Sustainable Hydrometallurgical Recovery of Valuable Elements from Spent Nickel–Metal Hydride HEV Batteries
Abstract
1. Introduction
2. Experimental Procedures
2.1. Mechanical Separation
2.2. Material Characterization
2.3. Acid Leaching
2.4. Acid Recovery by Nanofiltration
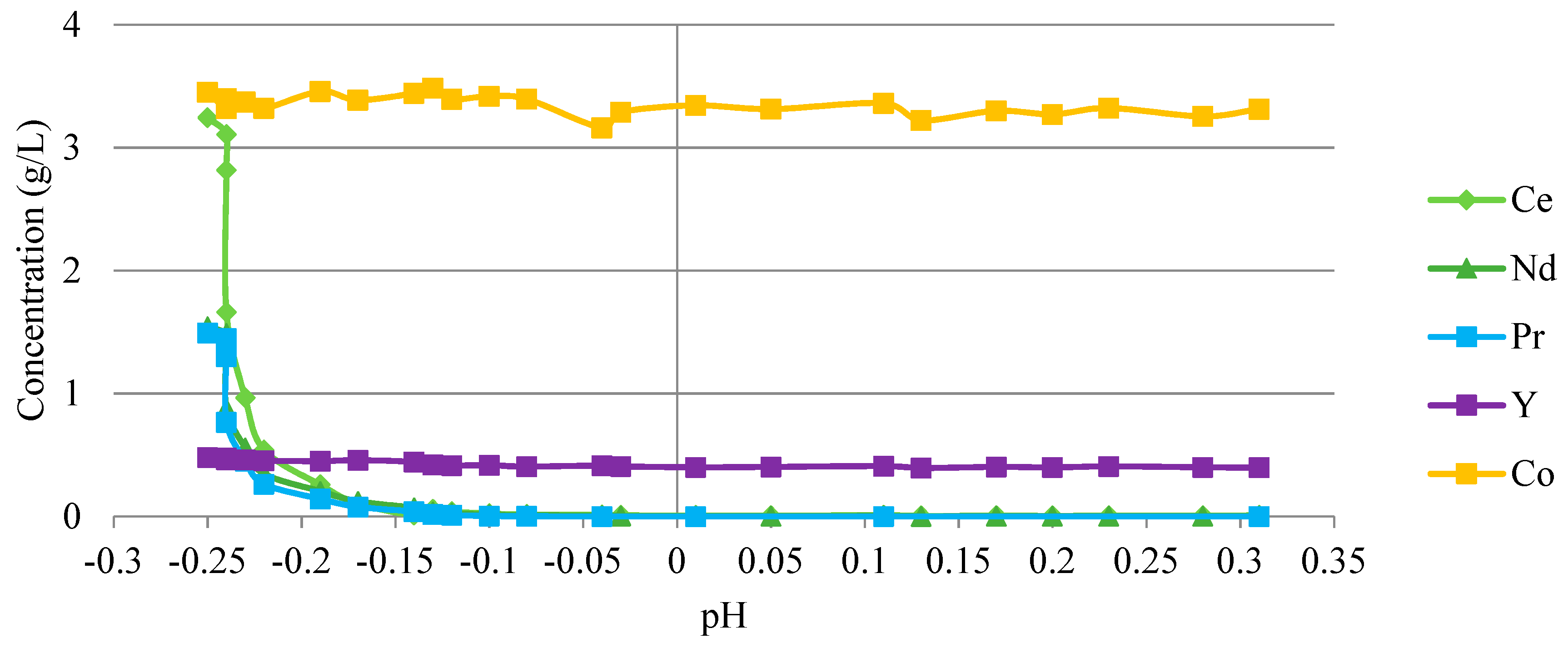
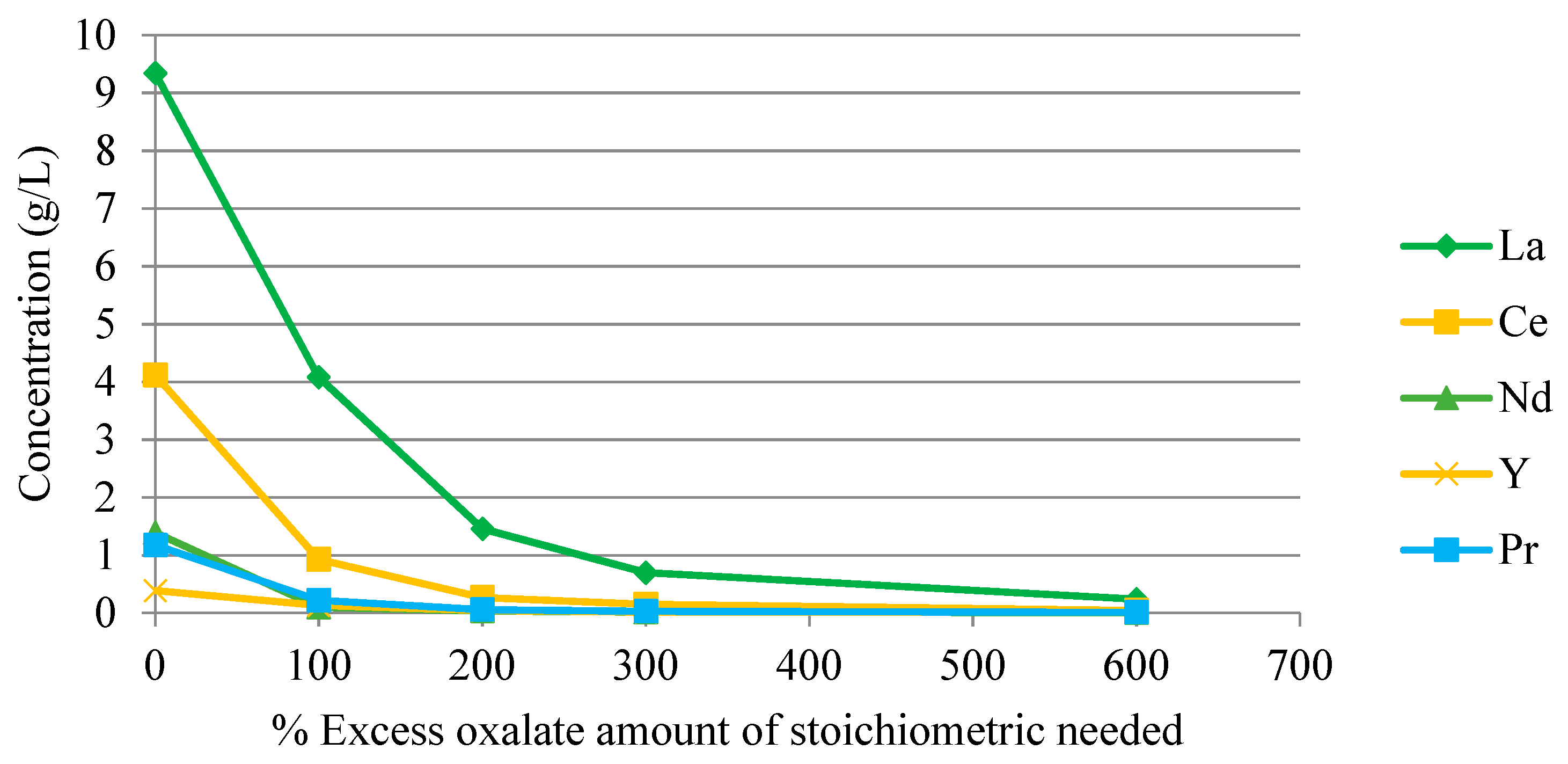
2.5. Separation of Rare Earth Elements by Precipitation
3. Results and Discussion
3.1. Material Characterization
3.2. Acid Leaching
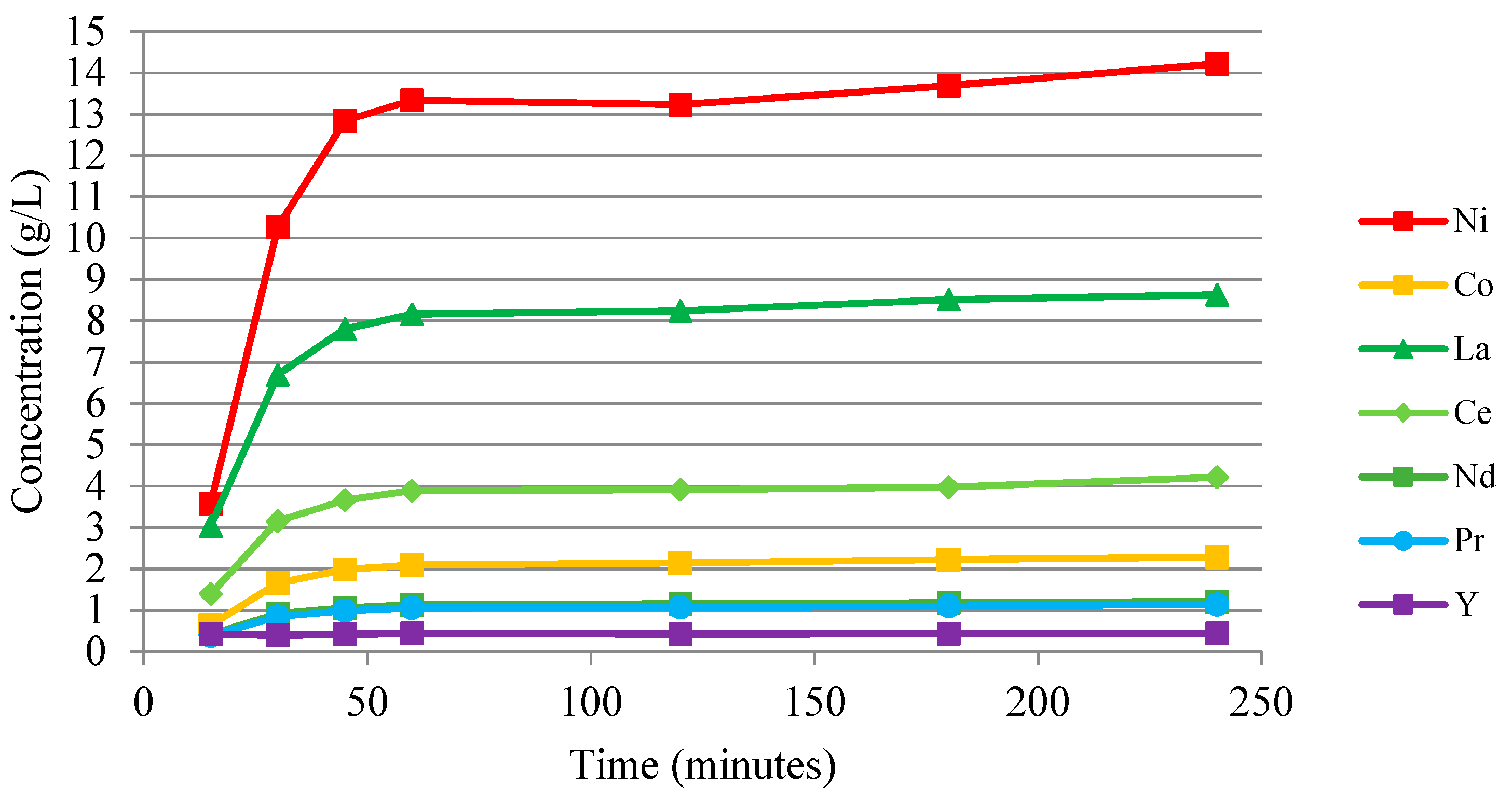
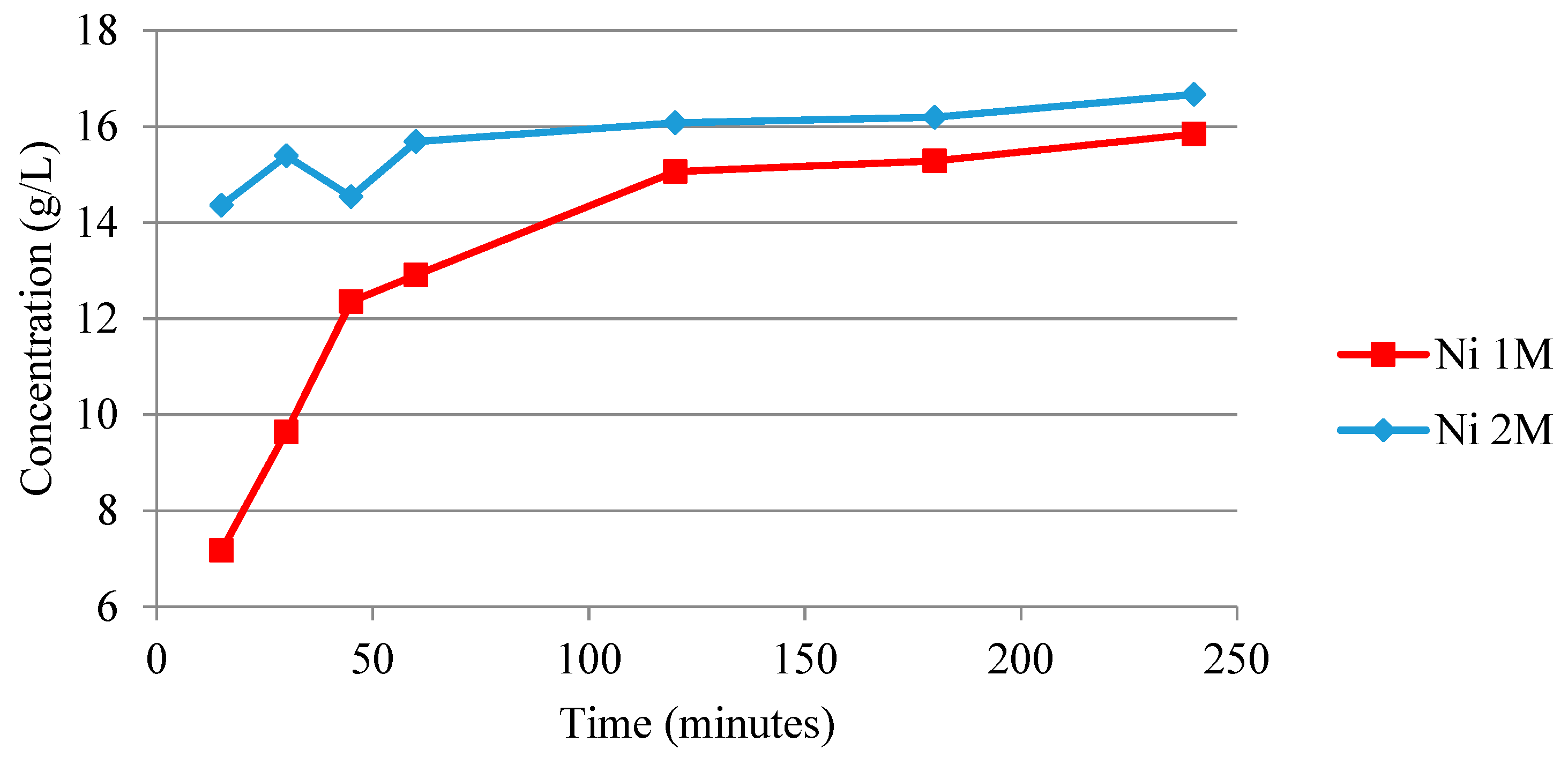
3.2.1. Anode Sulfuric Acid Leaching
3.2.2. Anode Hydrochloric Acid Leaching
3.2.3. Cathode Sulfuric Acid Leaching
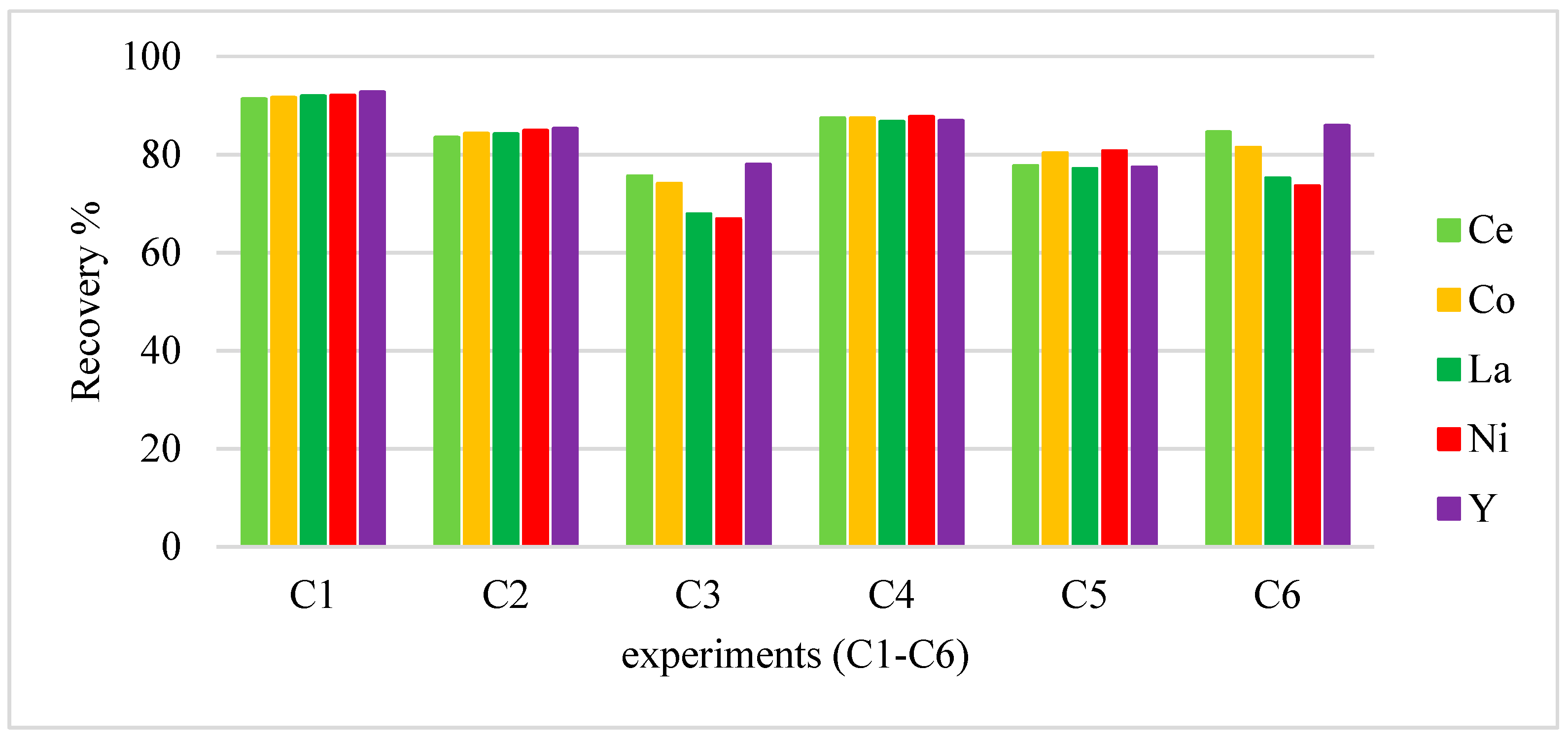
3.2.4. Cathode Hydrochloric Acid Leaching
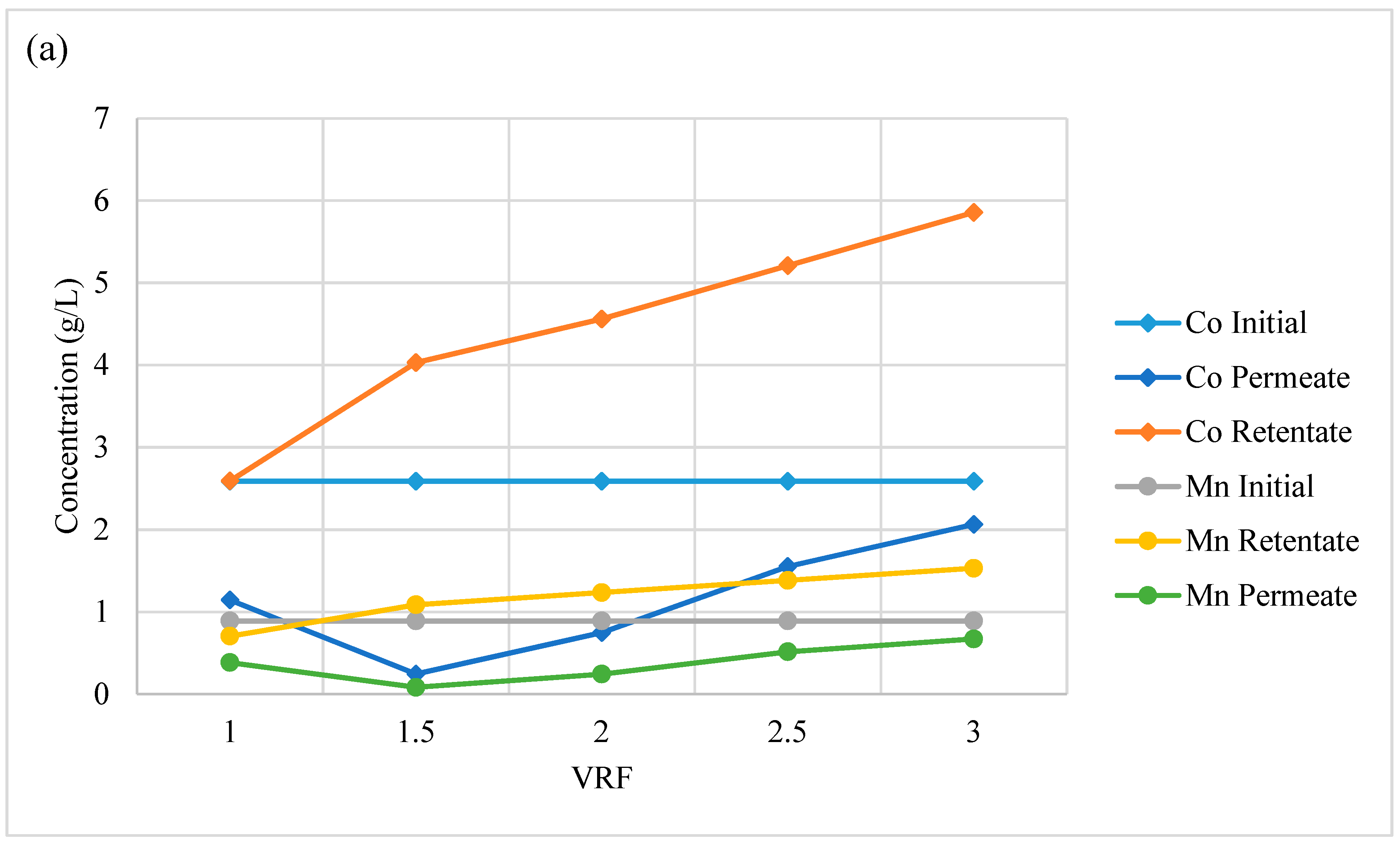
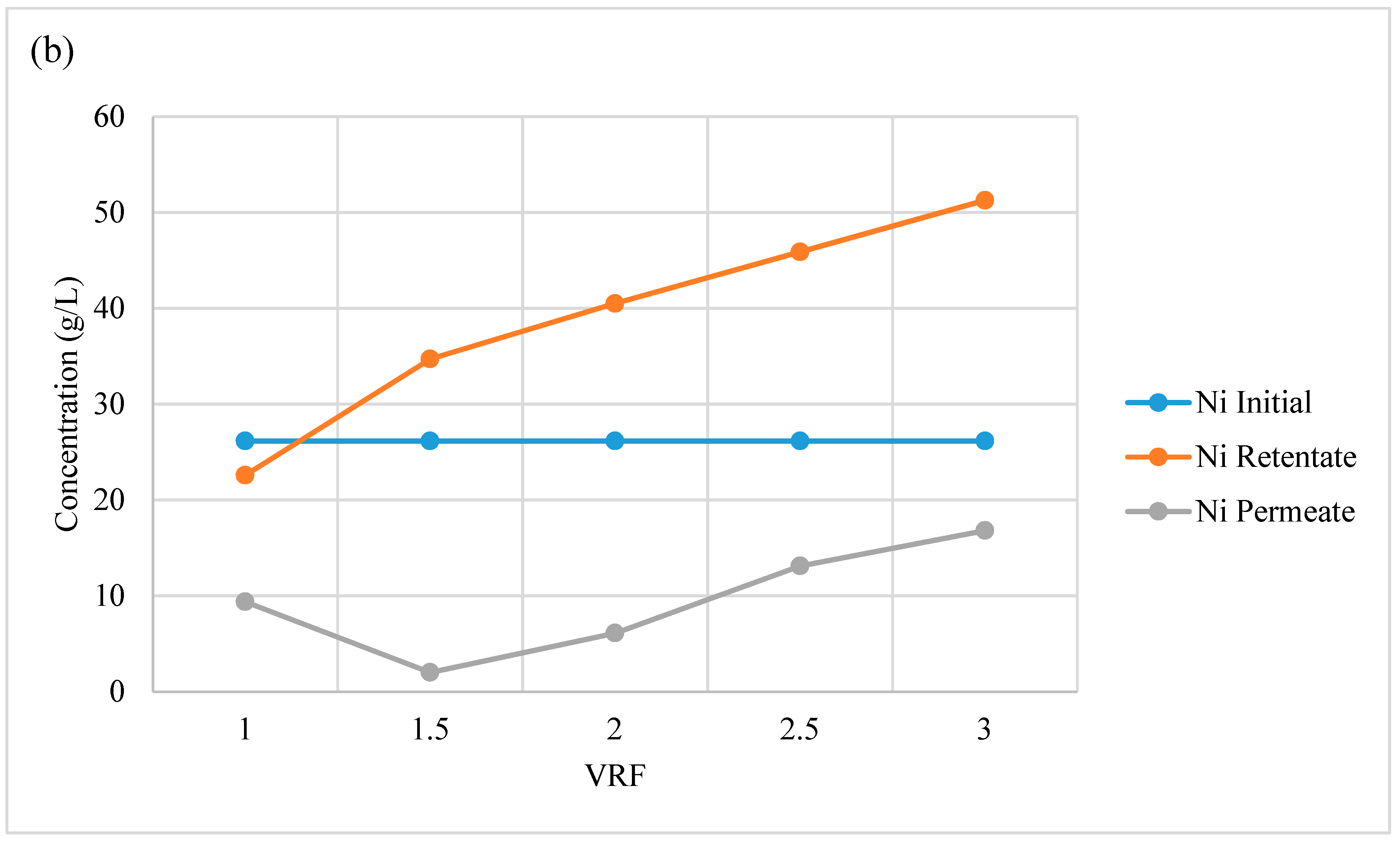
3.2.5. Acid Recovery via Nanofiltration
3.2.6. Separation of Rare Earth Elements by Precipitation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Critical Raw Materials for the EU, in Report of the Ad-Hoc Working Group on Defining Critical Raw Materials; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Moss, R.; Tzimas, W.P.; Arendorf, J.; Tercero Espinoza, L. Critical Metals in the Path towards the Decarbonisation of the EU Energy Sector: Assessing Rare Metals as Supply Chain Bottlenecks in Low-Carbon Energy Technologies; Technical Report, JRC Scientific and Technical Reports; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Guyonnet, D.; Planchon, M.; Rollat, A.; Escalon, V.; Tuduri, J.; Charles, N.; Vaxelaire, S.; Dubois, D.; Fargier, H. Material flow analysis applied to rare earth elements in Europe. J. Clean. Prod. 2015, 107, 215–228. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Critical Materials Strategy; U.S. Department of Energy: Washington, DC, USA, 2011.
