Phase Transformation Kinetics of a FCC Al0.25CoCrFeNi High-Entropy Alloy during Isochronal Heating
Abstract
1. Introduction
2. Experimental Procedures
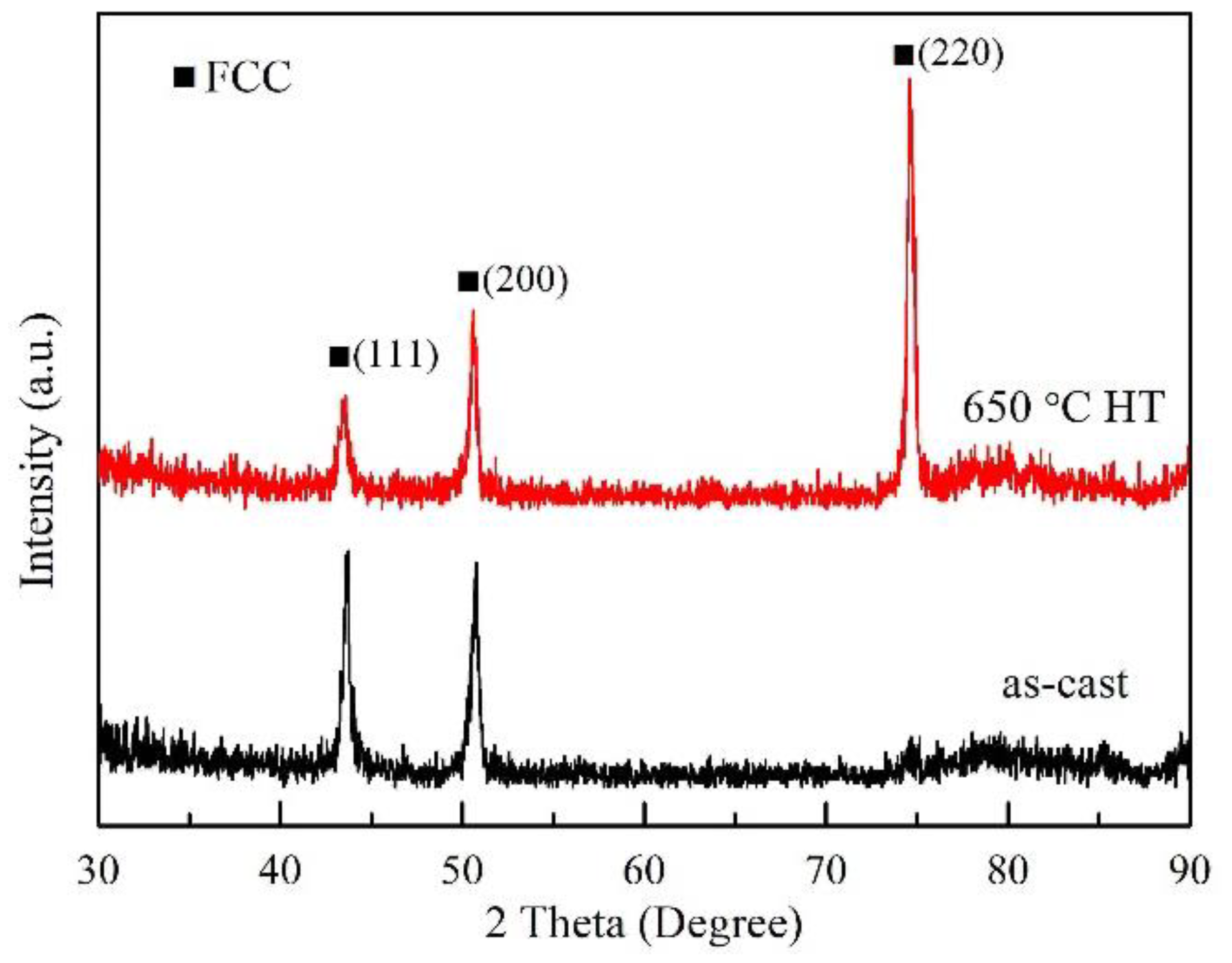
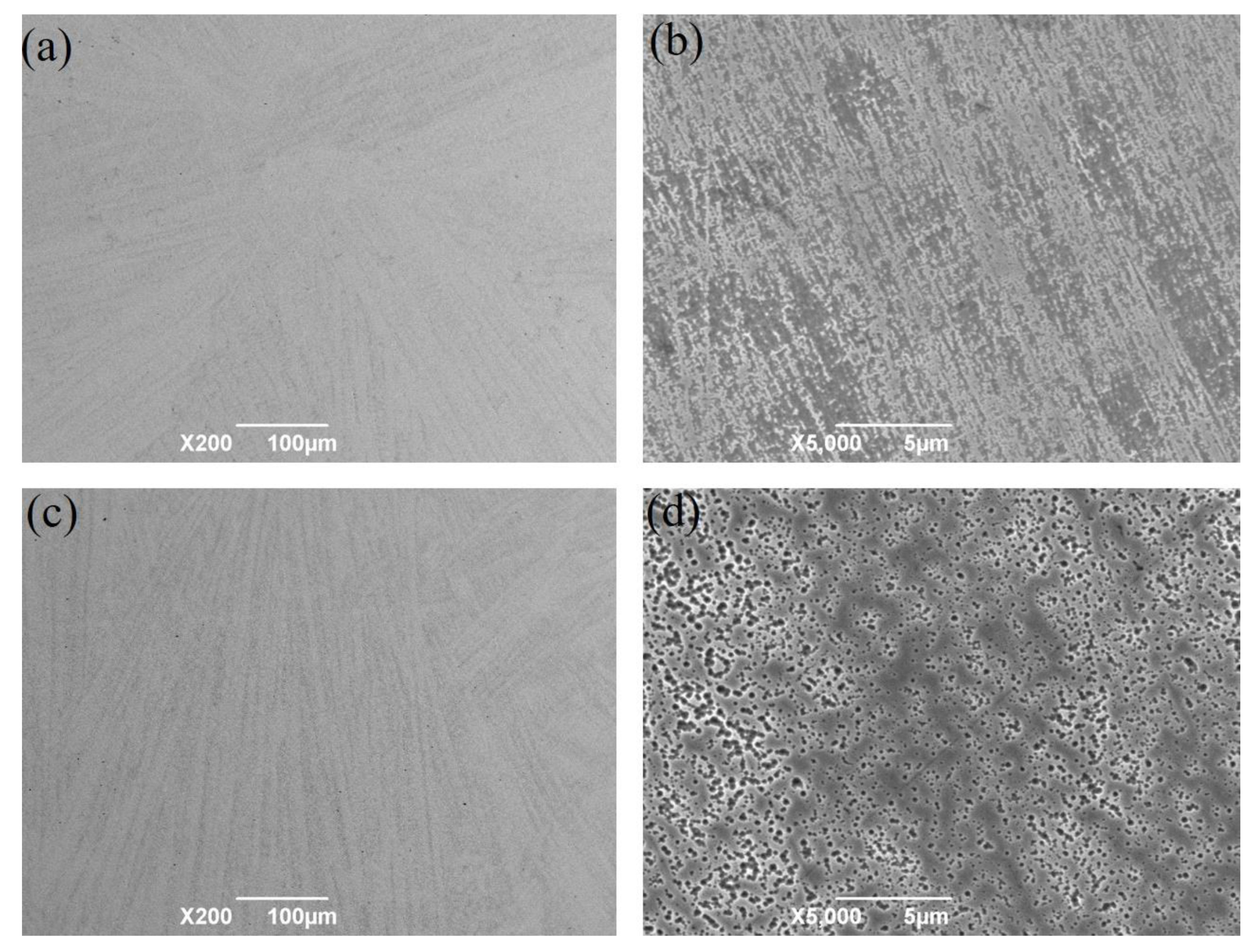
3. Results
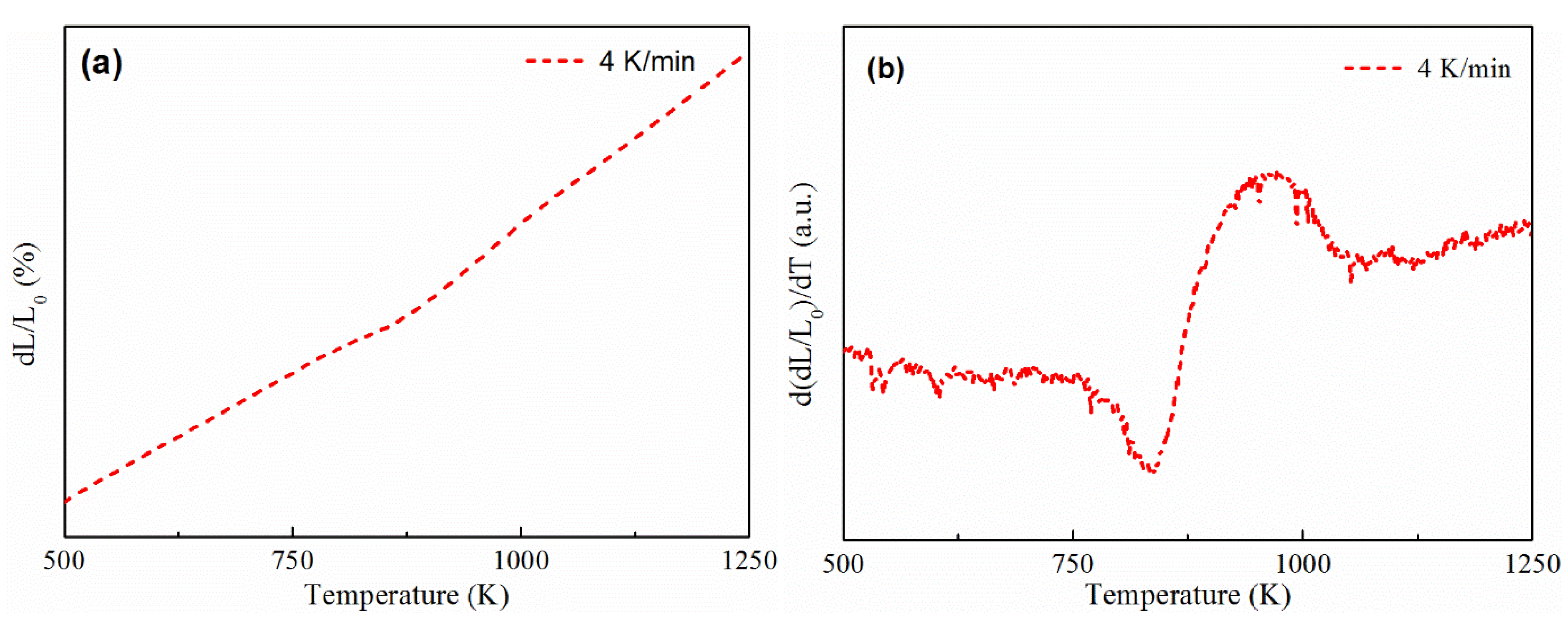
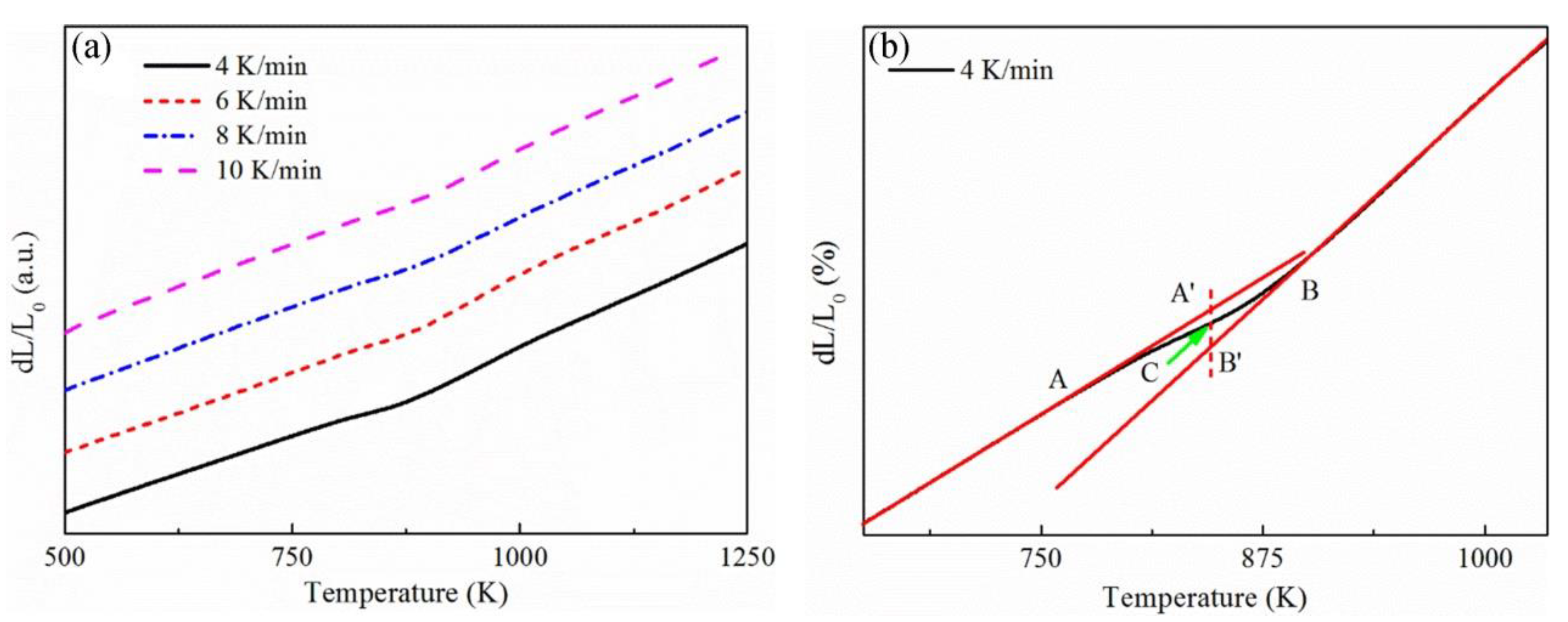
3.1. Thermal Expansion Curve
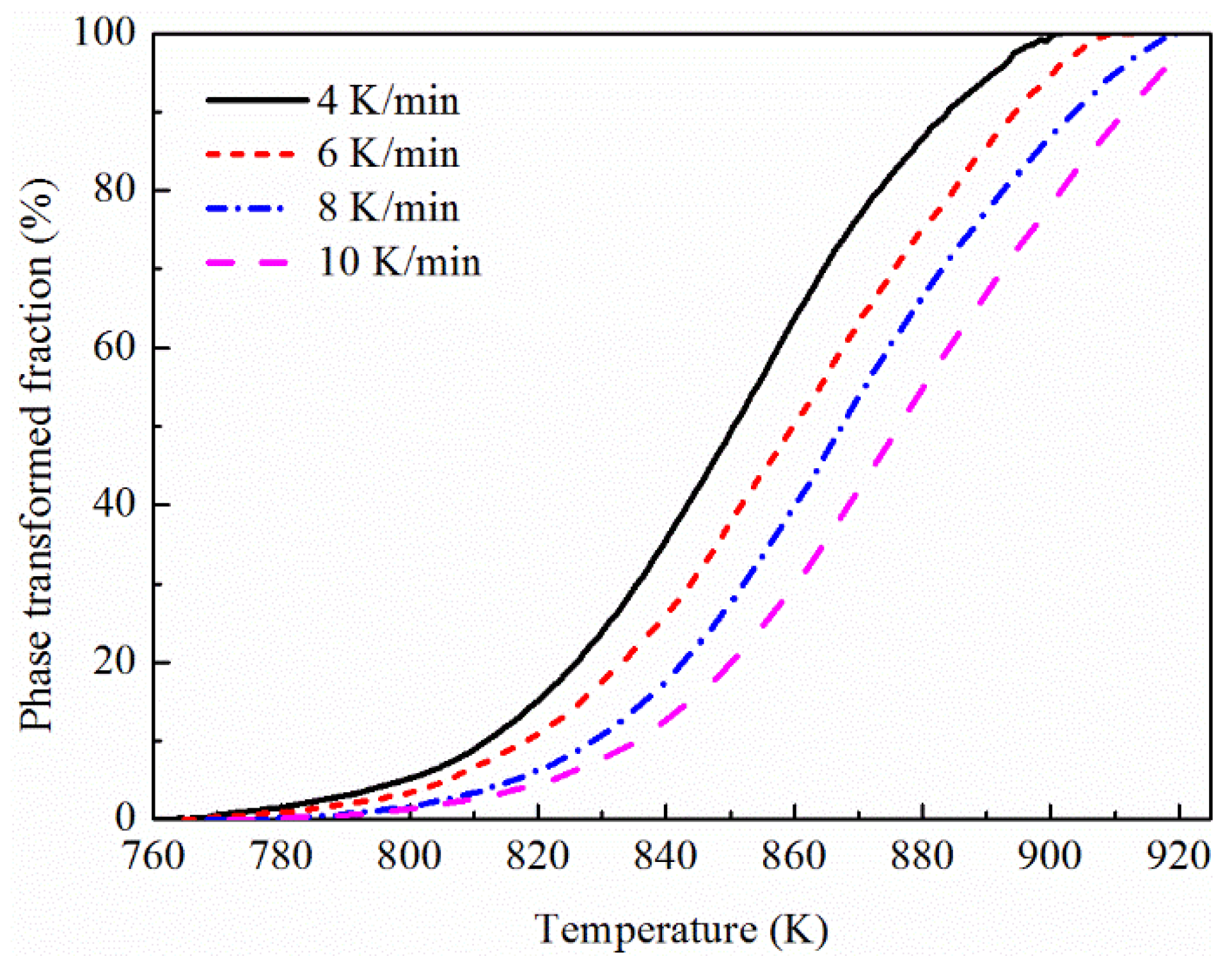
3.2. Determination of Phase Transformed Volume
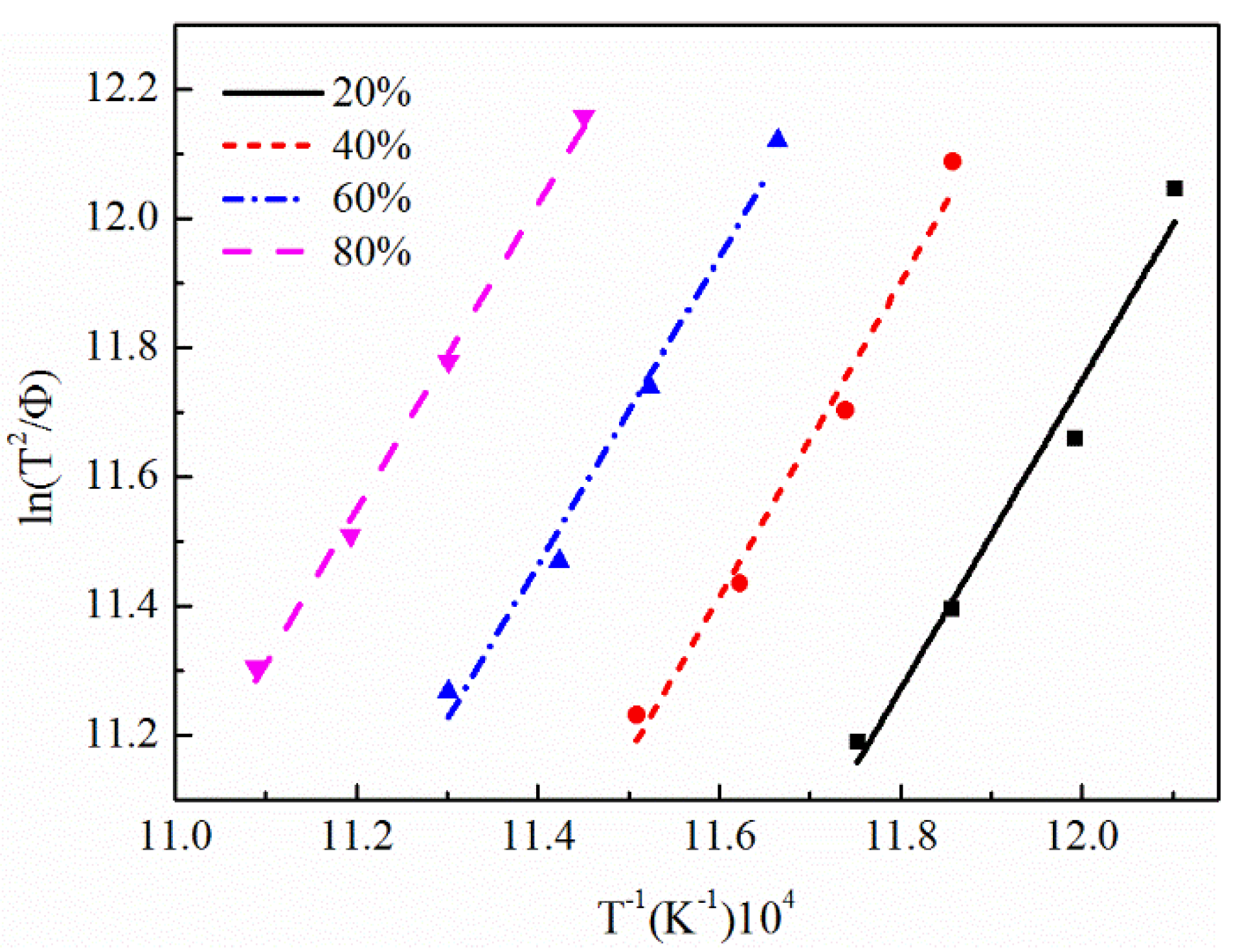
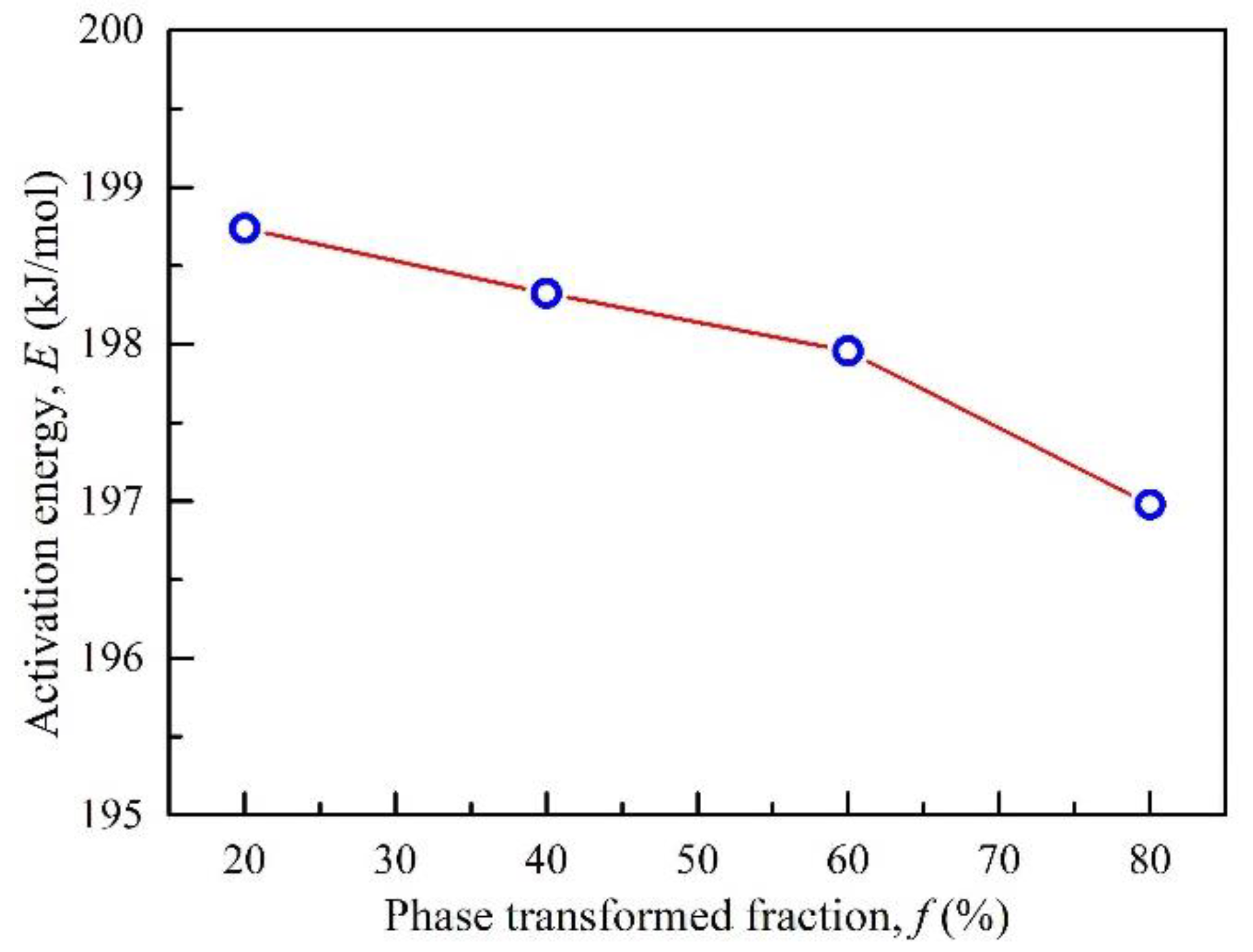
3.3. Calculation of Activation Energy
3.4. Calculation of Avrami Exponent
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Tsai, M.-H. Physical Properties of High Entropy Alloys. Entropy 2013, 15, 5338–5345. [Google Scholar] [CrossRef]
- Choi, W.-M.; Jung, S.; Jo, Y.H.; Lee, S.; Lee, B.-J. Design of new face-centered cubic high entropy alloys by thermodynamic calculation. Met. Mater. Int. 2017, 23, 839–847. [Google Scholar] [CrossRef]
