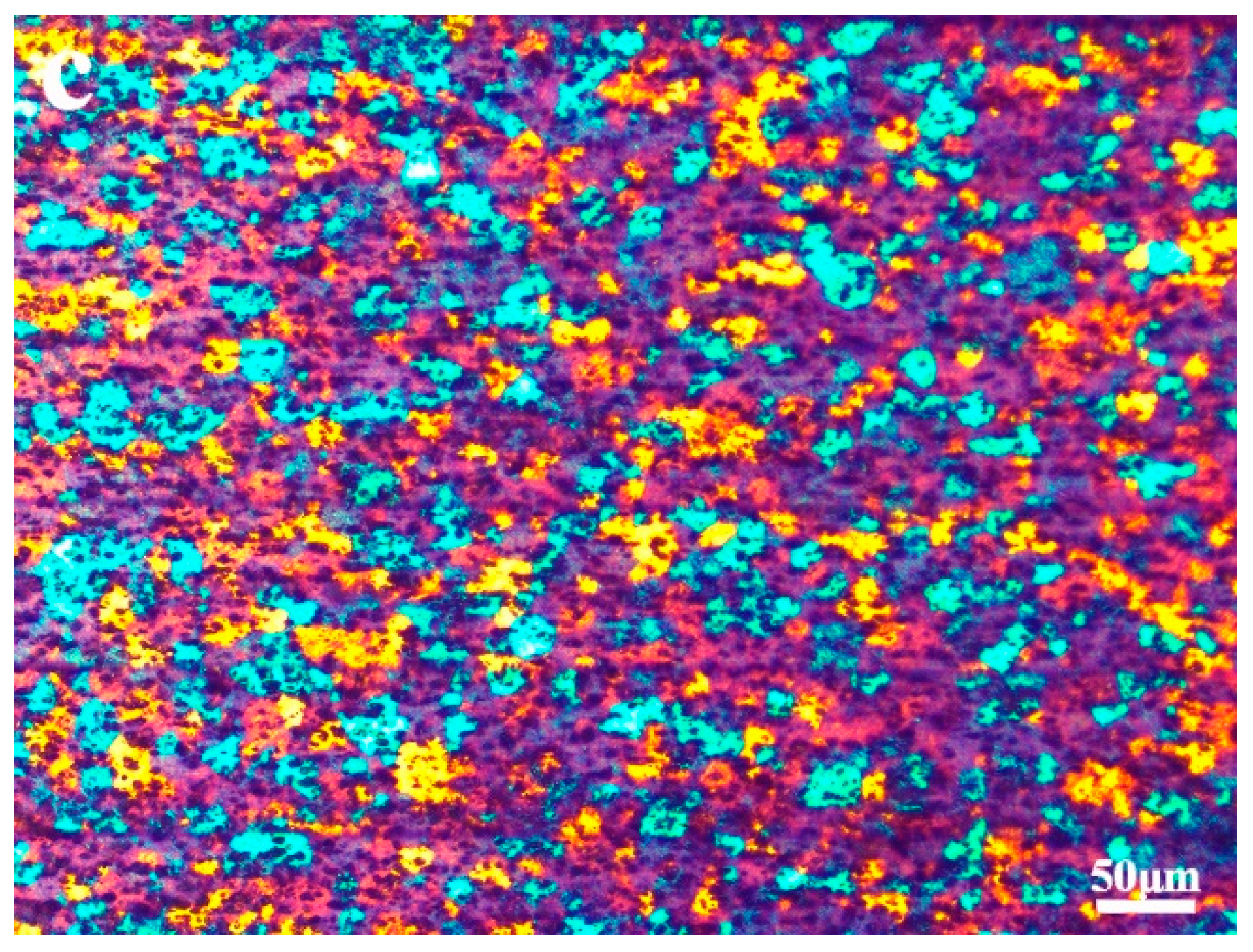
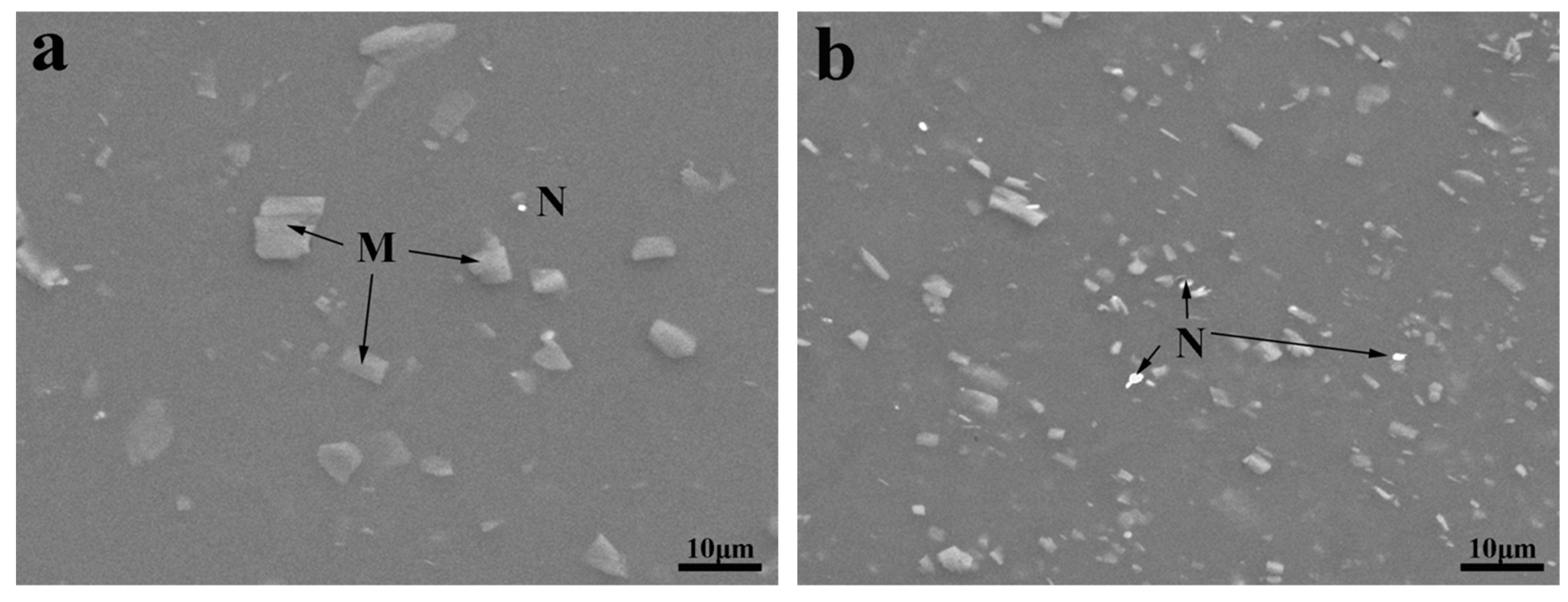
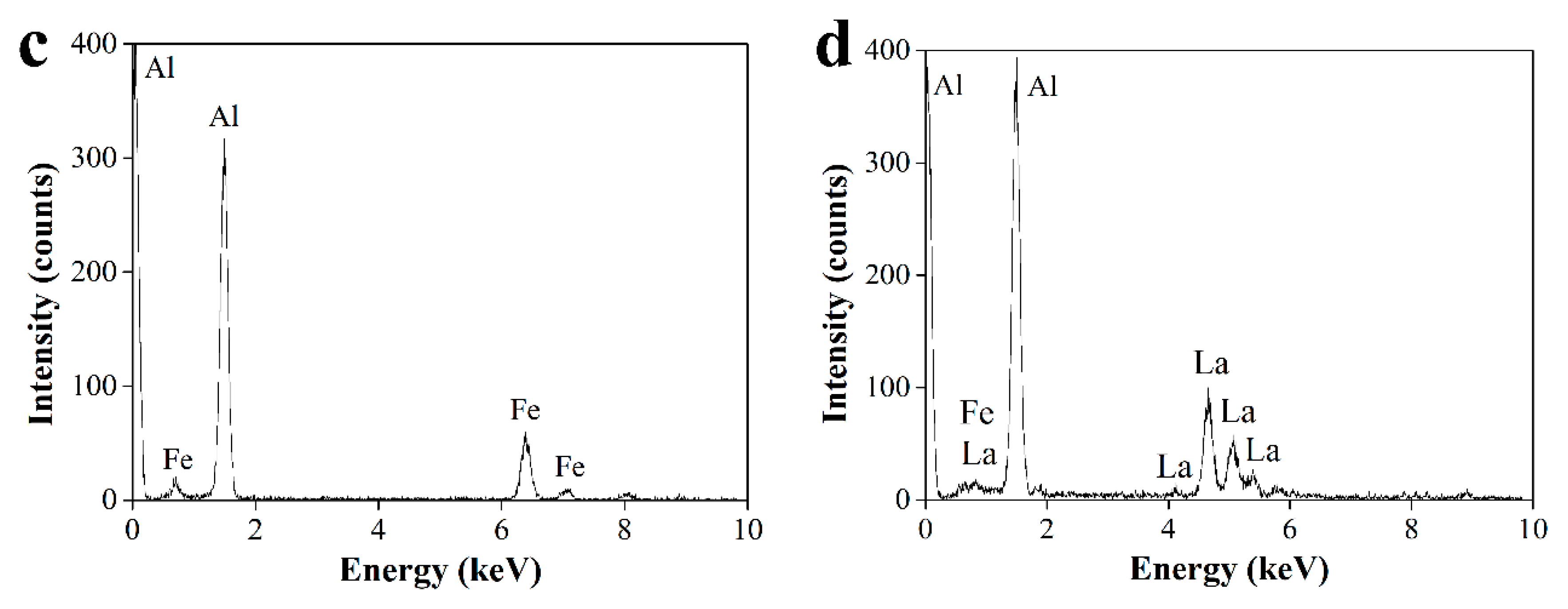
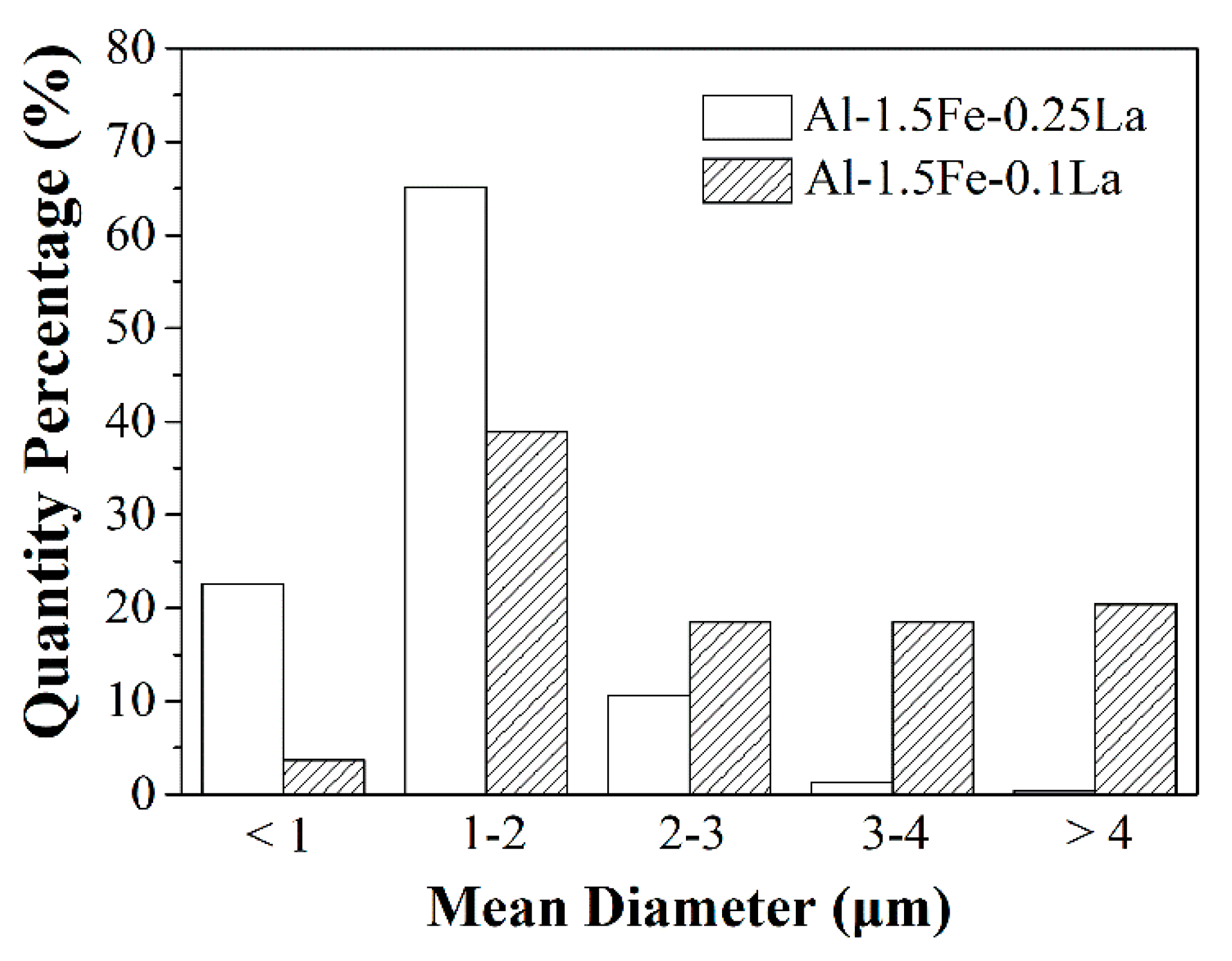
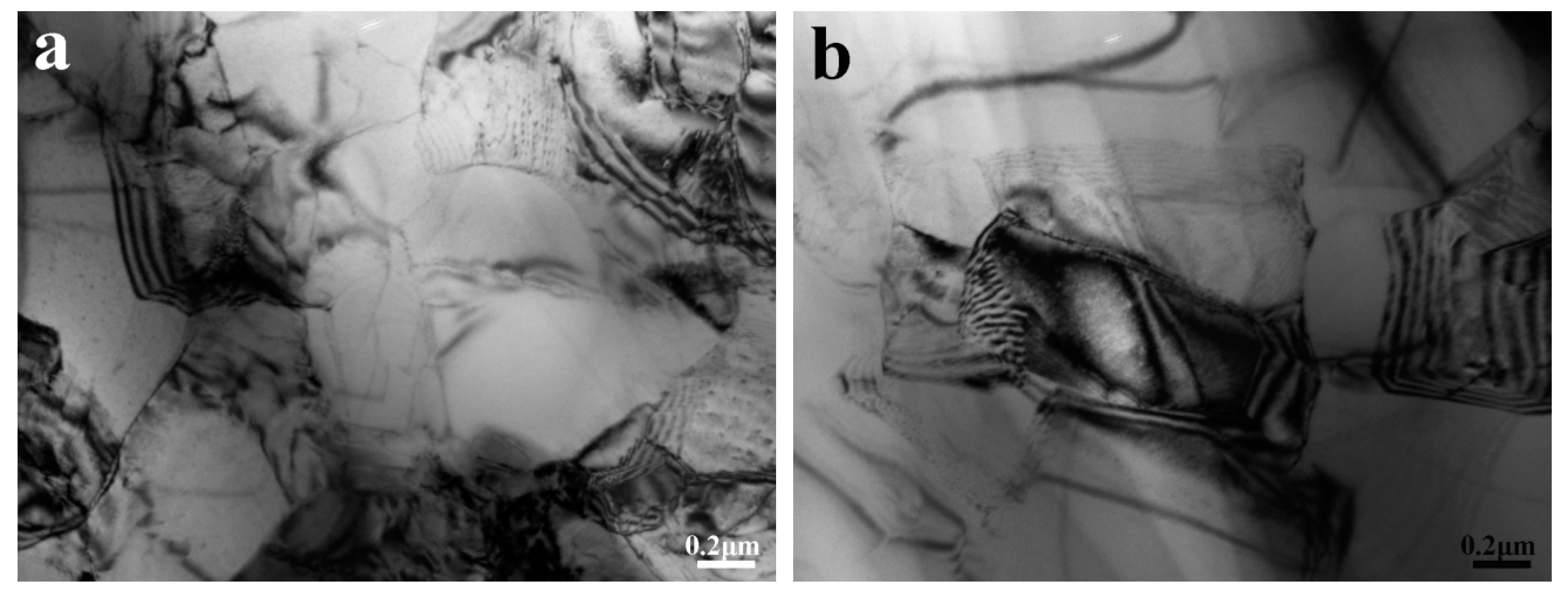
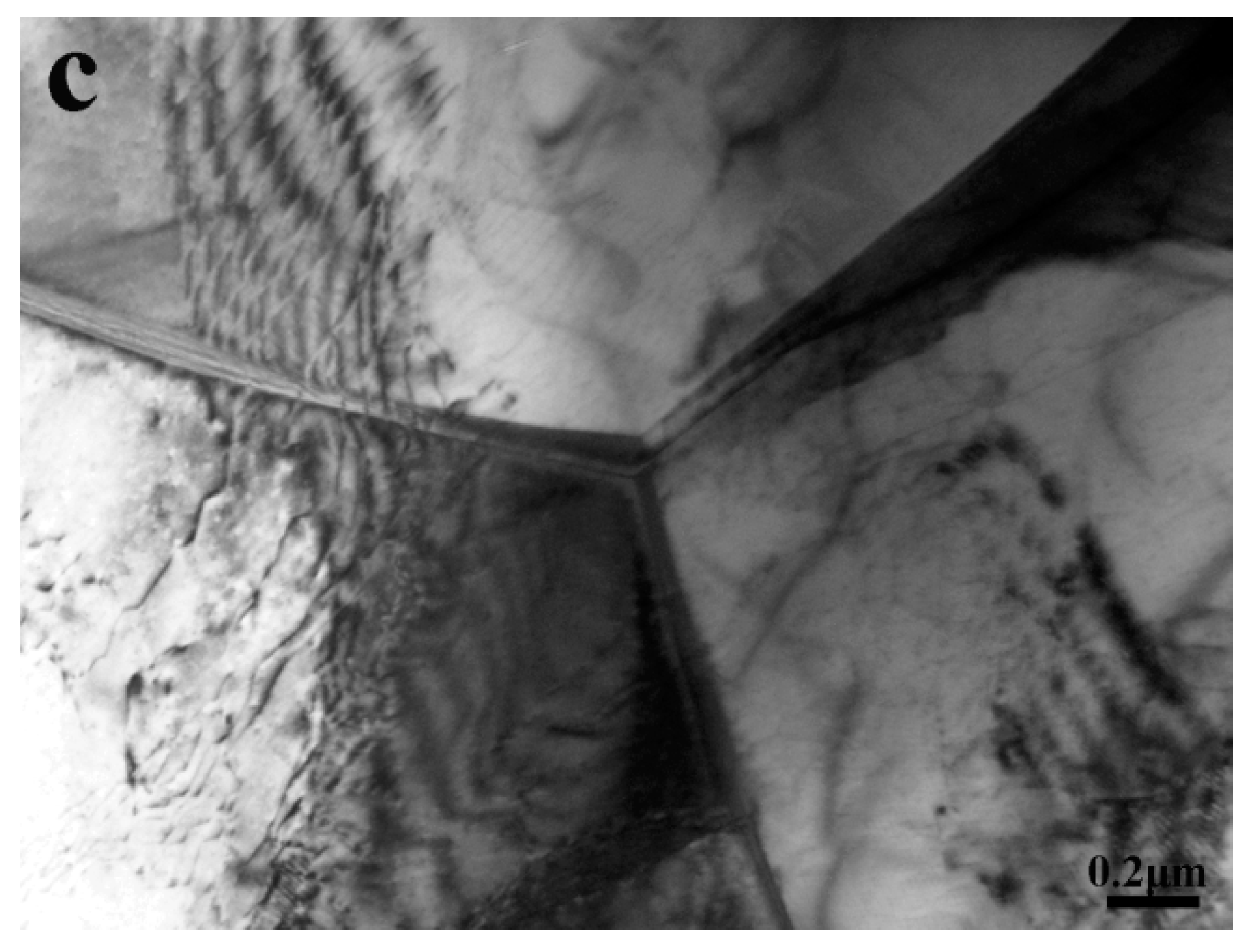
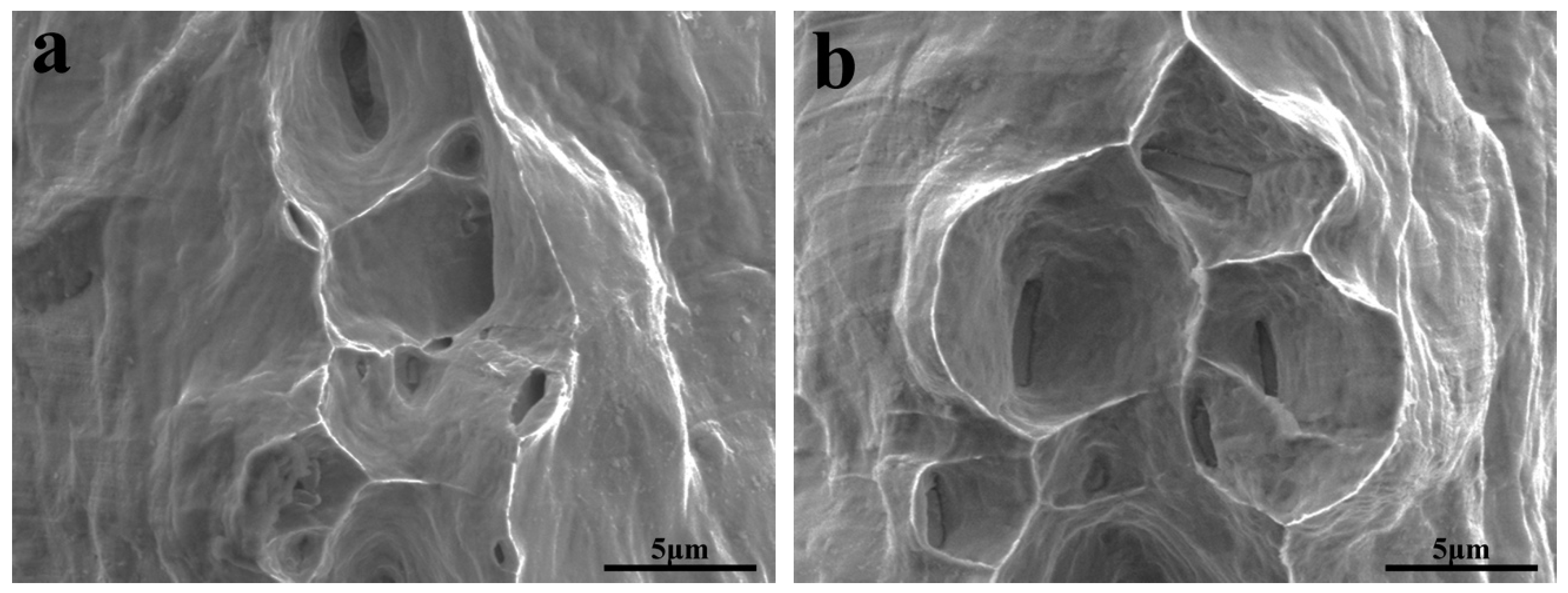
3.1. Microstructure Characterization
3.2. Mechanical Properties
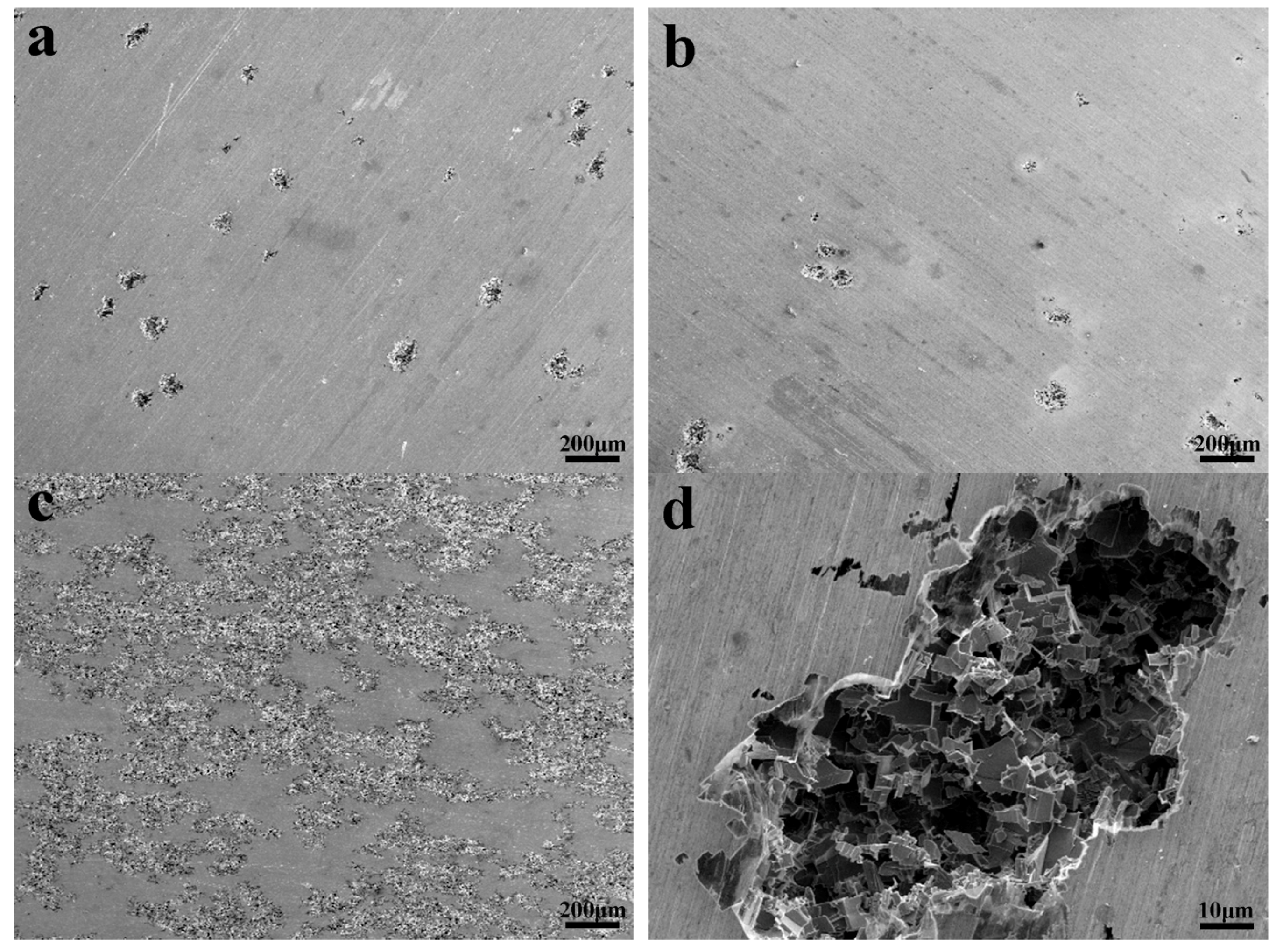
3.3. Corrosion Behavior
3.3.1. Polarization Analysis
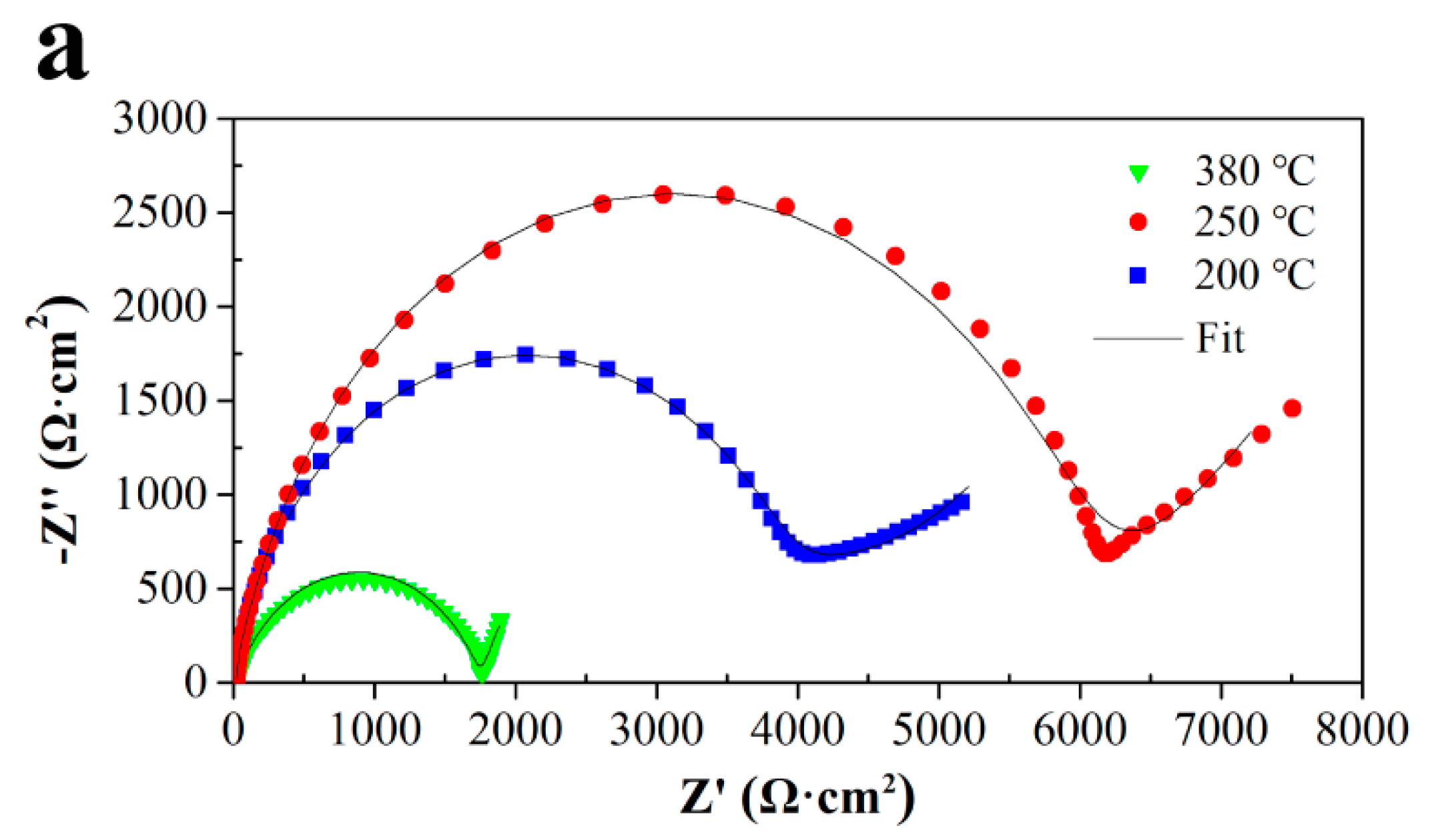
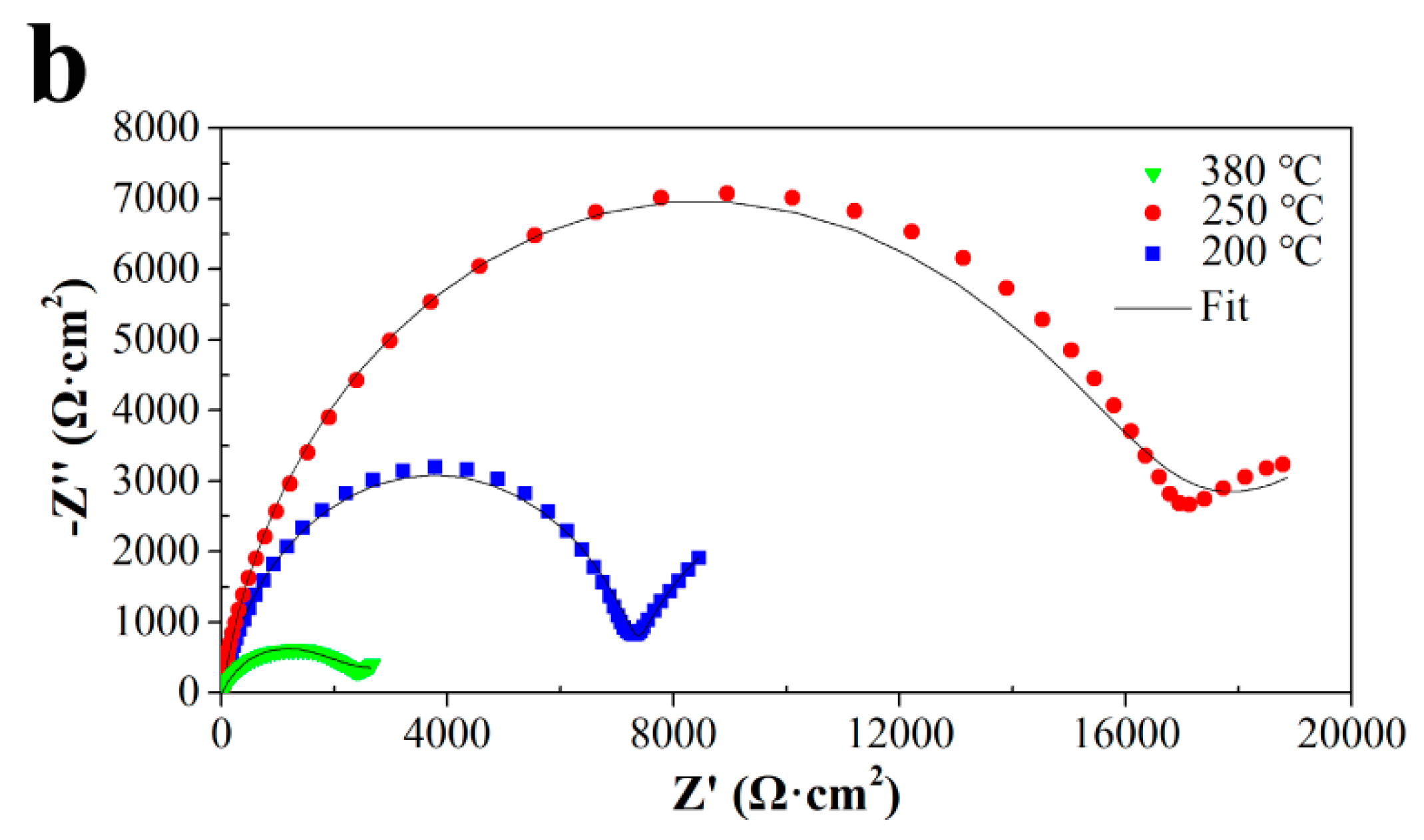
3.3.2. EIS Simulation and Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xia, X.F.; Gu, Y.Y.; Xu, S.A. Titanium conversion coatings on the aluminum foil AA 8021 used for lithium–ion battery package. Appl. Surf. Sci. 2017, 419, 447–453. [Google Scholar] [CrossRef]
- Ohsaki, T.; Takami, N.; Kanda, M.; Yamamoto, M. High Performance Thin Lithium-Ion Battery Using an Aluminum-Plastic Laminated Film Bag. Stud. Surf. Sci. Catal. 2001, 132, 925–928. [Google Scholar] [CrossRef]
- Müller, K.; Schoenweitz, C.; Langowski, H.C. Thin Laminate Films for Barrier Packaging Application–Influence of Down Gauging and Substrate Surface Properties on the Permeation Properties. Packag. Technol. Sci. 2012, 25, 137–148. [Google Scholar] [CrossRef]