- Rollat, A.; Guyonnet, D.; Planchon, M.; Tuduri, J. Prospective analysis of the flows of certain rare earths in Europe at the 2020 horizon. Waste Manag. 2016, 49, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Gergoric, M.; Ravaux, C.; Steenari, B.-M.; Espegren, F.; Retegan, T. Leaching and Recovery of Rare-Earth Elements from Neodymium Magnet Waste Using Organic Acids. Metals 2018, 8, 721. [Google Scholar] [CrossRef]
- Müller, T.; Friedrich, B. Development of a recycling process for nickel-metal hydride batteries. J. Power Sources 2006, 158, 1498–1509. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- EU Commission. The Raw Materials Initiative—Meeting Our Critical Needs for Growth and Jobs in Europe; EU Commission: Brussels, Belgium, 2008. [Google Scholar]
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming rare earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Bernardes, A.M.; Tenório, J.A.S. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Yang, M.; Cui, M.; Hou, X. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 2006, 84, 75–80. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Ebin, B.; Petranikova, M.; Ekberg, C. Physical separation, mechanical enrichment and recycling-oriented characterization of spent NiMH batteries. J. Mater. Cycles Waste Manag. 2018, 201, 2018–2027. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; de Michelis, I.; Prisciandaro, M.; Medici, F.; Vegliò, F. A review of the processes and lab-scale techniques for the treatment of spent rechargeable NiMH batteries. J. Power Sources 2017, 362, 202–218. [Google Scholar] [CrossRef]
