Abstract
This research describes the adsorption of Cu2+ onto a helical ribbon carbon nanofiber. The characterization of carbon nanofiber by zeta potential showed an isoelectronic pH of 1.9. The influence of different adsorption factors, such as stirring speed, temperature, pH, adsorbent concentration, etc., on the Cu2+ adsorption capacity have been evaluated. The pH has a great influence on Cu2+ adsorption, with the maximum adsorption capacity reached at a pH of 10. The experimental data fit well to pseudo-second order kinetic and Langmuir isotherm models (qm = 8.80 mg·g−1) at T = 298 K and pH = 4. The Cu2+ adsorption could be explained by the particle diffusion model. Results showed that carbon nanofiber could be successfully used for the elimination of Cu2+ from wastewater.
1. Introduction
Heavy metals are characterized by their high relative densities (greater than 5 g·cm−3) and atomic weights (between 63.5 and 200.6) [1,2,3]. Though many of these are necessary for life, higher amounts above required levels are hazardous [4].
Moreover, heavy metals are not biodegradable, being easily accumulated in living organisms, particularly in humans. These can cause serious illnesses, such as cancer, damage to the nervous system, increases in blood and respiratory pressure, kidney failure, and could become lethal at high concentrations [1,5,6,7]. For this reason, the pollution of water due to the presence of heavy metals is a major concern today world-wide. Each year large, volumes of wastewater are produced from different industrial sectors, such as mining, metallurgy and smelting, among others. The most common heavy metals present in wastewaters are nickel, zinc, lead, iron, copper, arsenic, cadmium, and uranium [8,9,10].
Among these, cooper is considered a harmful metal [9,11,12]. There are three forms of copper: Cu0 metal, Cu+ cuprous ion, and Cu2+ cupric ion, this last form normally appearing in the environment and considered to be the most toxic of the three [13,14]. The main contributors of copper to the environment are mining industries, printing circuits, electroplating industries, paints, fertilizers, plastics and etching, etc. [15,16,17]. Certain industries that release wastewater into the environment have reported Cu2+ concentrations ranging from 2.0 mg·L−1 to 900 mg·L−1 [18]. Therefore, the recovery of heavy metals, in general, and in particular of copper, is a subject of great social relevance due to the environmental and economic benefits of eliminating contaminates from industrial effluents.
A great number of methods can be used for the recovery or removal of heavy metals from liquid effluents [19]. The choice of one method over another is based mainly on an economic question and metal concentration [1]. Conventional methods include bioremediation [20,21], reverse osmosis [22], electrochemical treatment [23], coagulation [24], precipitation [25,26], solvent extraction [27,28,29], membrane filtration [30,31], electrodialysis [32], supercritical fluids extraction [33,34], ion exchange [35,36,37,38], and flotation [39,40].
Adsorption is one of the most popular methods for eliminating heavy metals [41]. It is a technology in which the pollutants, particularly in the case of heavy metals, can interact in a physical or chemical way with the active elements of the adsorbent. Adsorption is a technique that is easy to use and design, and it does not produce contaminants [42]. Moreover, another advantage of the adsorption process is the possibility to regenerate the adsorbent by desorption, being therefore a reversible technique, so it is considered a technology that is not aggressive with the environment. These advantages have made adsorption a leading method for wastewater treatment [13].
Different types of adsorbent have been developed, such as active carbon, clay ore, chelating materials, natural chitosan/zeolites, etc. [19,41,43,44]. In the last twenty years, the interest in nanotechnology has been growing. Materials such as fullerenes, carbon nanotubes, graphene and graphene oxide have a large number of applications in different fields, including the removal of heavy metals from wastewater [38,45]. Their unique properties, including high resistance, electrical conductivity and thermal stability, as well as having large specific surface areas, make them sustainable adsorbents for wastewater treatment, and more specifically, for the elimination of heavy metals [46,47]
This paper reports the results derived from Cu2+ adsorption on new helix spiral carbon nanofibers (CNF). CNF are structures composed of a helical ribbons of graphene, spiraled about and angled to the fiber axis, similar to the structure of an Archimedes screw with flights inclined to the axis [48].
There are many factors that influence heavy metal adsorption efficiency, such as initial metal concentration, temperature, sorbate amount, pH, and contact time [42,43]. Therefore, the objective of this research was to determine the effects of various experimental variables on Cu2+-CNF adsorption systems. Furthermore, kinetic mechanisms, adsorption isotherms models and thermodynamics parameters were investigated.
2. Materials and Methods
Carbon nanofibers donated by Antolin group S.A. were used as an adsorbent [49]. Stock metal solution was prepared from CuSO4 (Fluka, Munich, Germany). All other reagents used in the work were analytical reagent grade.
2.1. Characterization of Carbon Nanofibers
Carbon nanofibers were examined using a Hitachi S4800 (Tokyo, Japan) scanning electron apparatus equipped with a detectors of secondary, backscattered and transmission electrons, and an Oxford Inca Energy dispersion microanalysis system. Nanomaterials were dispersed in ethanol, and drops of the suspension material were deposited on TEM grating.
The zeta potential was measured using a Zetasizer Malvern Nano ZS (Malvern Panalytical Ltd., Worcestershire, UK) at 25 °C. To study the influence of pH, aqueous suspensions were prepared in pH solutions between 1 and 13 using solutions of 0.5 M of HCl and NaOH.
2.2. Batch Adsorption Experiments
The batch adsorption experiments were carried out in a 250 mL glass reactor capable of mechanical (impeller) shaking.
Solutions (100 mL) containing Cu2+ were used, and weighed amounts of the adsorbent, carbon nanofibers (CNF), were added. The pH was adjusted using HCl and NaOH. The residual copper content in the aqueous solution was determined by AAS (Perkin Elmer 1100 B spectrophotometer, Waltham, MA, USA). These were filtered prior to solution analyses. The metal uptake capacity was calculated using Equation (1):
where qt is the amount metals ions adsorbed (mg·Cu·g−1 sorbent), while C0 and Ct are the initial and contact time metal ion concentrations (mg·Cu·L−1) in the aqueous solution, respectively. V is the volume of the solution (0.1 L), and M is the mass of the sorbent (g).
The removal efficiency was calculated using the following equation:
where Ce (mg·Cu·L−1) is the equilibrium metal concentration in solution.