- Shi, Z.M.; Gao, K.; Shi, Y.T.; Wang, Y. Microstructure and mechanical properties of rare-earth-modified Al−1Fe binary alloys. Mater. Sci. Eng. A 2015, 632, 62–71. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Q.; Li, M.; Huang, X.; Li, B. Effect of Sc and Zr additions on corrosion behavior of Al–Zn–Mg–Cu alloys. J. Alloy. Compd. 2014, 612, 42–50. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, D.; Xu, X.; Gao, Y.; Chen, G.; Chen, W.; You, X.; Huang, Y.; Tang, J. Effect of Ce addition on the mechanical and electrochemical properties of a lithium battery shell alloy. J. Alloys Compd. 2014, 617, 665–669. [Google Scholar] [CrossRef]
- Chen, K.H.; Fang, H.C.; Zhang, Z.; Chen, X.; Liu, G. Effect of of Yb, Cr and Zr additions on recrystallization and corrosion resistance of Al–Zn–Mg–Cu alloys. Mater. Sci. Eng. A 2008, 497, 426–431. [Google Scholar] [CrossRef]
- Chen, H.; Fan, C.; Liu, Y.; Wen, Y.; Zhong, X. Effect of Fe and Ce on corrosion behaviors of aluminum in 3.5% NaCl solution. Guangxi Sci. 2011, 35, 657–661. [Google Scholar]
- Mi, G.F.; Dong, C.F.; Zhao, D.W. Effect of Different Cooling Speed and Rare Earth La on the Microstructures and Phase Constitution of Al-Fe Alloy. Appl. Mech. Mater. 2010, 44–47, 2126–2130. [Google Scholar] [CrossRef]