- Korkmaz, K.; Alemrajabi, M.; Rasmuson, Å.; Forsberg, K. Recoveries of Valuable Metals from Spent Nickel Metal Hydride Vehicle Batteries via Sulfation, Selective Roasting, and Water Leaching. J. Sustain. Metall. 2018, 4, 33–325. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Wakui, Y.; Suzuki, T.M.; Inoue, K. Hydrometallurgical process for recovery of metal values from spent nickel-metal hydride secondary batteries. Hydrometallurgy 1998, 50, 61–75. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Wakui, Y.; Suzuki, T.M.; Inoue, K. Recovery of metal values from spent nickel–metal hydride rechargeable batteries. J. Power Sources 1999, 77, 116–122. [Google Scholar] [CrossRef]
- Tzanetakis, N.; Scott, K. Recycling of nickel–metal hydride batteries. I: Dissolution and solvent extraction of metals. J. Chem. Technol. Biotechnol. 2004, 79, 919–926. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Bernardes, A.M.; Tenório, J.A.S. Spent NiMH batteries—The role of selective precipitation in the recovery of valuable metals. J. Power Sources 2009, 193, 914–923. [Google Scholar] [CrossRef]
- Rodrigues, L.E.O.C.; Mansur, M.B. Hydrometallurgical separation of rare earth elements, cobalt and nickel from spent nickel-metal-hydride batteries. J. Power Sources 2010, 195, 3735–3741. [Google Scholar] [CrossRef]