3. Results
3.1. Characterization of CNF
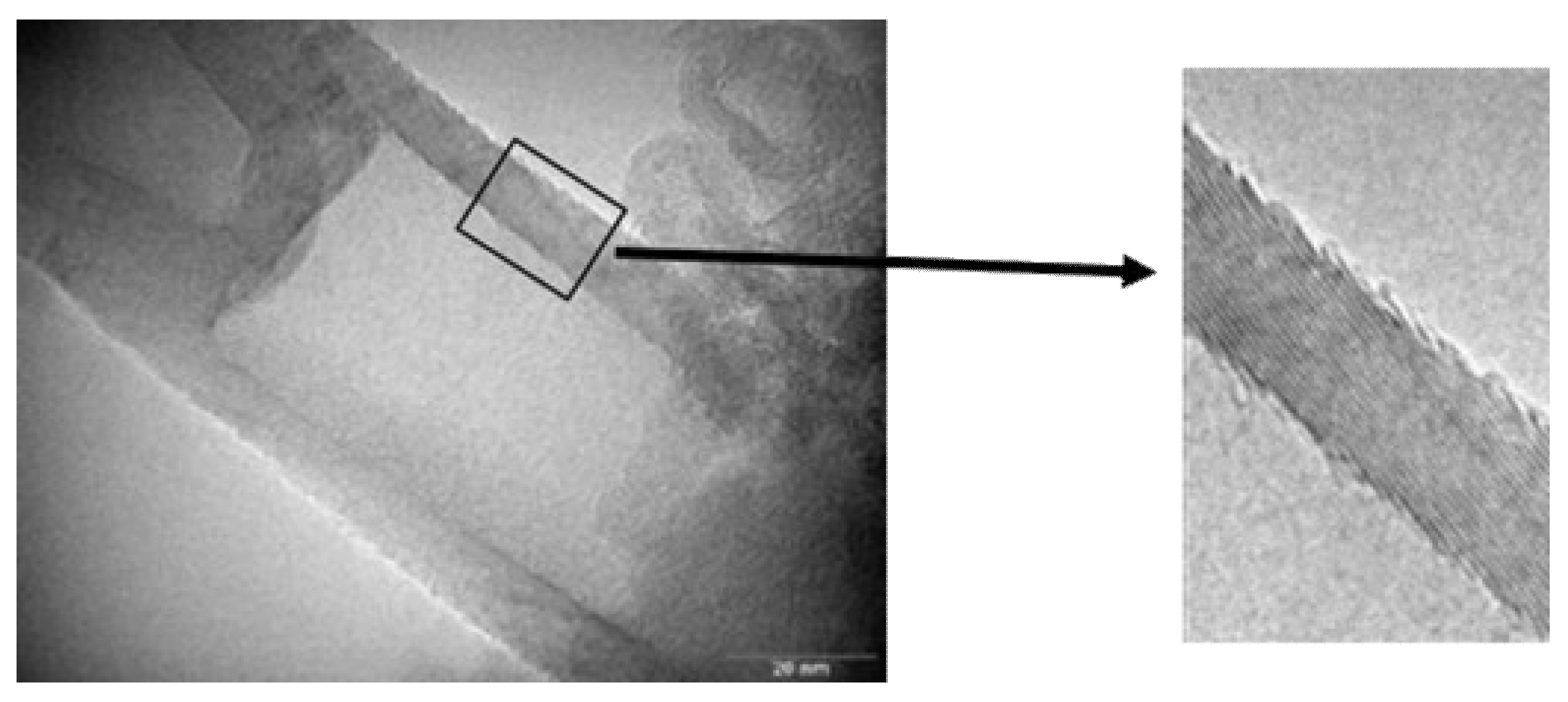
TEM micrographs of CNF are shown in Figure 1. The helix spiral structure is observed. The structure of CNF is a continuous cone-helix. The presence of pyrolytic amorphous carbon in the CNF is not observed [49]. CNF structural details are presented in references [48,49,50]. Table 1 shows the elemental composition.
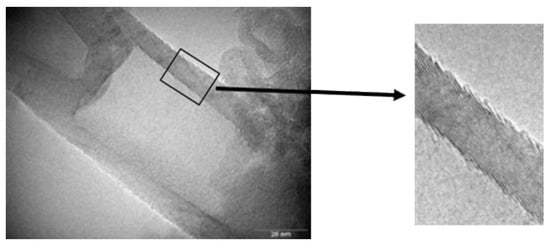
Figure 1.
TEM images of carbon nanofibers (CNF) (graphitic structure is observed).

Table 1.
CNF elemental analysis (wt %).
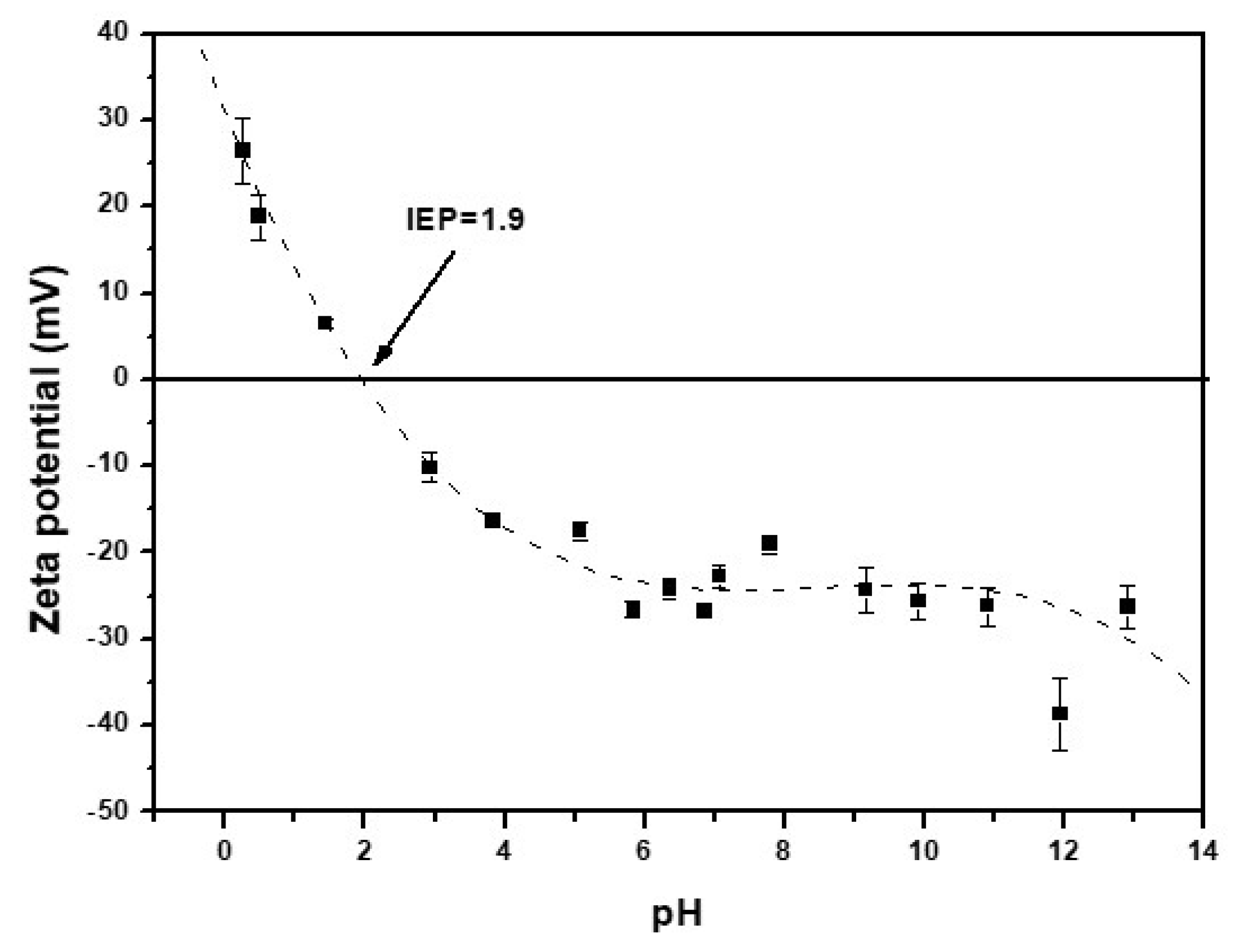
The isoelectric point (IEP) of CNF is obtained at a pH value of 1.9 (Figure 2). At a pH values around the pH(IEP), the sorbent surface charge is neutral. When the pH value is lower than the isoelectronic point, the sorbent surface has a positive charge, and a negative charge when the pH is greater than 1.9 [37,51]. The surface charge could affect in the adsorption properties of the adsorbent [51].
