Novel Task Specific Ionic Liquids to Remove Heavy Metals from Aqueous Effluents
Abstract
1. Introduction
2. Experimental Section
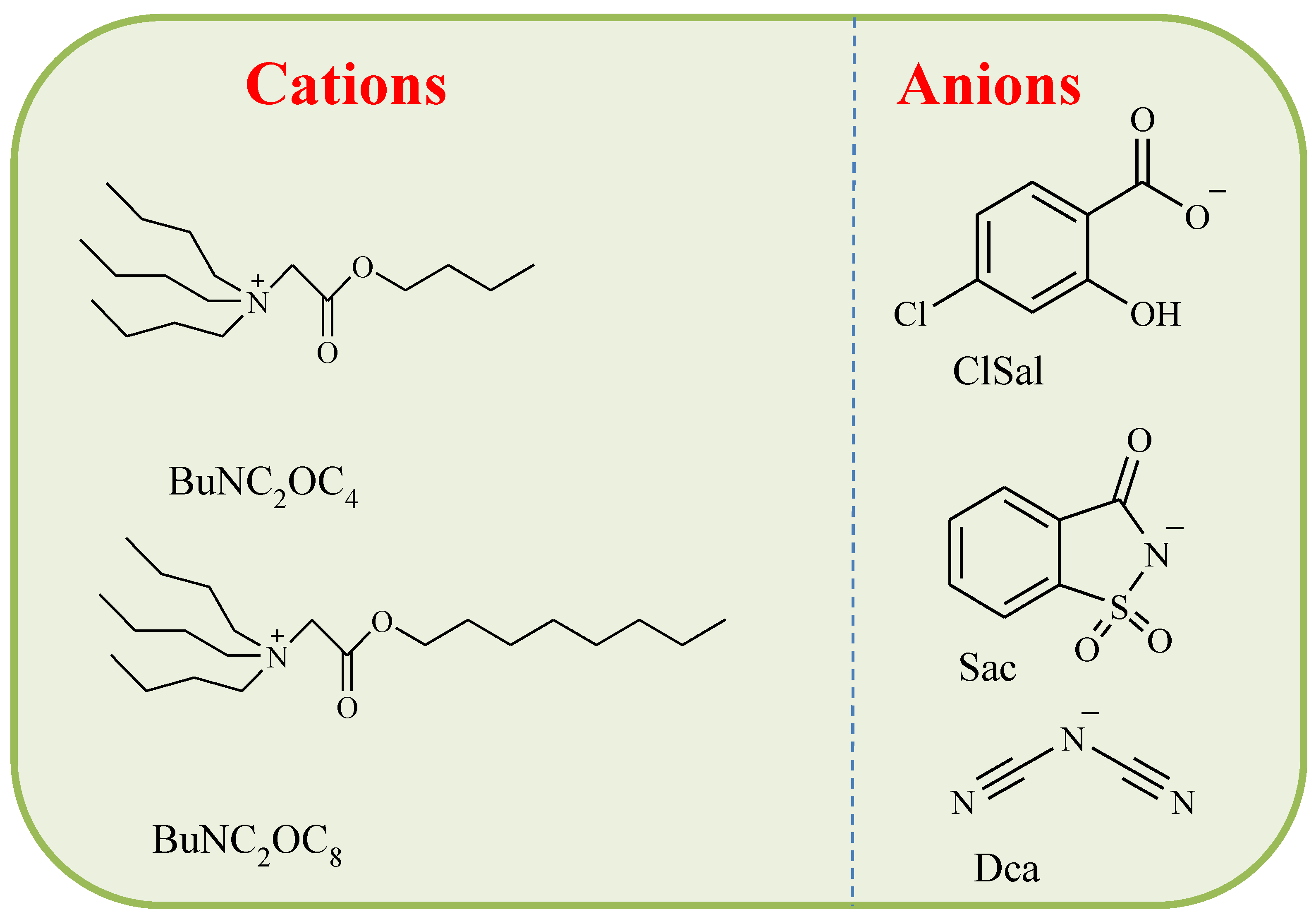
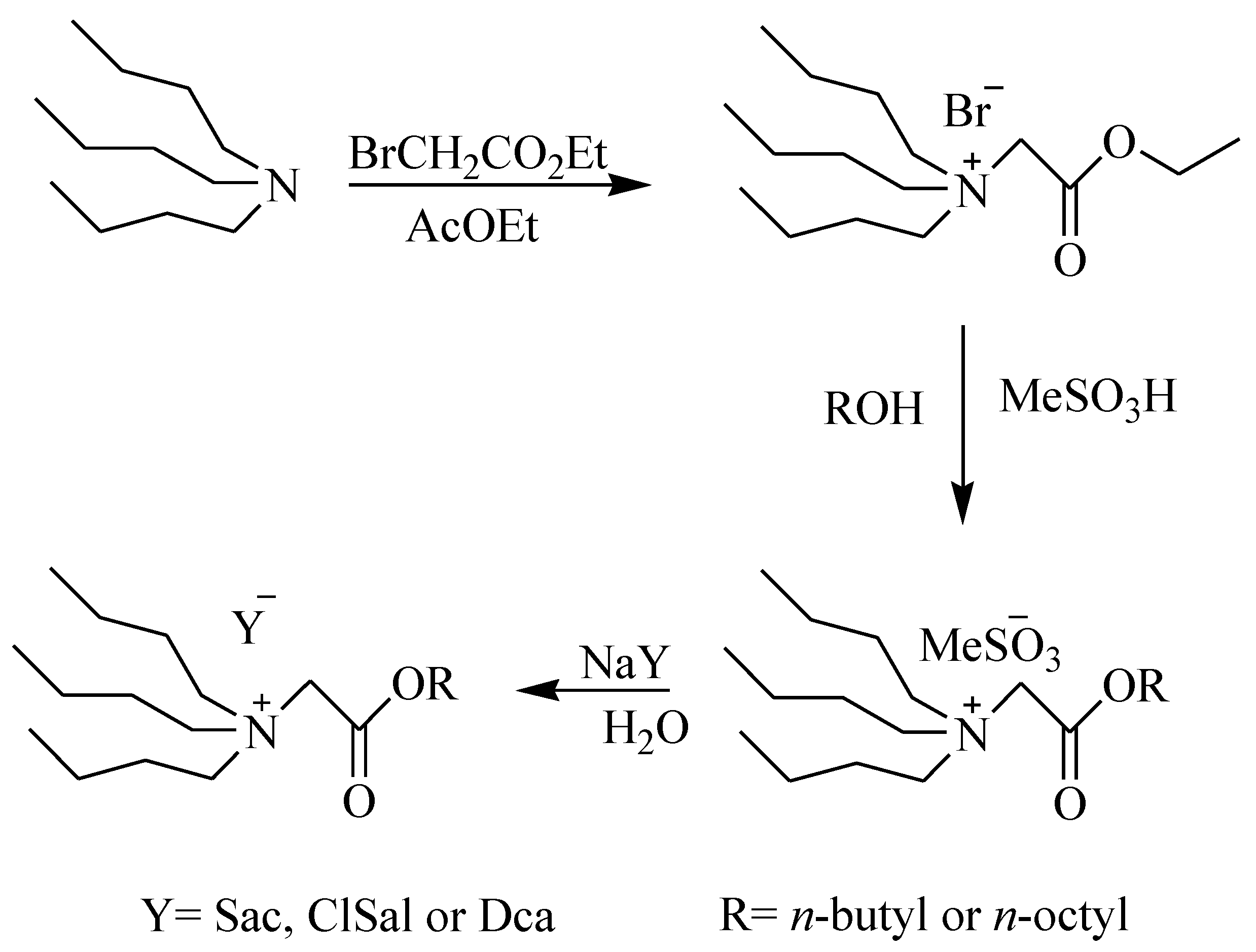
2.1. Chemicals and Reagents
2.2. Analytical Measurements
2.3. Extraction Experiments
3. Results and Discussion
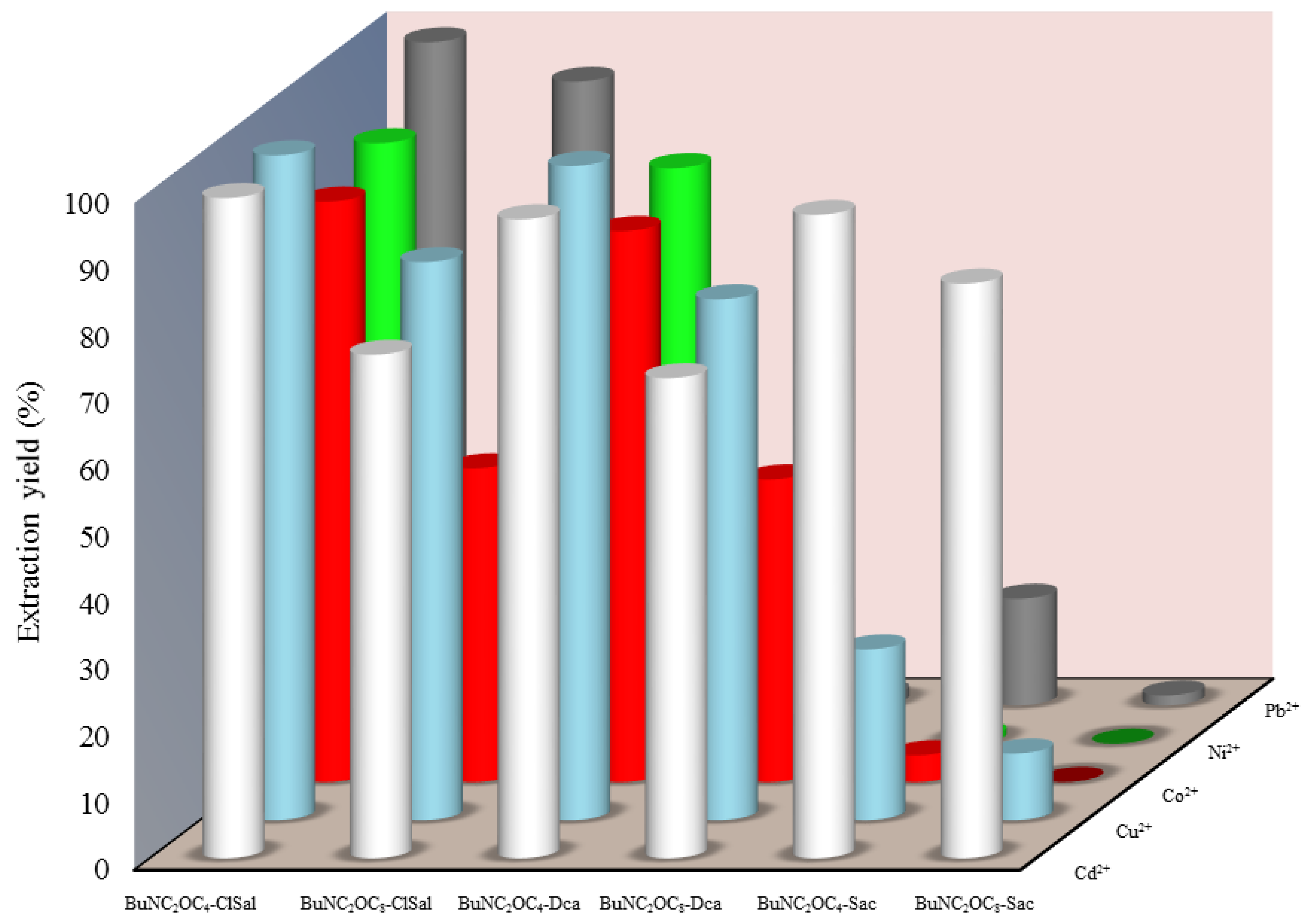
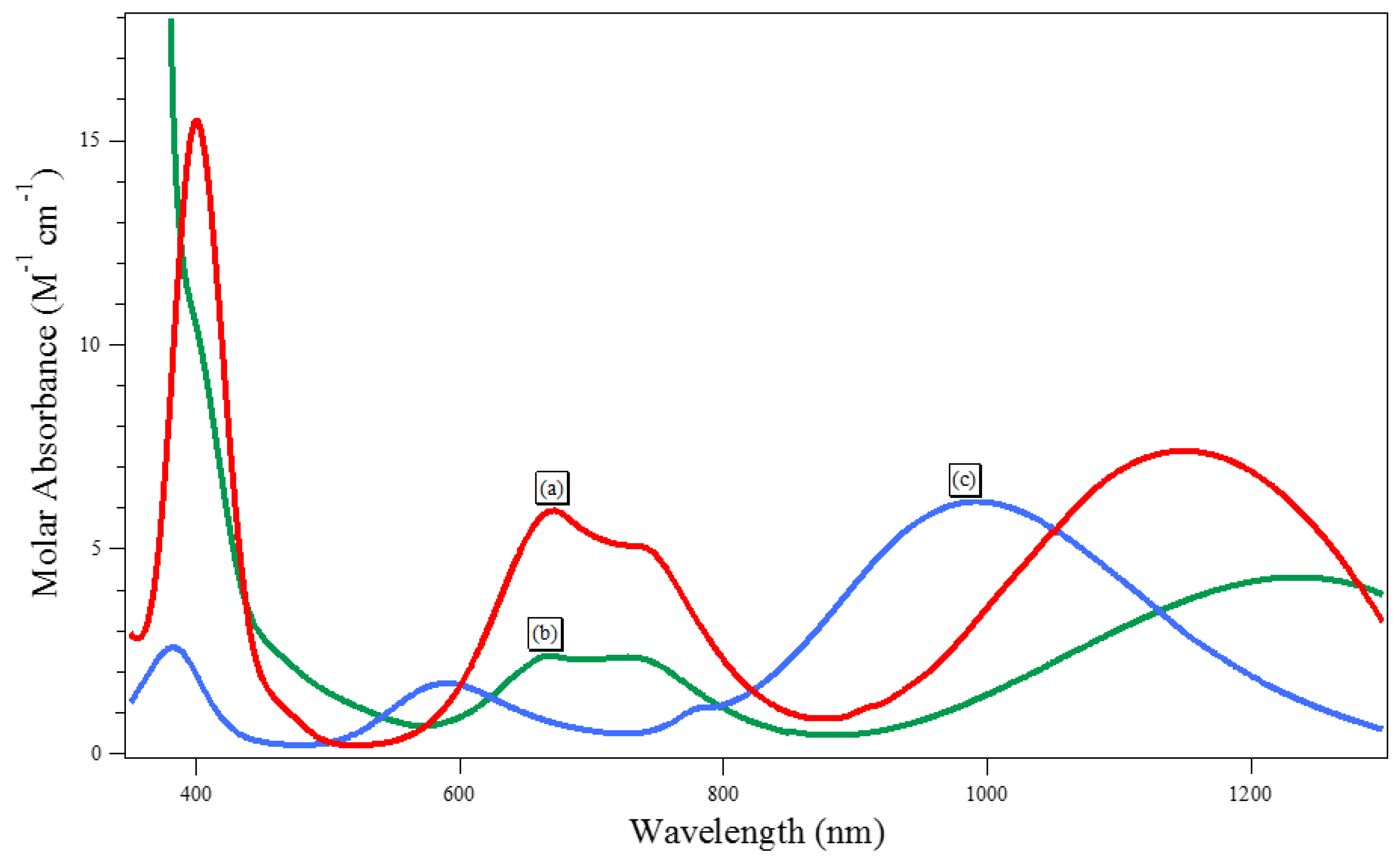
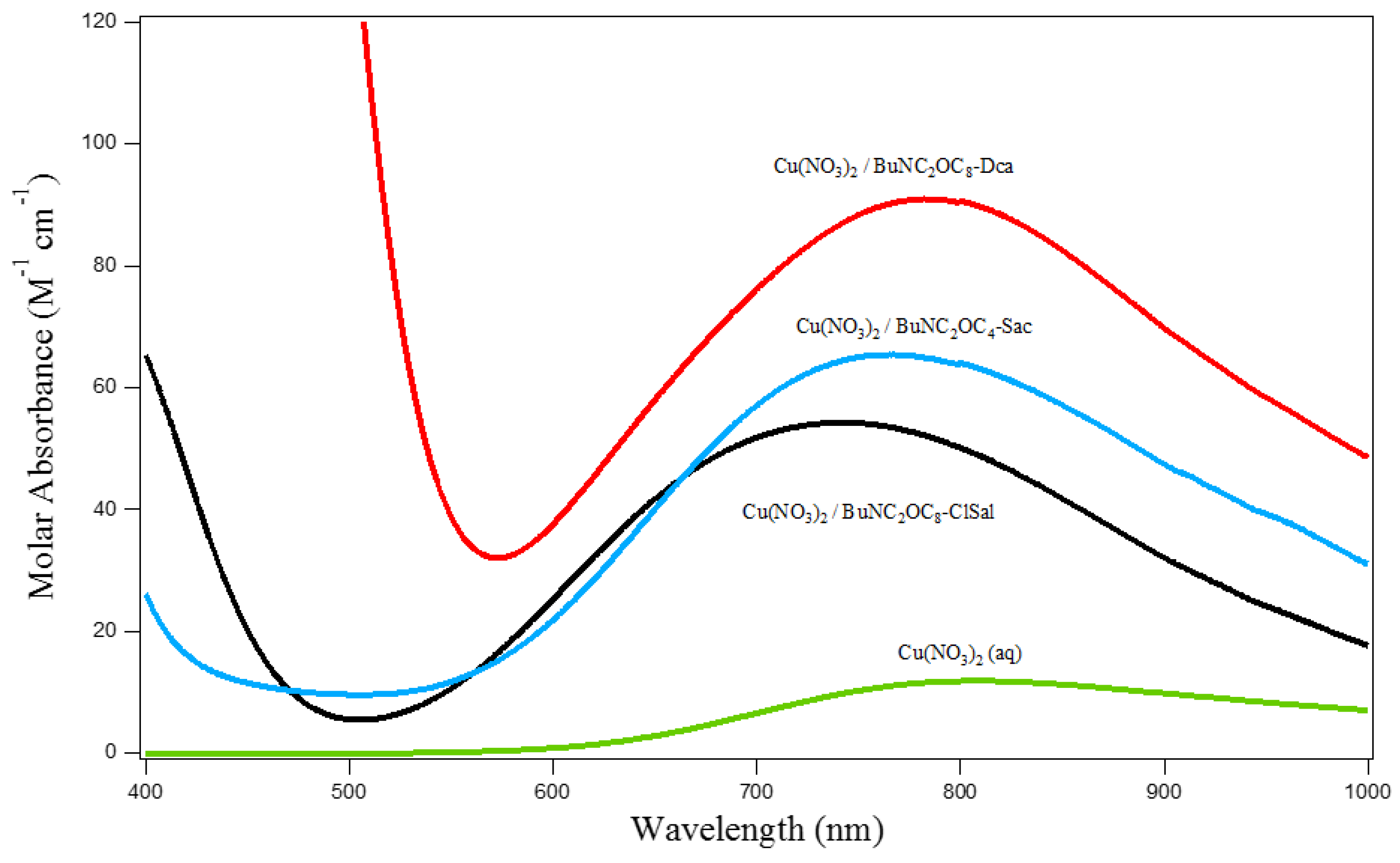
3.1. Extraction of Cu(II), Ni(II), Cd(II), Co.(II) and Pb(II) from Aqueous Solutions
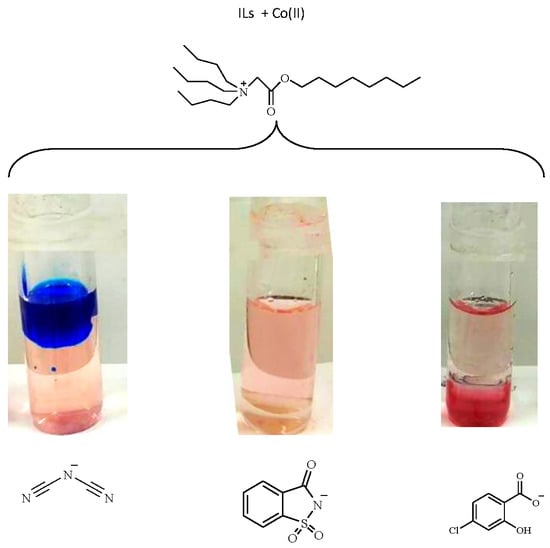
3.2. Influence of the Anion of the Ionic Liquid
3.3. Influence of the Cation of the Ionic Liquid
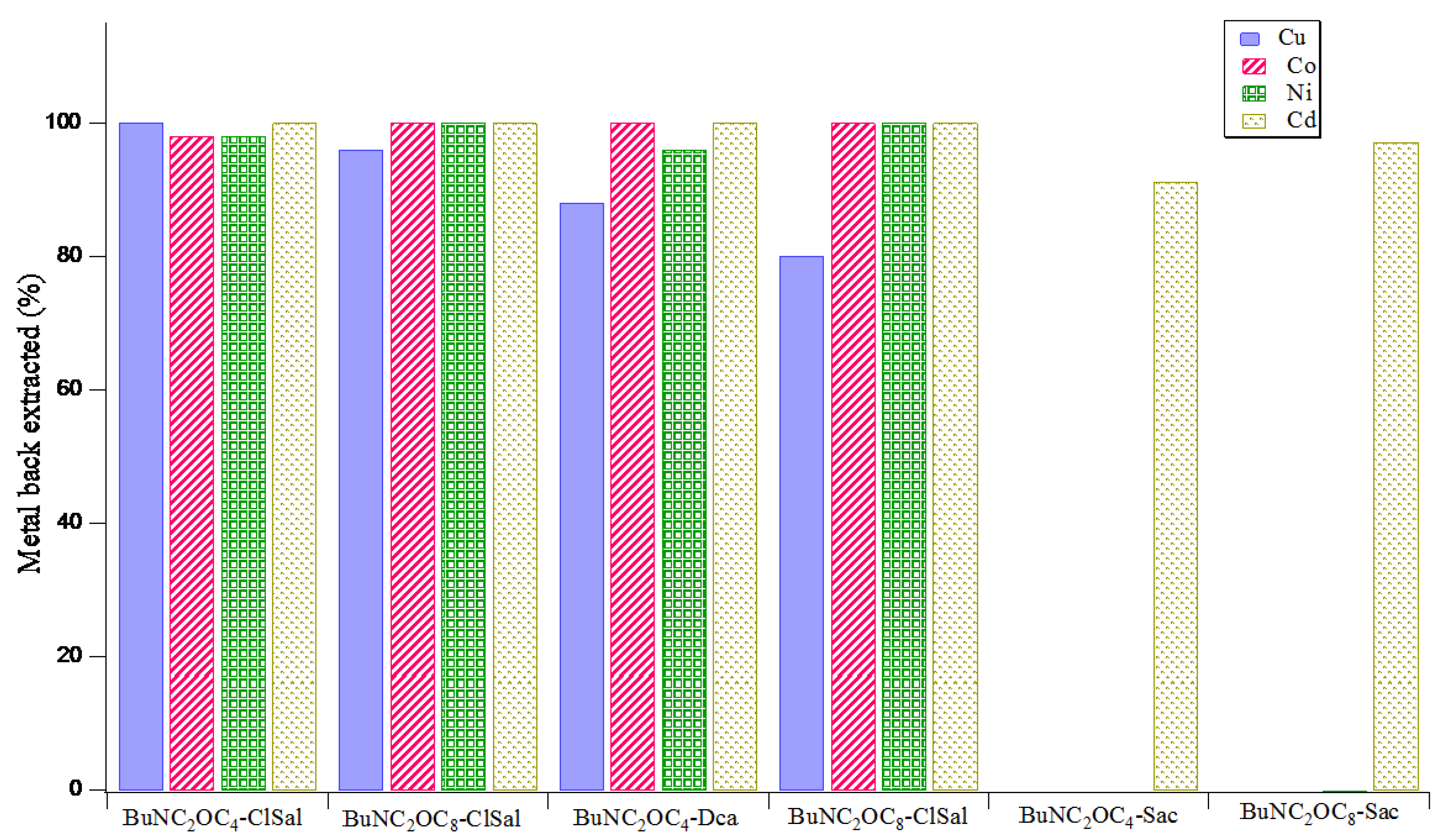
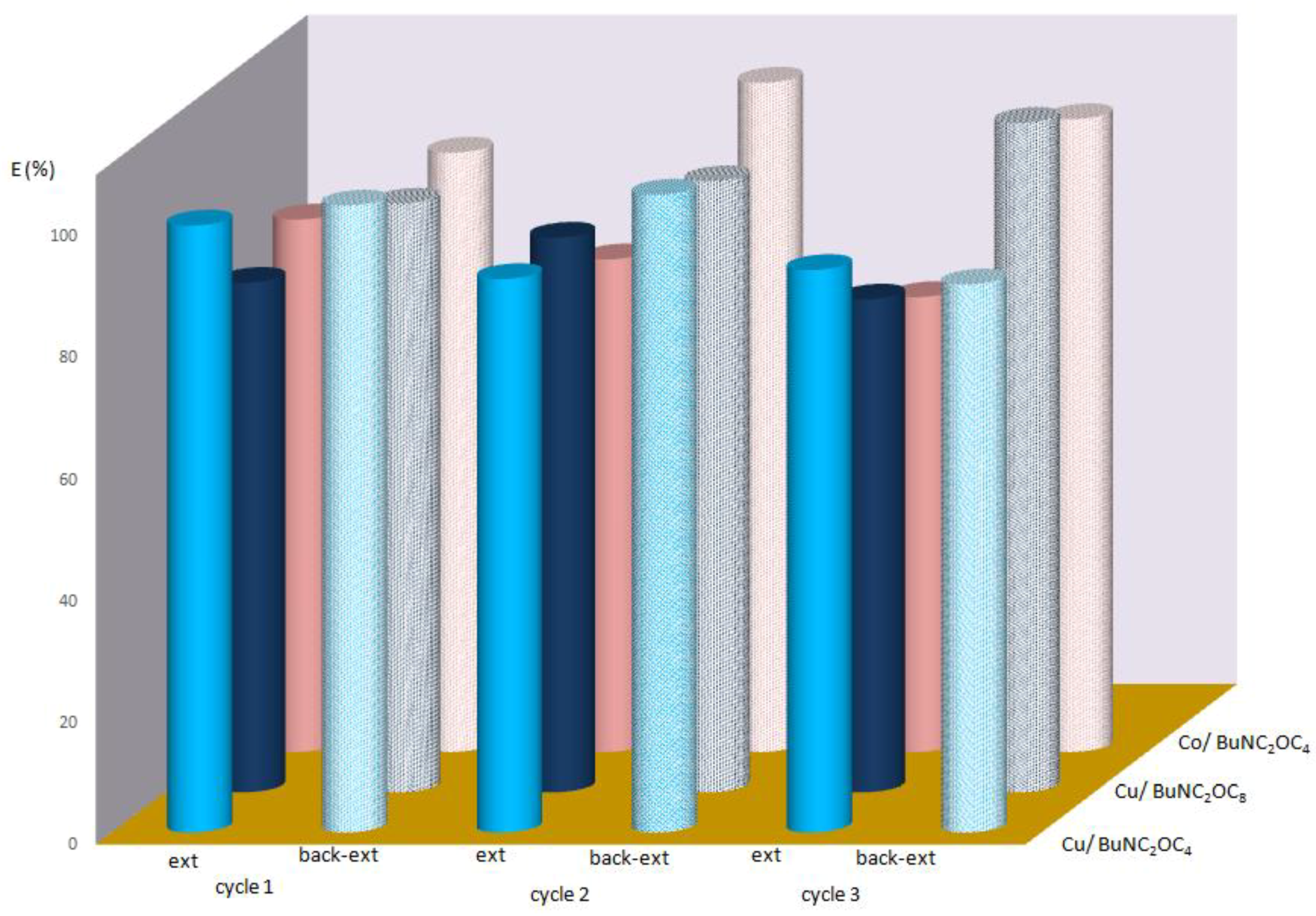
3.4. Back-Extraction of Metal Ions and Recyclability of Ionic Liquid
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lichtfouse, E.; Schwarzbauer, J.; Rober, T.D. Environmental Chemistry: Green Chemistry and Pollutants in Ecosystems; Springer: Berlin, Germany, 2005; p. 780. [Google Scholar]