- Larsson, K.; Ekberg, C.; Odegaard-Jensen, A. Dissolution and characterization of HEV NiMH batteries. Waste Manag. 2013, 33, 689–698. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Fang, X. Rare earth element recycling from waste nickel-metal hydride batteries. J. Hazard. Mater. 2014, 279, 384–388. [Google Scholar] [CrossRef]
- Pietrelli, L.; Bellomo, B.; Fontana, D.; Montereali, M. Characterization and leaching of NiCd and NiMH spent batteries for the recovery of metals. Waste Manag. 2005, 25, 221–226. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Ju, Z.; Wu, F. Recovery of Ni, Co and rare earths from spent Ni–metal hydride batteries and preparation of spherical Ni(OH)2. Hydrometallurgy 2009, 100, 41–46. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Leaching of base metals from spent Ni–metal hydride batteries with emphasis on kinetics and characterization. Hydrometallurgy 2015, 158, 172–179. [Google Scholar] [CrossRef]
- Josso, P.; Roberts, S.; Teagle, D.A.H.; Pourret, O.; Herrington, R.; Albarran, C.P. Extraction and separation of rare earth elements from hydrothermal metalliferous sediments. Miner. Eng. 2018, 118, 106–121. [Google Scholar] [CrossRef]
- Larsson, K.; Binnemans, K. Separation of rare earths by split-anion extraction. Hydrometallurgy 2015, 156, 206–214. [Google Scholar] [CrossRef]
- Larsson, K.; Ekberg, C.; Ødegaard-Jensen, A. Using Cyanex 923 for selective extraction in a high concentration chloride medium on nickel metal hydride battery waste: Part II: Mixer-settler experiments. Hydrometallurgy 2013, 133, 168–175. [Google Scholar] [CrossRef]
- Larsson, K.; Ekberg, C.; Ødegaard-Jensen, A. Using Cyanex 923 for selective extraction in a high concentration chloride medium on nickel metal hydride battery waste. Hydrometallurgy 2012, 129–130, 35–42. [Google Scholar] [CrossRef]