- Li, R.; Ma, J.; Zhou, Z.; Yu, H. Effect of rare earth on microstructure of eutectic Al-2Fe alloy. J. Chin. Rare Earth Soc. 2004, 22, 722–724. [Google Scholar]
- Yang, H. The Structure and Property Relationship in Rare Earth Aluminum Alloy Surface Oxide Film. Master’s Thesis, Beijing University of Technology, Beijing, China, June 2002. [Google Scholar]
- Deng, Y.; Yin, Z.; Zhao, K.; Duan, J.; Hu, J.; He, Z. Effects of Sc and Zr microalloying additions and aging time at 120 °C on the corrosion behavior of an Al–Zn–Mg alloy. Corros. Sci. 2012, 65, 288–298. [Google Scholar] [CrossRef]
- Li, C.; Pan, Q.; Shi, Y.; Wang, Y.; Li, B. Influence of aging temperature on corrosion behavior of Al–Zn–Mg–Sc–Zr alloy. Mater. Des. 2014, 55, 551–559. [Google Scholar] [CrossRef]
- Huang, L.P.; Chen, K.H.; Li, S.; Song, M. Influence of high-temperature pre-precipitation on local corrosion behaviors of Al–Zn–Mg alloy. Scr. Mater. 2007, 56, 305–308. [Google Scholar] [CrossRef]
- Fu, G.S.; Sun, F.S.; Ren, L.Y.; Chen, W.Z.; Qian, K.W. Modification Behavior of trace rare earth on impurity phases in commercial purity aluminum. J. Rare Earth 2002, 20, 61–66. [Google Scholar]