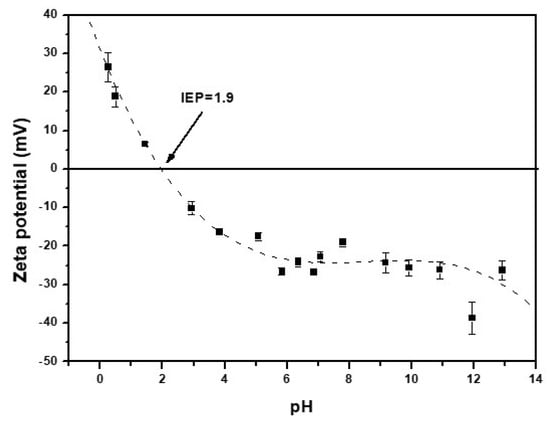
Figure 2.
Zeta potential of CNF versus pH.
3.2. Sorption Bach Experiment
To research the efficiency of the CNF in Cu2+ adsorption, parameters such as contact time, stirring speed, temperature, pH, carbon nanomaterials concentration, and copper concentration have been studied.
3.2.1. Effect of the Contact Time and the Stirring Speed
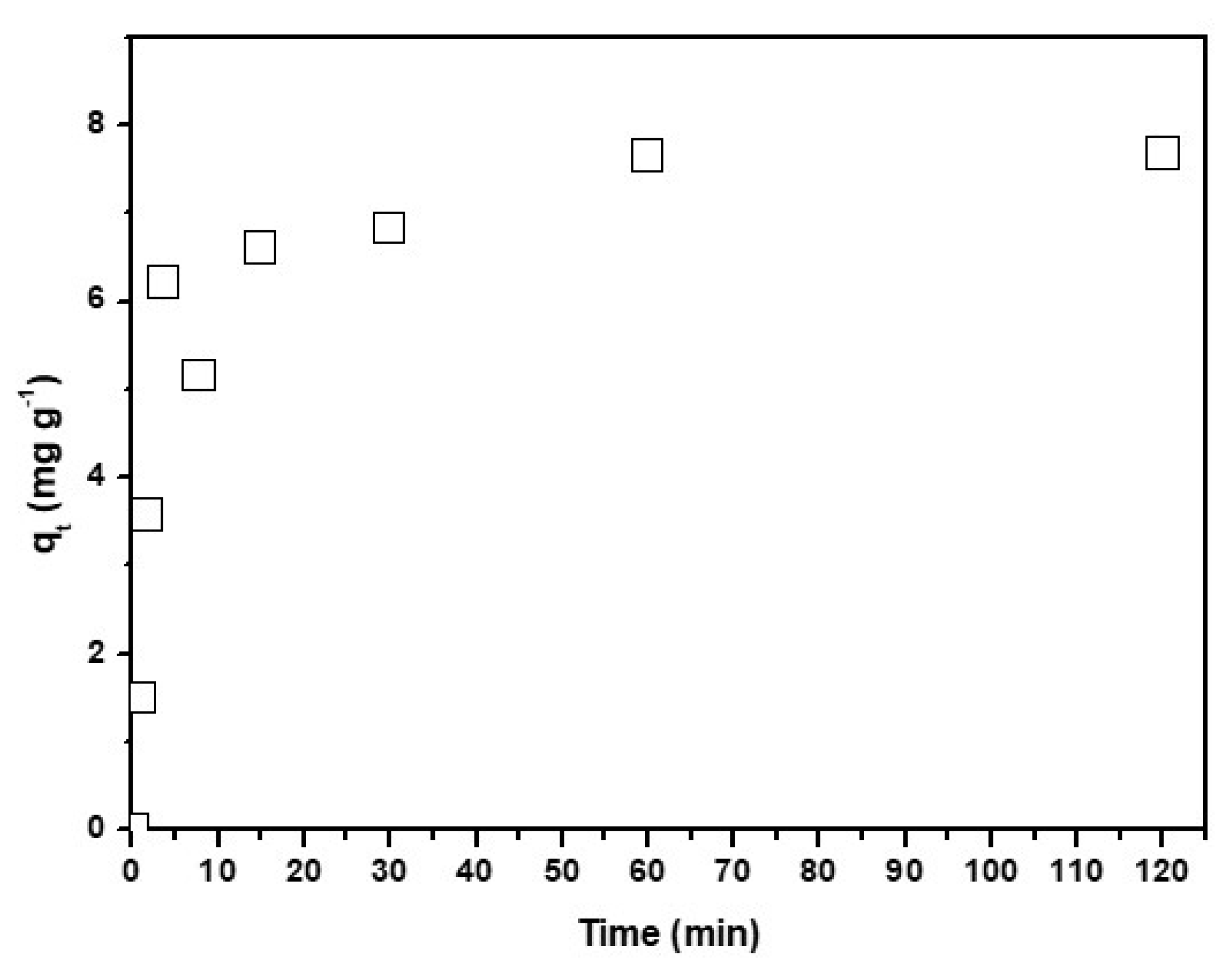
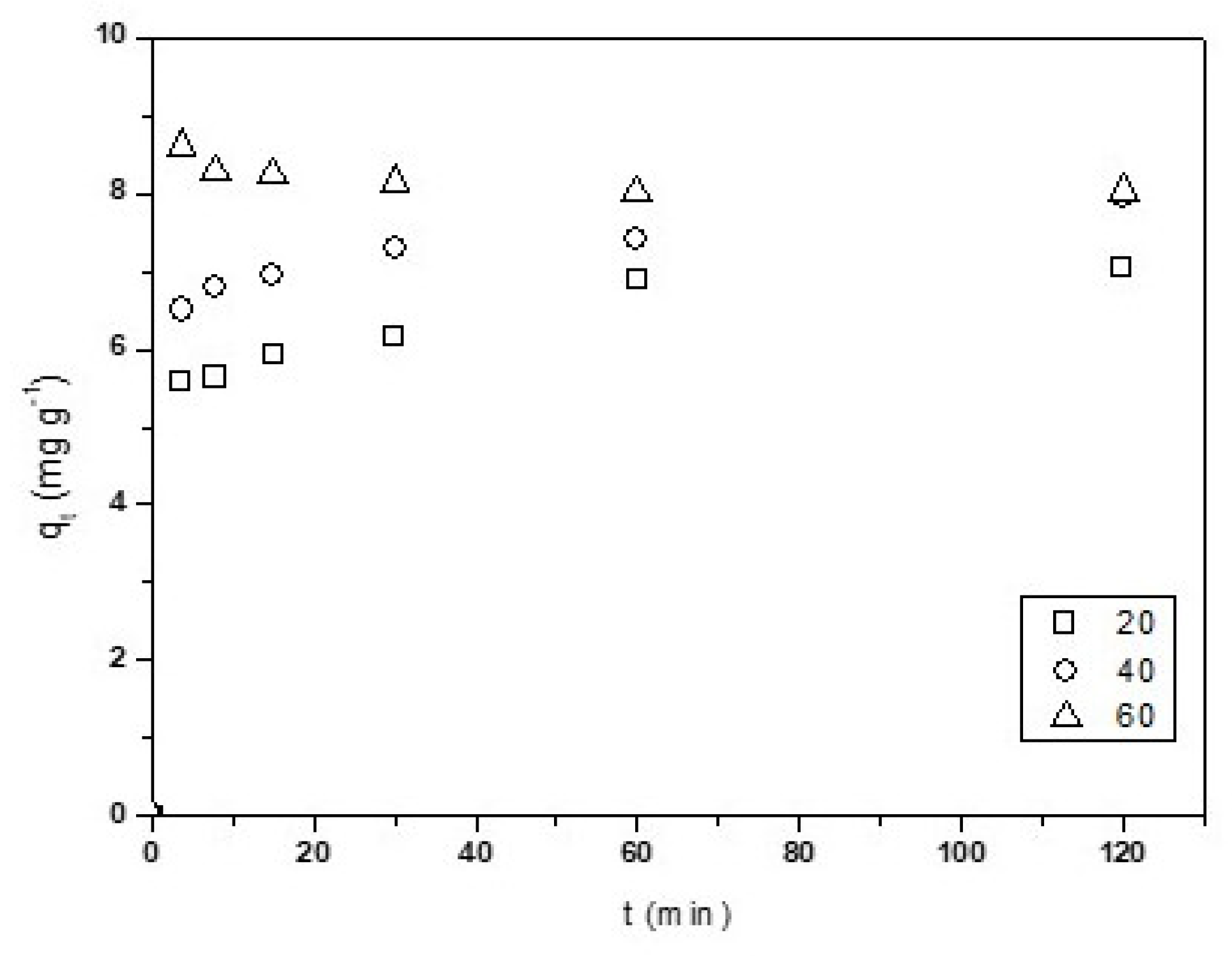
Figure 3 shows the effect of the contact time on Cu2+ adsorption. The adsorption kinetics was investigated for better understanding of the dynamics of adsorption of metal on the CNF. The removal of Cu2+ between 0–30 min increased sharply, and then tended to became almost stable, which denoted accomplishment of equilibrium at 30 min. From this point, the qt values remained constant. This behavior could be due to the fact that, at the beginning, all of the active sites on the CNF surface were empty and the copper concentration high, and after 30 min, the surface active sites available diminish and the copper concentration lower, so the metal uptake remain constant [52]. The copper adsorption was a quick process.
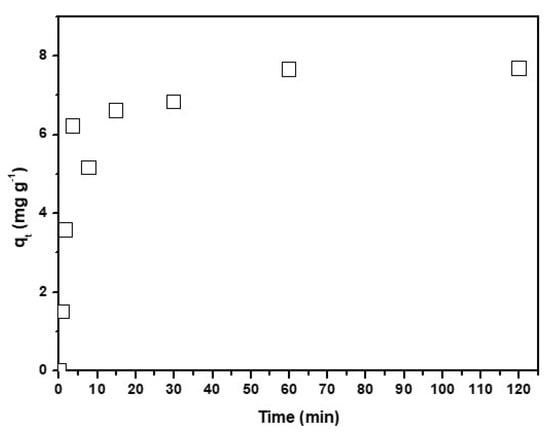
Figure 3.
Contact time effect in the adsorption of Cu2+ onto CNF. Experimental conditions: CNF = 0.1 g, V = 100 mL of [Cu2+] = 0.01 mg·L−1 pH = 4, T = 298 K, θ = 500 rpm.