- Rao, J.C.; Diao, H.Y.; Ocelík, V.; Vainchtein, D.; Zhang, C.; Kuo, C.; Tang, Z.; Guo, W.; Poplawsky, J.D.; Zhou, Y.; Liaw, P.K.; de Hosson, J.T.M. Secondary phases in AlxCoCrFeNi high-entropy alloys: An in-situ TEM heating study and thermodynamic appraisal. Acta Mater. 2017, 131, 206–220. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Liu, S.; Fang, Y.; Zhang, Y.; Xue, J.; Yan, S.; Wang, Y. Precipitation behavior of AlxCoCrFeNi high entropy alloys under ion irradiation. Sci. Rep. 2016, 6, 32146. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat treatment impacts the micro-structure and mechanical properties of AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Chen, B.-R.; Yeh, A.-C.; Yeh, J.-W. Effect of one-step recrystallization on the grain boundary evolution of CoCrFeMnNi high entropy alloy and its subsystems. Sci. Rep. 2016, 6, 22306. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Niu, S.Z.; Wang, W.Y.; Kou, H.C.; Li, J.S.; Wang, S.Q.; Beaugnon, E. Formation of a hexagonal closed-packed phase in Al0.5CoCrFeNi high entropy alloy. MRS Commun. 2017, 7, 879–884. [Google Scholar] [CrossRef]