- Zhu, M.; Jian, Z.; Yao, L.; Liu, C.; Yang, G.; Zhou, Y. Effect of mischmetal modification treatment on the microstructure, tensile properties, and fracture behavior of Al-7.0%Si-0.3%Mg foundry aluminum alloys. J. Mater. Sci. 2011, 46, 2685–2694. [Google Scholar] [CrossRef]
- Engler, O.; Kong, X.W.; Yang, P. Influence of particle stimulated nucleation on the recrystallization textures in cold deformed al-alloys part I—Experimental observations. Scr. Mater. 1997, 37, 1665–1674. [Google Scholar] [CrossRef]
- Engler, O. On the influence of dispersoids on the particle stimulated nucleation of recrystallization in an Al-Fe-Si model alloy. Mater. Sci. Forum 1998, 273, 483–488. [Google Scholar] [CrossRef]
- Luo, B.H.; Shan, Y.M.; Bai, Z.H. Effect of annealing temperature on microstructure and corrosive properties of cold-rolled 5083 aluminum alloy after quenching. J. Cent. South Univ. 2007, 38, 802–808. [Google Scholar]
- Dong, W.; Chang, B.; Yao, X.; Zhang, Y.; Wen, Z. 8021 Alloy Double Zero Foil and Its Production Process. China Patent CN103695723A, 2 April 2014. [Google Scholar]
- Chai, Z.; Jiang, C.; Zhu, K.; Zhao, Y.; Wang, C.; Cai, F.; Chen, M.; Wang, L. Pretreatment Behaviors and Improved Corrosion Resistance for Cu/Co-Ni-Cu Coating Electrodeposition on Magnesium Alloy. J. Electrochem. Soc. 2016, 163, D493–D499. [Google Scholar] [CrossRef]