The equilibrium time could be decreased by increasing the stirring speed, so the appropriate hydrodynamic conditions were investigated. Table 2 shows that an increase in the stirring speed increased qt values, reaching a maximum between 500–2000 rpm. In this stirring speed interval, the thickness of the aqueous film diffusion layer and the aqueous resistance to mass transfer are at a minimum. An increase in the stirring speed to higher than 2000 rpm decreases the Cu2+ adsorption. This could be due the formation of local equilibrium between the Cu2+ solution and the surrounding CNF and/or the agglomeration of the nanoparticles.

Table 2.
Effect of the stirring speed on the Cu2+ adsorption onto the CNF. Experimental conditions: CNF = 0.1 g, V = 100 mL of [Cu2+] = 0.01 mg·L−1 pH = 4, T = 298 K, θ = variable.
3.2.2. Adsorption Kinetic and Diffusion Mechanism
To understand the process of Cu2+ adsorption onto CNF, three kinetic adsorption models have been assessed [53]:
- (a)
- pseudo-first order [51]
- (b)
- pseudo-second order [54]
- (c)
- Elovich model [55]where qe and qt (mg·g−1) are the sorption capacities at equilibrium and at time t (min), respectively, k1 is first-order adsorption rate constant (min−1), and k2 (g·mg−1·min−1) is the pseudo-second order constant. α is the initial adsorption rate (mg·min−1) at contact time t = 0 min, and β is the extent of surface coverage and activated energy (g·mg−1).
According to the calculated data, among the different adsorption reaction models, the higher correlation coefficient, R2, was obtained for the pseudo-second order kinetics model (Equation (4) and Table 3). The qe values derived from the model and the experiment are really similar: qe (experimental) 6.51 mg·g−1 [56].

Table 3.
Kinetic parameters for Cu2+ adsorption onto CNF.