- Won, J.W.; Kang, M.; Kwon, H.-J.; Lim, K.R.; Seo, S.M.; Na, Y.S. Edge-Cracking Behavior of CoCrFeMnNi High-Entropy Alloy During Hot Rolling. Met. Mater. Int. 2018, 24, 1432–1437. [Google Scholar] [CrossRef]
- Niu, S.; Kou, H.; Guo, T.; Zhang, Y.; Wang, J.; Li, J. Strengthening of nanoprecipitations in an annealed Al0.5CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2016, 671, 82–86. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Bos, C.; Mittemeijer, E.J. Analysis of solid state phase transformation kinetics: Models and recipes. Int. Mater. Rev. 2007, 52, 193–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, J.; Wang, W.Y.; Kou, H.; Beaugnon, E. Temperature dependent deformation mechanisms of Al0.3CoCrFeNi high-entropy alloy, starting from serrated flow behavior. J. Alloys Compd. 2018, 757, 39–43. [Google Scholar] [CrossRef]
- Wang, J.; Guo, T.; Li, J.; Jia, W.; Kou, H. Microstructure and mechanical properties of non-equilibrium solidified CoCrFeNi high entropy alloy. Mater. Chem. Phys. 2018, 210, 192–196. [Google Scholar] [CrossRef]
- Guo, T.; Li, J.; Wang, J.; Wang, W.Y.; Liu, Y.; Luo, X.; Kou, H.; Beaugnon, E. Microstructure and properties of bulk Al0.5CoCrFeNi high-entropy alloy by cold rolling and subsequent annealing. Mater. Sci. Eng. A 2018, 729, 141–148. [Google Scholar] [CrossRef]
- Li, M.; Gazquez, J.; Borisevich, A.; Mishra, R.; Flores, K.M. Evaluation of microstructure and mechanical property variations in AlxCoCrFeNi high entropy alloys produced by a high-throughput laser deposition method. Intermetallics 2018, 95, 110–118. [Google Scholar] [CrossRef]
- Joseph, J.; Hodgson, P.; Jarvis, T.; Wu, X.; Stanford, N.; Fabijanic, D.M. Effect of hot isostatic pressing on the microstructure and mechanical properties of additive manufactured AlxCoCrFeNi high entropy alloys. Mater. Sci. Eng. A 2018, 733, 59–70. [Google Scholar] [CrossRef]