- Moreira, A.H.; Benedetti, A.V.; Sumodjo, P.T.A.; Garrido, J.A.; Cabot, P.L. Electrochemical behaviour of heat-treated Al–Zn–Mg alloys in chloride solutions containing sulphate. Electrochim. Acta 2002, 47, 2823–2831. [Google Scholar] [CrossRef]
- Park, J.; Paik, C.H.; Huang, Y.H.; Alkire, R. Influence of Fe-Rich Intermetallic Inclusions on Pit Initiation on Aluminum Alloys in Aerated NaCl. J. Electrochem. Soc. 1999, 146, 517–523. [Google Scholar] [CrossRef]
- Rynders, R.M.; Paik, C.H.; Ke, R.; Alkire, R. Use of in situ atomic force microscopy to image corrosion at inclusions. J. Electrochem. Soc. 1994, 141, 1439–1445. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical characteristics of intermetallic phases in aluminum alloys: An experimental survey and discussion. J. Electrochem. Soc. 2005, 152, b140. [Google Scholar] [CrossRef]
- Ambat, R.; Davenport, A.J.; Scamans, G.M.; Afseth, A. Effect of iron-containing intermetallic particles on the corrosion behaviour of aluminium. Corros. Sci. 2006, 48, 3455–3471. [Google Scholar] [CrossRef]
- Speckert, L.; Burstein, G.T. Combined anodic/cathodic transient currents within nucleating pits on Al–Fe alloy surfaces. Corros. Sci. 2011, 53, 534–539. [Google Scholar] [CrossRef]