- Pietrelli, L.; Bellomo, B.; Fontana, D.; Montereali, M.R. Rare earths recovery from NiMH spent batteries. Hydrometallurgy 2002, 66, 135–139. [Google Scholar] [CrossRef]
- Innocenzi, V.; Vegliò, F. Recovery of rare earths and base metals from spent nickel-metal hydride batteries by sequential sulphuric acid leaching and selective precipitations. J. Power Sources 2012, 211, 184–191. [Google Scholar] [CrossRef]
- Porvali, A.; Wilson, B.; Lundström, M. Lanthanide-alkali double sulfate precipitation from strong sulfuric acid NiMH battery waste leachate. Waste Manag. 2018, 71, 381–389. [Google Scholar] [CrossRef]
- Coman, V.; Robotin, B.; Ilea, P. Nickel recovery/removal from industrial wastes: A review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Forsberg, K.M.; Rasmuson, Å.C. Recycling of waste pickle acid by precipitation of metal fluoride hydrates. Miner. Eng. 2007, 20, 950–955. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Gibert, O.; Torres, E.; Ayora, C.; Cortina, J.L. Application of nanofiltration for acidic waters containing rare earth elements: Influence of transition elements, acidity and membrane stability. Desalination 2018, 430, 33–44. [Google Scholar] [CrossRef]
- Chilyumova, E.; Thöming, J. Nanofiltration of bivalent nickel cations—Model parameter determination and process simulation. Desalination 2008, 224, 12–17. [Google Scholar] [CrossRef]
- Murthy, Z.V.P.; Choudhary, A. Application of nanofiltration to treat rare earth element (neodymium) containing water. J. Rare Earths 2011, 29, 974–978. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Endo, D.; Sakaki, K.; Akiba, E. Effect of rare earth on lattice size and equilibrium hydrogen pressure for AB5-type MmNi3.55Co0.75Al0.30Mn0.40. J. Alloys Compd. 2008, 459, 215–219. [Google Scholar] [CrossRef]