Three adsorption mechanisms have been considered to evaluate the adsorption of Cu2+ on CNF [57,58]:
- (a)
- Film diffusion controlled process: The metal species diffused from the aqueous solution to the CNF surface
- (b)
- Particle diffusion controlled process: The ion diffused inside CNF
- (c)
- Moving boundary processwhere K (min−1) is the rate constant model, qe and qt (mg·g−1) are the sorption capacities at equilibrium and contact time, respectively, and t (min).
The results obtained by the kinetic diffusion models show that the Cu2+ adsorption onto the CNF could be better explained by the particle diffusion model, where K = 0.0525 min−1 (Table 4).

Table 4.
Adsorption mechanism kinetic parameters for Cu2+ adsorption onto CNF.
3.2.3. pH Influence on Cu2+ Adsorption
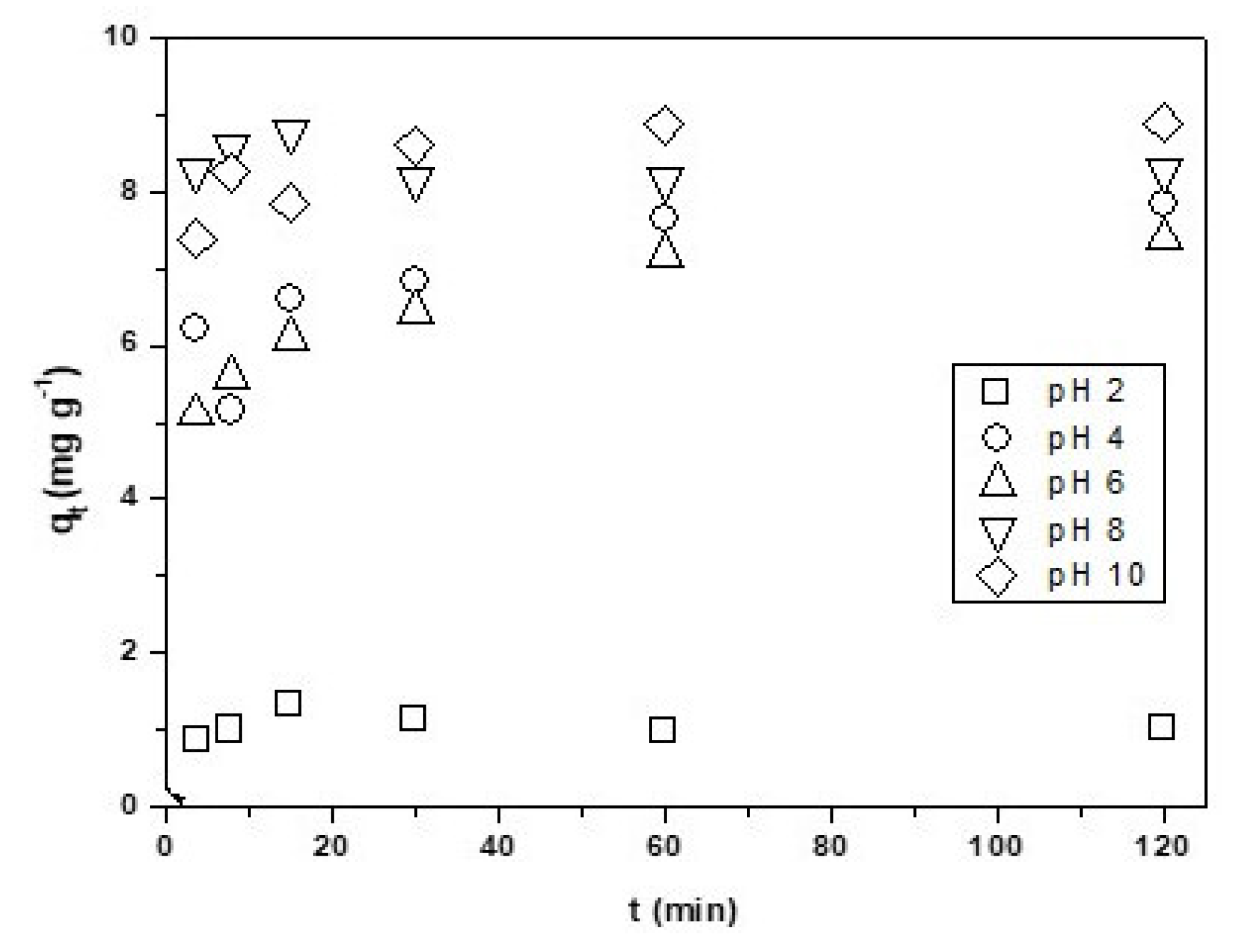
As seen in Figure 4, at a low pH of two, the adsorption of Cu2+ onto CNF is very low. This is due to the pH value being very near the zero potential pH = 1.9, the charge in the surface being 0 (neutral), and the Cu2+ adsorption being nil or very low. At pH values lower than two, the potential of the surface charge of the CNF is positive, the cations in solution are repealed from the adsorbent, and it is not adsorbed. When the pH increases to between 4 and 10, the cooper adsorption increased to its maximum at pH 10 (qe = 8.88 mg·g−1). Above the isoelectronic pH, the nanomaterial surfaces have a negative charge and attract the metal ions facilitating the adsorption [18,37].
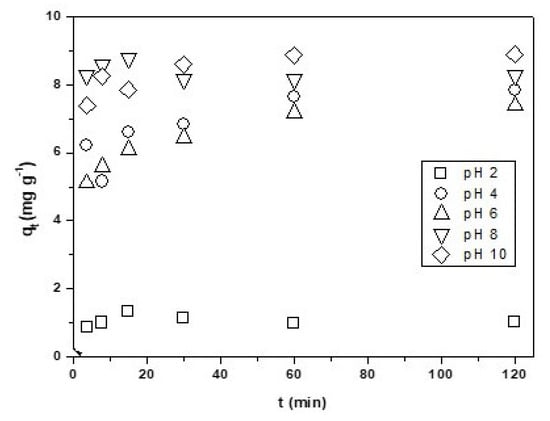
Figure 4.
Effect of the pH on the Cu2+ adsorption onto CNF. Experimental conditions: CNF = 0.1 g, V = 100 mL of [Cu2+] = 0.01 g·L−1 pH = variable, T = 298 K, θ = 500 rpm.
3.2.4. Effect of the Copper Concentration
The adsorption capacity depends on Cu2+ concentration; Table 5 shows an increase in copper concentration in the aqueous phase resulting in a reduction of the recovery percentage. This may be associated with a competitive effect of copper by the active position of the adsorbent.

Table 5.
Effect of the Cu2+ concentration on the adsorption onto CNF. Experimental conditions: CNF = 0.1 g, V = 100 mL of [Cu2+] = variable, pH = 4, T = 298 K, θ = 500 rpm.