- Kumbasar, R.A. Extraction and concentration study of cadmium from zinc plant leach solutions by emulsion liquid membrane using trioctylamine as extractant. Hydrometallurgy 2009, 95, 290–296. [Google Scholar] [CrossRef]
- Blais, J.F.; Dufresne, S.; Mercier, G. State of the art of technologies for metal removal from industrial effluents. Revues Des. Sci. De L’eau 1999, 12, 687–711. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, G.J.; Willauer, H.D.; Swatloski, R.P.; Visser, A.E.; Rogers, R.D. Room temperature ionic liquids as novel media for “clean” liquid-liquid extraction. Chem. Commun. 1998, 1765–1766. [Google Scholar] [CrossRef]
- Zhao, H.; Malhotra, S.V. Applications of ionic liquids in organic synthesis. Aldrichimica Acta 2002, 35, 75–83. [Google Scholar] [CrossRef]
- De los Rios, A.P.; Hernandez-Fernandez, F.J.; Lozano, L.J.; Sanchez, S.; Moreno, J.I.; Godinez, C. Removal of metal ions from aqueous solutions by extraction with ionic liquids. J. Chem. Eng. Data 2010, 55, 605–608. [Google Scholar] [CrossRef]
- Papaiconomou, N.; Lee, J.M.; Salminen, J.; Von Stosch, M.; Prausnitz, J.M. Selective extraction of copper, mercury, silver, and palladium ions from water using hydrophobic ionic liquids. Ind. Eng. Chem. Res. 2008, 47, 5080–5086. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’ solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Reyna Gonzales, J.M.; Torriero, A.A.; Siriwardana, A.I.; Burgar, I.M.; Bond, A.M. Extraction of copper(II) ions from aqueous solutions with a methimazole-based ionic liquid. Anal. Chem. 2010, 82, 7691–7698. [Google Scholar] [CrossRef] [PubMed]
- Germani, R.; Mancini, M.V.; Savelli, G.; Spreti, N. Mercury extraction by ionic liquids: Temperature and alkyl chain length effect. Tetrahedron Lett. 2007, 48, 1767–1769. [Google Scholar] [CrossRef]