- Xia, S.; Gao, M.; Zhang, Y. Abnormal temperature dependence of impact toughness in AlxCoCrFeNi system high entropy alloys. Mater. Chem. Phys. 2018, 210, 213–221. [Google Scholar] [CrossRef]
- Bönisch, M.; Wu, Y.; Sehitoglu, H. Twinning-induced strain hardening in dual-phase FeCoCrNiAl0.5 at room and cryogenic temperature. Sci. Rep. 2018, 8, 10663. [Google Scholar] [CrossRef]
- Liu, W.; Yang, T.; Liu, C. Precipitation hardening in CoCrFeNi-based high entropy alloys. Mater. Chem. Phys. 2018, 210, 2–11. [Google Scholar] [CrossRef]
- Linden, Y.; Pinkas, M.; Munitz, A.; Meshi, L. Long-period antiphase domains and short-range order in a B2 matrix of the AlCoCrFeNi high-entropy alloy. Scr. Mater. 2017, 139, 49–52. [Google Scholar] [CrossRef]
- Ghassemali, E.; Sonkusare, R.; Biswas, K.; Gurao, N.P. In-situ study of crack initiation and propagation in a dual phase AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2017, 710, 539–546. [Google Scholar] [CrossRef]
- Wang, J.; Niu, S.; Guo, T.; Kou, H.; Li, J. The FCC to BCC phase transformation kinetics in an Al0.5CoCrFeNi high entropy alloy. J. Alloys Compd. 2017, 710, 144–150. [Google Scholar] [CrossRef]
- Gwalani, B.; Soni, V.; Choudhuri, D.; Lee, M.; Hwang, J.; Nam, S.; Ryu, H.; Hong, S.H.; Banerjee, R. Stability of ordered L12 and B2 precipitates in face centered cubic based high entropy alloys-Al0.3CoFeCrNi and Al0.3CuFeCrNi2. Scr. Mater. 2016, 123, 130–134. [Google Scholar] [CrossRef]
- Liu, Y.C.; Sommer, F.; Mittemeijer, E.J. Calibration of the differential dilatometric measurement signal upon heating and cooling; thermal expansion of pure iron. Thermochim. Acta 2004, 413, 215–225. [Google Scholar] [CrossRef]
- Mittemeijer, E.J. Fundamentals of Materials Science; Springer: Heidelberg, Germany, 2010; pp. 448–457. [Google Scholar]
- Liu, Y.; Liu, C.; Sommer, F.; Mittemeijer, E.J. Martensite formation kinetics of substitutional Fe–0.7at.%Al alloy under uniaxial compressive stress. Acta Mater. 2015, 98, 164–174. [Google Scholar] [CrossRef]