- Mioduski, T. Identification of Saturating Solid Phases in the System Ce2(SO4)3-H2O from the Solubility Data. J. Therm. Anal. Calorim. 1999, 55, 751. [Google Scholar] [CrossRef]
- Casari, B.M.; Langer, V. New Structure Type among Octahydrated Rare-Earth Sulfates, β-Ce2(SO4)3·8H2O, and a new Ce2(SO4)3·4H2O Polymorph. Z. Anorg. Allg. Chem. 2007, 633, 1074–1081. [Google Scholar] [CrossRef]
- Todorovsky, D.S.; Milanova, M.M.; Minkova, N.L.; Balarev, C. Solubility of some lanthanide sulfates in polycomponent systems containing H2SO4. Mon. Chem. Chem. Mon. 1993, 124, 673–679. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Tareeva, O.A.; Ivlev, K.G.; Kashulina, T.G. Solubility of Double Alkali Metal (Na, K) Rare-Earth (La, Ce) Sulfates in Sulfuric-Phosphoric Acid Solutions at 20 °C. Russ. J. Appl. Chem. 2005, 78, 1058–1063. [Google Scholar] [CrossRef]
- Nash, K.L. A Review of the Basic Chemistry and Recent Developments in Trivalent f-Elements Separations. Solvent Extr. Ion Exch. 1993, 11, 729–768. [Google Scholar] [CrossRef]




| Experiment | Acid Type | Acid Concentration (mol/L) | Temperature (°C) |
|---|---|---|---|
| 1 (A, B) | H2SO4 | 1 | 25 |
| 2 (A, B) | H2SO4 | 2 | 25 |
| 3 (A, B) | H2SO4 | 2 | 90 |
| 4 (A, B) | H2SO4 | 4 | 90 |
| 5 (A, B) | HCl | 1 | 25 |
| 6 (A, B) | HCl | 2 | 25 |
| 7 (A, B) | HCl | 2 | 90 |
| 8 (A, B) | HCl | 4 | 90 |
| 9 (A, B) | HCl | 8 | 90 |
| 10 (A&B) | HCl | Permeate from nanofiltration (NF) | 25 |