- Du, J.; Ding, D.; Zhang, W.; Xu, Z.; Gao, Y.; Chen, G.; Chen, W.; You, X.; Chen, R.; Huang, Y.; et al. CeLa enhanced corrosion resistance of Al-Cu-Mn-Mg-Fe alloy for lithium battery shell. Appl. Surf. Sci. 2017, 422. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, D.; Zhang, W.; Gao, Y.; Chen, G.; Chen, W.; You, X.; Huang, Y.; Chen, R.; Tang, J. Microstructure and electrochemical property of Ce-containing Al-Cu-Mn Alloy. Nonferrous Met. Mater. Eng. 2016, 37, 78–83. [Google Scholar]
- Baig, M.; Ammar, H.R.; Seikh, A.H.; Alam, M.A.; Alharthi, N.H. Effect of Immersion Time and Temperature on Corrosion Behaviour of Nanocrystalline Al-Fe-Cr Alloy. J. Electrochem. Sci. 2017, 12, 3336–3349. [Google Scholar] [CrossRef]
- Xiao, Y.P.; Pan, Q.L.; Li, W.B.; Liu, X.Y.; He, Y.B. Influence of retrogression and re-aging treatment on corrosion behaviour of an Al–Zn–Mg–Cu alloy. Mater. Des. 2011, 32, 2149–2156. [Google Scholar] [CrossRef]
- Brunner, J.G.; Birbilis, N.; Ralston, K.D.; Virtanen, S. Impact of ultrafine-grained microstructure on the corrosion of aluminium alloy AA2024. Corros. Sci. 2012, 57, 209–214. [Google Scholar] [CrossRef]