The adsorption equilibrium data of the Cu2+-CNF system were checked in terms of three isotherm models: Langmuir, Freundlich, Dubinin-Radushkevich and Temkin [59]. The following equations have been used:
- (a)
- Langmuir [60,61]:
- (b)
- Freundlich [62]:where qe (mg·g−1) is the sorption capacities at equilibrium time, Ce (mg·L−1) is the metal ion concentration in the aqueous phase at equilibrium, qm (mg·g−1) is maximum sorption capacity, and b (L·mg−1) is the Langmuir sorption constant. RL is a dimensional constant separation [63]. C0 (mg·L−1) is the maximun initial copper concentration. KF is the Freundlich constant (L·g−1), and 1/n is a heterogeneity factor constant, their values must be less than one for favorable adsorption.
- (c)
- Dubinin-Radushkevich [64]:where:where qe are mg·g−1 in equilibrium, qd is the theoretical capacity adsorption (mg·g−1), K is a constant related to the mean free energy in the adsorption (mol2·J−2) and E (KJ·mol−1) [63]. ε is the Polanyi adsorption potential, R is the universal gas constant (8.314 J·K−1·mol−1), T is the temperature in K, and Ce is the metal ion concentration in the aqueous phase at equilibrium in mg·L−1.
- (d)
- Temkin [65]:where qe is the sorption capacities at equilibrium time in mol·g−1, Ce is the metal ion concentration in the aqueous phase at equilibrium in mol·L−1, and KT is the isotherm equilibrium binding constant (L·g−1); B is a constant related to the sorption heat in J·mol−1.
According to the values of the correlation coefficient, R2, the experimental results fit well to the studied isotherms, Table 6. However, the best fit is obtained for the Langmuir model. The qm value obtained from this model is similar to the experimental one, 8.77 mg·g−1. The separation factor, RL, shows that the adsorption process is favorable if the RL value is 0 < RL < 1, unfavorable when RL > 1, linear when RL = 1, and irreversible at values of RL = 0. The Cu2+ adsorption on CNF is favorable with a RL value between zero and one, the value here being 5.6 × 10−3. The 1/n value obtained from adjusting the data to the Freundlich isotherm is 9.12 × 10−2, also indicating that the copper adsorption on CNF is favorable.

Table 6.
Calculated isotherms parameters. Experimental conditions: CNF = 0.1 g, V = 100 mL of [Cu2+] = variable pH = 4, T = 298 K, θ = 500 rpm.
The energy value calculated using the Dubinin-Raduskevich model was E = 7.34 (KJ·mol−1). This is lower than 8 KJ·mol−1, which indicates that the mechanism of adsorption has a physical nature [63].
3.2.5. Effect of the CNF Dosage
The CNF dosage was modified in order to evaluate the effect of this parameter. It was expected that an increase in the CNF dosage will increase the adsorption percentage and diminish the adsorption capacity values.
The objective of this research was to achieve the highest possible metal recovery. The best result was obtained using 0.2 g of CNF, with 97% of the copper recovered (see Table 7).

Table 7.
Effect CNF concentration. Experimental conditions: CNF = variable, V = 100 mL of [Cu2+] = 0.01 mg·L−1, pH = 4, T = 298 K, θ = 500 rpm.
3.2.6. Temperature Influence and Thermodynamic Studies
The influence of the temperature (293–333 K) on Cu2+ adsorption on CNF have been studied. The assay conditions were 0.01 g·L−1 of Cu(II), 4 pH, 0.1 g of CNF adsorbent, and a stirring speed of 500 rpm. Figure 5 shows the variation of qt vs. t.
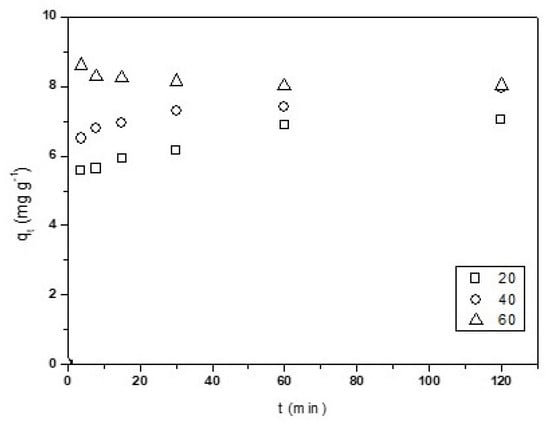
Figure 5.
Temperature effect on the variation of qt (mg·g−1) vs. time.
An increase of the qe value with temperature was observed, having reached a maximum at 333 K, 8 mg·g−1. Therefore, the process is considered endothermic. The increase of adsorption with temperature can be due to different factors, such as a change in the pore size of the nanofibers and/or an increase of the active sites. In addition, the collisions between the metal and the surface of the nanofibers are greater, thereby contributing to a rise in the adsorption capacity [18].
The experimental data obtained at different temperatures (293–333 K) was used to calculate the thermodynamics parameters, ΔH°, ΔG°, and ΔS°, using the following equation:
where ΔS° is the entropy of the reaction, ΔH° is the enthalpy, T is the temperature in Kelvin, and R is the universal gas constant (8.314 J·K−1·mol−1). Kd is defined as follows:
where qe, (mg·g−1) and Ce (mg·L−1) are the equilibrium capacity on the solid and the liquid phases, respectively. Plotting the linear fit vs. to the slope could be used to calculate the ΔH° value (Table 8).

Table 8.
Thermodynamic parameters for Cu2+-CNF system.
For the entire temperature range studied, the ΔG° values were negative, this indicating that the adsorption process is spontaneous. These values are lower than 18 KJ·mol−1, characteristic of a physisorption [18], and this agrees with the result obtained from RL Langmuir parameter (see Table 6).
The positive enthalpy confirms the endothermic behavior of Cu2+ adsorption on the CNF. The ΔS° value is positive, which indicates an increase in the randomness in the CNF/Cu2+ system. In the temperature range studied, the ΔH° is lower than T·ΔS°, so the sorption process was dominated by entropic rather than enthalpic changes [51,62].
3.2.7. Cu2+ Elution
According to the results for Cu2+ adsorption on CNF, it is expected that an increase in the acid concentration changes the adsorption conditions, and Cu2+ could then be eluted. The effect of the acid concentration in elution was studied. For 30 min, 0.05 g of CNF load with 4.83 mg·g−1 of Cu2+ were put in contact with 20 mL of H2SO4 in solutions of different concentrations at a stirring speed of 500 rpm.
An increase in the acid concentration increased the elution percentage (see Table 9), reaching a percentage of around 96% with a H2SO4 concentration of 4 M. The kinetic elution study was performed using a solution of 2 M sulfuric acid, with equilibrium reached after 15 min. The elution kinetics was faster than the adsorption.

Table 9.
Elution percentage.