| Experiment | Acid Type | Acid Concentration (mol/L) | Anode Active Material (g) | Anode Mesh Material (g) |
|---|---|---|---|---|
| C1 | H2SO4 | 2 | 0.78 | 0.22 |
| C2 | H2SO4 | 256 | 0,78 | 0.22 |
| C3 | H2SO4 | 2 | 1 | 0.28 |
| C4 | HCl | 2 | 0.78 | 0.22 |
| C5 | HCl | 2.56 | 0.78 | 0.22 |
| C6 | HCl | 2 | 1 | 0.28 |
| Leach liq. | Total Elemental Concentration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Co | La | Ce | Nd | Pr | Y | Mn | Al | Zn | K | Fe | |
| L1 (g/L): | 4.18 | 0.48 | 0.94 | 0.29 | 0.13 | 0.12 | 0.05 | 0.22 | 0.10 | 0.18 | 0.05 | 0.01 |
| L2 (g/L): | 26.0 | 2.59 | - | - | - | - | - | 0.9 | - | - | - | - |
| Experiment | Leach Liquor | Initial Volume (L) | Flow Rate (L/h) | Initial Flux (L/m2, h) |
|---|---|---|---|---|
| N1 | L1 | 15 | 26.4 | 15.5 |
| N2 | L2 | 20 | 26.4 | 15.5 |
| Experiment | Agent Type (Excess %) * |
|---|---|
| P1 | Oxalic Acid (100%) |
| P2 | Oxalic Acid (200%) |
| P3 | Oxalic Acid (300%) |
| P4 | Oxalic Acid (600%) |
| P5 | Oxalic Acid (24 h stirring version of P4) |
| P6 | NaOH |
| Element | Ce | Co | La | Mn | Nd | Ni | Pr | Y | Al | Fe | Zn | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathode (mass %) | nd | 6.1 | nd | 0.5 | nd | 75.5 | nd | 0.7 | 0.3 | 0.1 | 3.5 | 1.9 |
| Anode (mass %) | 6.4 | 4.7 | 20.4 | 4.1 | 2.9 | 51.7 | 2.5 | 0.7 | 1.9 | 0.1 | 0.4 | 1.4 |
| Element | Unnorm. wt% | Norm. wt% | Atom. wt% |
|---|---|---|---|
| Nickel | 0.20 | 0.20 | 0.13 |
| Sulfur | 15.13 | 15.42 | 17.66 |
| Oxygen | 28.96 | 29.52 | 67.78 |
| Cerium | 15.96 | 16.27 | 4.26 |
| Praseodymium | 1.46 | 1.49 | 0.39 |
| Neodymium | 3.66 | 3.73 | 0.95 |
| Lanthanum | 32.74 | 33.37 | 8.83 |