- Dorin, T.; Stanford, N.; Birbilis, N.; Gupta, R.K. Influence of cooling rate on the microstructure and corrosion behavior of Al–Fe alloys. Corros. Sci. 2015, 100, 396–403. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S. 15-Role of intermetallics in corrosion of aluminum alloys. Smart corrosion protection. In Intermetallic Matrix Composites; Mitra, R., Ed.; Woodhead Publishing: Shaston, UK, 2018; pp. 425–462. [Google Scholar] [CrossRef]
- Ralston, K.D.; Fabijanic, D.; Birbilis, N. Effect of grain size on corrosion of high purity aluminium. Electrochim. Acta 2011, 56, 1729–1736. [Google Scholar] [CrossRef]
- Argade, G.R.; Panigrahi, S.K.; Mishra, R.S. Effects of grain size on the corrosion resistance of wrought magnesium alloys containing neodymium. Corros. Sci. 2012, 58, 145–151. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, M.; Wang, X.; Li, J. Effect of grain boundaries on the corrosion resistance of aluminum alloys. J. Beijing Univ. Chem. Technol. 2016, 43, 57–62. [Google Scholar] [CrossRef]



| Alloy | State | σ0.2 (MPa) | σb (MPa) | δ (%) |
|---|---|---|---|---|
| AA8021 | as-rolled | - | 85–110 | 2.6–3.2 |
| Al-1.5Fe-0.1La | as-rolled | 132.6 (±0.5) | 143.3 (±0.8) | 4.0 (±0.5) |
| Al-1.5Fe-0.25La | as-rolled | 151.1 (±0.7) | 165.9 (±1.0) | 3.1 (±0.3) |
| Al-1.5Fe-0.1La | annealed (250 °C) | 35.1 (±1.2) | 74.0 (±1.1) | 21.5 (±0.8) |
| Al-1.5Fe-0.25La | annealed (250 °C) | 30.2 (±0.9) | 80.1 (±2.1) | 24.6 (±1.4) |