4. Conclusions
The results obtained in this research prove that helical carbon nanofibers are a potential adsorbent of Cu2+ from wastewaters. Of the parameters studied, pH has the greatest influence on copper adsorption capacity, reaching a maximum qm value of around 8.80 mg·g−1 at a pH value of 10. An increase in the temperature does not influence the adsorption capacity; however, the equilibrium is reached in less time.
The Cu2+ kinetics fitted a pseudo-second order (0.0574 g·mg−1·min−1). The Cu2+ adsorption is considered a particle diffusion controlled process. The experimental data adjusted to a Langmuir model, the qm = 8.80 mg·g−1, is a favorable, endothermic (ΔH° = 26.76 KJ·mol−1) and physical nature adsorption. Metal elution was carried out with a H2SO4 4 M solution, at 30 min, reaching an elution percentage of 96%.
Author Contributions
Investigation, methodology, formal analysis, writing original draft, validation: I.G.-D., investigation, supervision, writing-review and editing: F.A.L., investigation, supervision, writing-review and editing: F.J.A.
Funding
This research received no external funding.
Acknowledgments
To CSIC Agency (Spain) for support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbas, A.; Al-amer, A.M.; Laoui, T.; Al-marri, M.J.; Nasser, M.S.; Khraisheh, M.; Ali, M. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar]
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar]
- Bertinato, J.; Abbe, M.R.L. Maintaining copper homeostasis: Regulation of copper-trafficking proteins in response to copper deficiency or overload. J. Nutr. Biochem. 2004, 15, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Tyler Mehler, W.; Gagliardi, B.; Keough, M.J.; Pettigrove, V. Evaluating freshwater mining sediment toxicity in Tasmania: Achieving strong multiple lines of evidence. Sci. Total Environ. 2019, 651, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Taoufiq, L.; Laamyem, A.; Monkade, M.; Zradba, A. Characterization and Application of Solid Waste in the Adsorption of Heavy metals. J. Mater. Environ. Sci. 2016, 7, 4646–4656. [Google Scholar]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Jayaweera, H.D.A.C.; Siriwardane, I.; de Silva, K.M.N.; de Silva, R.M. Synthesis of multifunctional activated carbon nanocomposite comprising biocompatible flake nano hydroxyapatite and natural turmeric extract for the removal of bacteria and lead ions from aqueous solution. Chem. Cent. J. 2018, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Nassef, E. Removal of Copper From Wastewater By Cementation From Simulated Leach Liquors. J. Chem. Eng. Process Technol. 2015, 6, 214. [Google Scholar] [CrossRef]
- Yang, S.; Ren, X.; Zhao, G.; Shi, W.; Montavon, G.; Grambow, B.; Wang, X. Competitive sorption and selective sequence of Cu(II) and Ni(II) on montmorillonite: Batch, modeling, EPR and XAS studies. Geochim. Cosmochim. Acta 2015, 166, 129–145. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Awual, M.R.; Ismael, M.; Khaleque, M.A.; Yaita, T. Ultra-trace copper(II) detection and removal from wastewater using novel meso-adsorbent. J. Ind. Eng. Chem. 2014, 20, 2332–2340. [Google Scholar] [CrossRef]
- Trakal, L.; Šigut, R.; Šillerová, H.; Faturíková, D.; Komárek, M. Copper removal from aqueous solution using biochar: Effect of chemical activation. Arab. J. Chem. 2014, 7, 43–52. [Google Scholar] [CrossRef]
- Howard, P.H.; Muir, D.C.G. Identifying New Persistent and Bioaccumulative Organics among Chemicals in Commerce. Environ. Sci. Technol. 2010, 44, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, A.M.; Gonzalez, D.; Heredia, L.G.; Marquez, K.; Perez, C.; Pena, E.; Flores, K.; Valdes, C.; Eubanks, T.M.; Parsons, J.G.; et al. Removal of Cu2+ and Ni2+ from aqueous solution using SnO2 nanomaterial effect of: pH, time, temperature, interfering cations. Microchem. J. 2018, 141, 188–196. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.M.; Viñarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Tiquia-Arashiro, S.M. Lead absorption mechanisms in bacteria as strategies for lead bioremediation. Appl. Microbiol. Biotechnol. 2018, 102, 5437–5444. [Google Scholar] [CrossRef] [PubMed]
- MLayah, A.; Jellali, S. Study of continuous lead removal from aqueous solutions by marble wastes: Efficiencies and mechanisms. Int. J. Environ. Sci. Technol. 2015, 12, 2965–2978. [Google Scholar] [CrossRef]
- Matsumiya, M.; Sumi, M.; Uchino, Y.; Yanagi, I. Recovery of indium based on the combined methods of ionic liquid extraction and electrodeposition. Sep. Purif. Technol. 2018, 201, 25–29. [Google Scholar] [CrossRef]
- Zhang, P.; Hahn, H.H.; Hoffmann, E. Different Behavior of Iron(III) and Aluminum(III) Salts To Coagulate Silica Particle Suspension. Acta Hydrochim. Hydrobiol. 2003, 31, 145–151. [Google Scholar] [CrossRef]
- Boamah, P.O.; Huang, Y.; Hua, M.; Zhang, Q.; Wu, J.; Onumah, J.; Sam-Amoah, L.K.; Boamah, P.O. Sorption of heavy metal ions onto carboxylate chitosan derivatives—A mini-review. Ecotoxicol. Environ. Saf. 2015, 116, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Vaccari, D.A.; Li, Y.; Shammas, N.K. Chemical Precipitation. In Physicochemical Treatment Processes; Humana Press: Totowa, NJ, USA, 2005; pp. 141–197. [Google Scholar]
- Alguacil, F.J.; Garcia-Diaz, I.; Lopez, F.; Rodriguez, O. Recycling of copper flue dust via leaching-solvent extraction processing. Desalin. Water Treat. 2015, 56. [Google Scholar] [CrossRef]
- Ye, Q.; Li, G.; Deng, B.; Luo, J.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Solvent extraction behavior of metal ions and selective separation Sc3+ in phosphoric acid medium using P204. Sep. Purif. Technol. 2019, 209, 175–181. [Google Scholar] [CrossRef]
- Wang, L.Y.; Guo, Q.J.; Lee, M.S. Recent advances in metal extraction improvement: Mixture systems consisting of ionic liquid and molecular extractant. Sep. Purif. Technol. 2019, 210, 292–303. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Ozgun, H.; Dereli, R.K.; Ozturk, I.; Roest, K.; van Lier, J.B. A review on dynamic membrane filtration: Materials, applications and future perspectives. Bioresour. Technol. 2012, 122, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Kökkılıç, O.; Waters, K.E. The use of the emulsion liquid membrane technique to remove copper ions from aqueous systems using statistical experimental design. Miner. Eng. 2017, 107, 88–99. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Křivčík, J.; Mikulášek, P. Investigation of batch electrodialysis process for removal of lead ions from aqueous solutions. Chem. Eng. J. 2014, 256, 324–334. [Google Scholar] [CrossRef]