- Burnham, A.; Dinh, L. A comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application predictions. J. Therm. Anal. Calorim. 2007, 89, 479–490. [Google Scholar] [CrossRef]
- Michaelsen, C.; Dahms, M. On the determination of nucleation and growth kinetics by calorimetry. Thermochim. Acta 1996, 288, 9–27. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, T.; Ma, S.; Qian, L.; Luo, Z.; Song, Y.; Yang, H.; Lu, W. Microstructural evolution and phase transformation kinetics of MnBi alloys. J. Alloys Compd. 2018, 741, 951–956. [Google Scholar] [CrossRef]
- Riva, S.; Mehraban, S.; Lavery, N.; Schwarzmüller, S.; Oeckler, O.; Brown, S.; Yusenko, K. The effect of scandium ternary intergrain precipitates in Al-containing high-entropy alloys. Entropy 2018, 20, 488. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Wang, L.; Wen, Y.; Cheng, B.; Wu, Q.; Cao, T.; Xiao, Q.; Xue, Y.; Sha, G.; Wang, Y. High-content ductile coherent nanoprecipitates achieve ultrastrong high-entropy alloys. Nat. Commun. 2018, 9, 4063. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wei, C.; Yang, H.; Guo, T.; Xu, T.; Li, J. Phase Transformation Kinetics of a FCC Al0.25CoCrFeNi High-Entropy Alloy during Isochronal Heating. Metals 2018, 8, 1015. https://doi.org/10.3390/met8121015
Wang J, Wei C, Yang H, Guo T, Xu T, Li J. Phase Transformation Kinetics of a FCC Al0.25CoCrFeNi High-Entropy Alloy during Isochronal Heating. Metals. 2018; 8(12):1015. https://doi.org/10.3390/met8121015
Chicago/Turabian StyleWang, Jun, Chen Wei, Haoxue Yang, Tong Guo, Tingting Xu, and Jinshan Li. 2018. "Phase Transformation Kinetics of a FCC Al0.25CoCrFeNi High-Entropy Alloy during Isochronal Heating" Metals 8, no. 12: 1015. https://doi.org/10.3390/met8121015
APA StyleWang, J., Wei, C., Yang, H., Guo, T., Xu, T., & Li, J. (2018). Phase Transformation Kinetics of a FCC Al0.25CoCrFeNi High-Entropy Alloy during Isochronal Heating. Metals, 8(12), 1015. https://doi.org/10.3390/met8121015