| Alloy | Annealing Temperature (°C) | Ecorr (mV vs. SHE) | Icorr (μA/cm2) |
|---|---|---|---|
| Al-1.5Fe-0.1La | 200 | −775 (±11) | 2.41 (±0.4) |
| 250 | −768 (±4) | 0.82 (±0.2) | |
| 380 | −806 (±12) | 2.64 (±0.7) | |
| Al-1.5Fe-0.25La | 200 | −756 (±3) | 1.46 (±0.5) |
| 250 | −744 (±7) | 0.62 (±0.1) | |
| 380 | −776 (±9) | 2.57 (±0.8) |
| Alloy | Annealing Temperature (°C) | (Rf + Rct) (Ω cm2) |
|---|---|---|
| Al-1.5Fe-0.1La | 200 | 4034 |
| 250 | 6006 | |
| 380 | 2822 | |
| Al-1.5Fe-0.25La | 200 | 9714 |
| 250 | 15,510 | |
| 380 | 3140 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Ding, D.; Zhang, W.; Gao, Y.; Wu, Z.; Chen, G.; Chen, R.; Huang, Y.; Tang, J. Al-1.5Fe-xLa Alloys for Lithium-Ion Battery Package. Metals 2018, 8, 890. https://doi.org/10.3390/met8110890
Zhang R, Ding D, Zhang W, Gao Y, Wu Z, Chen G, Chen R, Huang Y, Tang J. Al-1.5Fe-xLa Alloys for Lithium-Ion Battery Package. Metals. 2018; 8(11):890. https://doi.org/10.3390/met8110890
Chicago/Turabian StyleZhang, Rong, Dongyan Ding, Wenlong Zhang, Yongjin Gao, Zhanlin Wu, Guozhen Chen, Renzong Chen, Yuanwei Huang, and Jinsong Tang. 2018. "Al-1.5Fe-xLa Alloys for Lithium-Ion Battery Package" Metals 8, no. 11: 890. https://doi.org/10.3390/met8110890