- Kanari, N.; Allain, E.; Joussemet, R.; Mochón, J.; Ruiz-Bustinza, I.; Gaballah, I. An overview study of chlorination reactions applied to the primary extraction and recycling of metals and to the synthesis of new reagents. Thermochim. Acta 2009, 495, 42–50. [Google Scholar] [CrossRef]
- Burford, M.D.; Ozel, M.Z.; Clifford, A.A.; Bartle, K.D.; Lin, Y.; Wai, C.M.; Smart, N.G. Extraction and recovery of metals using a supercritical fluid with chelating agents. Analyst 1999, 124, 609–614. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Alguacil, F.J. La eliminación de metales tóxicos presentes en efluentes líquidos mediante resinas de cambio iónico. Parte IV: Cromo(III)/H+/Lewatit SP112. Rev. Metal. 2017, 53, 093. [Google Scholar] [CrossRef]
- Alguacil, F.; Alcaraz, L.; García-Díaz, I.; López, F. Removal of Pb2+ in Wastewater via Adsorption onto an Activated Carbon Produced from Winemaking Waste. Metals 2018, 8, 697. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Lopez, F.A.; Rodriguez, O.; Martinez-Ramirez, S.; Garcia-Diaz, I. Sorption of indium (III) onto carbon nanotubes. Ecotoxicol. Environ. Saf. 2016, 130. [Google Scholar] [CrossRef] [PubMed]
- Smolinski, T.; Wawszczak, D.; Deptula, A.; Lada, W.; Olczak, T.; Rogowski, M.; Pyszynska, M.; Chmielewski, A.G. Solvent extraction of Cu, Mo, V, and U from leach solutions of copper ore and flotation tailings. J. Radioanal. Nucl. Chem. 2017, 314, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tangparitkul, S.; Hendry, B.; Harper, J.; Kim, Y.K.; Hunter, T.N.; Lee, J.W.; Harbottle, D. Selective separation of cesium contaminated clays from pristine clays by flotation. Chem. Eng. J. 2019, 355, 797–804. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Lopez, F.A.; Garcia-Diaz, I. Extracting metals from aqueous solutions using Ti-based nanostructures: A review. Desalin. Water Treat. 2016, 57. [Google Scholar] [CrossRef]
- Salam, M.A.; Makki, M.S.I.; Abdelaal, M.Y.A. Preparation and characterization of multi-walled carbon nanotubes/chitosan nanocomposite and its applicaton for removal of heavy metals from aqueous solution. J. Alloy. Compd. 2011, 509, 2582–2587. [Google Scholar] [CrossRef]
- Matos, M.P.S.R.; Correia, A.A.S.; Rasteiro, M.G. Application of carbon nanotubes to immobilize heavy metals in contaminated soils. J. Nanopart. Res. 2017, 19, 126. [Google Scholar] [CrossRef]
- Bhatia, M.; Satish Babu, R.; Sonawane, S.H.; Gogate, P.R.; Girdhar, A.; Reddy, E.R.; Pola, M. Application of nanoadsorbents for removal of lead from water. Int. J. Environ. Sci. Technol. 2017, 14, 1135–1154. [Google Scholar] [CrossRef]
- Weisenberger, M.; Martin-Gullon, I.; Vera-Agullo, J.; Varela-Rizo, H.; Merino, C.; Andrews, R.; Qian, D.; Rantell, T. The effect of graphitization temperature on the structure of helical-ribbon carbon nanofibers. Carbon 2009, 47, 2211–2218. [Google Scholar] [CrossRef]
- Vera-Agullo, J.; Varela-Rizo, H.; Conesa, J.A.; Almansa, C.; Merino, C.; Martin-Gullon, I. Evidence for growth mechanism and helix-spiral cone structure of stacked-cup carbon nanofibers. Carbon 2007, 45, 2751–2758. [Google Scholar] [CrossRef]
- Tanaka, A.; Yoon, S.-H.; Mochida, I. Preparation of highly crystalline nanofibers on Fe and Fe–Ni catalysts with a variety of graphene plane alignments. Carbon 2004, 42, 591–597. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Atia, A.A.; Mahfouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Vincent, T.; Guibal, E. Dy(III) recovery from dilute solutions using magnetic-chitosan nano-based particles grafted with amino acids. J. Mater. Sci. 2015, 50, 2832–2848. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Isotherms, kinetics and thermodynamic studies of adsorption of Cu2+ from aqueous solutions by Mg2+/K+ type orange peel adsorbents. J. Hazard. Mater. 2010, 174, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Porter, J.; Mckay, G. Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Res. 2001, 35, 605–612. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Rudzinski, W.; Panczyk, T. Kinetics of Isothermal Adsorption on Energetically Heterogeneous Solid Surfaces: A New Theoretical Description Based on the Statistical Rate Theory of Interfacial Transport. J. Phys. Chem. B 2000, 104, 9149–9162. [Google Scholar] [CrossRef]
- Imamoglu, M.; Ozturk, A.; Aydın, Ş.; Manzak, A.; Gündoğdu, A.; Duran, C. Adsorption of Cu(II) ions from aqueous solution by hazelnut husk activated carbon prepared with potassium acetate. J. Dispers. Sci. Technol. 2018, 39, 1144–1148. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical review in adsorption kinetic models. J. Zhejiang Univ. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Garcia-Diaz, I.; Lopez, F.; Rodriguez, O. Removal of Cr(VI) and Au(III) from aqueous streams by the use of carbon nanoadsorption technology. Desalin. Water Treat. 2017, 63. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Garcia-Diaz, I.; Lopez, F. The removal of chromium (III) from aqueous solution by ion exchange on Amberlite 200 resin: Batch and continuous ion exchange modeling. Desalin. Water Treat. 2012, 45. [Google Scholar] [CrossRef]
- Armbruster, M.H.; Austin, J.B. The Adsorption of Gases on Plane Surfaces of Mica. J. Am. Chem. Soc. 1938, 60, 467–475. [Google Scholar] [CrossRef]
- Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Hosseini, S.-H.; Kharghani, K.; Zarei, H.; Rastegar, A. Kinetic, equilibrium and thermodynamic studies on sorption of uranium and thorium from aqueous solutions by a selective impregnated resin containing carminic acid. J. Hazard. Mater. 2015, 286, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Oyelude, E.O.; Awudza, J.A.M.; Twumasi, S.K. Removal of malachite green from aqueous solution using pulverized teak leaf litter: Equilibrium, kinetic and thermodynamic studies. Chem. Cent. J. 2018, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Hutson, N.D.; Yang, R.T. Theoretical basis for the Dubinin-Radushkevitch (D-R) adsorption isotherm equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Nollet, H.; Roels, M.; Lutgen, P.; Van der Meeren, P.; Verstraete, W. Removal of PCBs from wastewater using fly ash. Chemosphere 2003, 53, 655–665. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).