| Total | 98.10 | 100.00 | 100.00 |
| Nanofiltration Stages | Total Concentration (mg/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce | Co | La | Mn | Nd | Ni | Pr | Y | Al | Fe | K | Zn | |
| Initial composition | 286 | 483 | 942 | 221 | 130 | 4178 | 119 | 53 | 101 | 8 | 51 | 183 |
| Permeate (VRF = 1) | 2 | 6 | 8 | 3 | 1 | 47 | 1 | 0 | 1 | 1 | 21 | 52 |
| Permeate (VRF = 2) | 4 | 10 | 14 | 6 | 2 | 79 | 2 | 1 | 1 | 2 | 32 | 83 |
| Retentate (VRF = 1) | 270 | 485 | 931 | 217 | 127 | 4053 | 120 | 59 | 109 | 33 | 52 | 164 |
| Retentate (VRF = 2) | 531 | 936 | 1800 | 421 | 247 | 8364 | 236 | 117 | 213 | 183 | 83 | 260 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz, K.; Alemrajabi, M.; Rasmuson, Å.C.; Forsberg, K.M. Sustainable Hydrometallurgical Recovery of Valuable Elements from Spent Nickel–Metal Hydride HEV Batteries. Metals 2018, 8, 1062. https://doi.org/10.3390/met8121062
Korkmaz K, Alemrajabi M, Rasmuson ÅC, Forsberg KM. Sustainable Hydrometallurgical Recovery of Valuable Elements from Spent Nickel–Metal Hydride HEV Batteries. Metals. 2018; 8(12):1062. https://doi.org/10.3390/met8121062
Chicago/Turabian StyleKorkmaz, Kivanc, Mahmood Alemrajabi, Åke C. Rasmuson, and Kerstin M. Forsberg. 2018. "Sustainable Hydrometallurgical Recovery of Valuable Elements from Spent Nickel–Metal Hydride HEV Batteries" Metals 8, no. 12: 1062. https://doi.org/10.3390/met8121062
APA StyleKorkmaz, K., Alemrajabi, M., Rasmuson, Å. C., & Forsberg, K. M. (2018). Sustainable Hydrometallurgical Recovery of Valuable Elements from Spent Nickel–Metal Hydride HEV Batteries. Metals, 8(12), 1062. https://doi.org/10.3390/met8121062