- Kidani, K.; Hirayama, N.; Imura, H. Extraction behavior of divalent metal cations in ionic liquid chelate extraction systems using 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides and thenoyltrifluoroacetone. Anal. Sci. 2008, 24, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Egorov, V.; Djigailo, D.I.; Momotenko, D.S.; Chernyshow, D.V.; Torochesnikova, I.I.; Smirnova, S.V.; Pletner, I.V. Task-specific ionic liquid trioctylmethylammonium salicylate as extraction solvent fortransition metal ions. Talanta 2010, 80, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Lertlapwasin, R.N.; Bhawawet, N.A.; Imyin, A.S.; Fuangswasdi, S. Ionic liquid extraction of heavy metal ions by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Sep. Purif. Technol. 2010, 72, 70–76. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Visser, A.E.; Spear, S.K.; Reichert, W.M.; Swatloski, R.P.; Roger, R.D. Mercury(II) partitioning from aqueous solutions with a new, hydrophilic ethylene-glycol functionalized bis(methylimidazolium) ionic liquid. Green Chem. 2003, 5, 129–135. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J.H., Jr; Rogers, R.D. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem. Commun. 2001, 135–136. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J.H., Jr.; Rogers, R.D. Task-specific ionic liquids incorporating novel cations for the coordination and extraction of Hg2+ and Cd2+: Synthesis, characterization, and extraction studies. Environ. Sci. Technol. 2002, 36, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Harjani, J.R.; Friscic, T.; MacGillivray, L.R.; Singer, R.D. Removal of metal ions from aqueous solutions using chelating task specific ionic liquids. Dalton Trans. 2008, 34, 4595–4601. [Google Scholar] [CrossRef]
- Olivier, J.H.; Camerel, F.; Selb, J.; Retailleau, P.; Ziessel, R. Terpyridine-functionalized imidazolium ionic liquids. Chem. Commun. 2009, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Neuefeind, J.; Beitz, J.V.; Skanthakumar, S.; Soderholm, L. Mechanisms of metal ion transfer into room-temperature ionic liquids: The role of anion exchange. J. Am. Chem. Soc. 2003, 125, 15466–15473. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, H.; Binnemans, K.; Van Hecke, K.; Van Meervelt, L.; Nockemann, P. Hydrophobic ionic liquids with strongly coordinating anions. Chem. Commun. 2010, 46, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Dzielawa, J.A.; Rickert, P.; Dietz, M.L. EXAFS Investigations of the mechanism of facilitated ion transfer into a room temperature ionic liquid. J. Am. Chem. Soc. 2002, 124, 10664–10665. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.L.; Dzielawa, J.A. Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: Implications for the ‘greenness’ of ionic liquids as diluents in liquid–liquid extraction. Chem. Commun. 2001, 2124–2125. [Google Scholar] [CrossRef]
- Dietz, M.L.; Dzielawa, J.A.; Laszak, I.; Young, B.A.; Jensen, M.P. Influence of solvent structural variations on the mechanism of facilitated ion transfer into room-temperature ionic liquids. Green Chem. 2003, 6, 682–685. [Google Scholar] [CrossRef]
- Dietz, M.L.; Stepinski, D.C. A ternary mechanism for the facilitated transfer of metal ions into room-temperature ionic liquids (RTILs): Implications for the “greenness” of RTILs as extraction solvent. Green Chem. 2005, 7, 747–750. [Google Scholar] [CrossRef]
- Messadi, A.; Mohamadou, A.; Boudesocque, S.; Dupont, L.; Guillon, E. Task-specific ionic liquid with coordinating anion for heavy metal ion extraction: Cation exchange versus ion-pair extraction. Sep. Purif. Technol. 2013, 107, 172–178. [Google Scholar] [CrossRef]
- Root, A.; Binnemans, K. Efficient separation of transition metals from rare earths by an undiluted phosphonium thiocyanate ionic liquid. Phys. Chem. Chem. Phys. 2016, 18, 16039–16045. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, D.; Van der Hoogerstraete, T.; Banerjee, D.; Valia, Y.A.; Metz S, J.; Binnemans, K.; Kroon, M.C. A mechanism for solvent extraction of first row transition metals from chloride media with the ionic liquid tetraoctylammonium oleate. Dalton Trans. 2016, 45, 9661–9668. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Radalla, A. Binary and ternary complexes of inosine. Talanta 1998, 46, 53–61. [Google Scholar] [CrossRef]
- Baran, E.J. The saccharinate anion: A versatile and fascinating ligand in coordination chemistry. Quim. Nova 2005, 28, 326–328. [Google Scholar] [CrossRef]
- Martın, S.; Gotzone Barandika, S.M.; Ruiz de Larramendi, J.L.; Corte, R.; Font-Bardia, M.; Lezama, L.; Serrna, Z.E.; Solans, X. Structural analysis and magnetic properties of the 1D and 3D compounds, [Mn(dca)2nbipym] (M = Mn, Cu; dca = Dicyanamide; bipym = Bipyrimidine; n = 1, 2). Inorg. Chem. 2001, 40, 3687–3692. [Google Scholar]
- Mohamadou, A.; Van Albada, G.A.; Kooijman, H.; Wieczorek, B.; Spek, A.L.; Reedijk, J. The binding mode of the ambidentate ligand dicyanamide to transition metal ions can be tuned by bisimidazoline ligands with H-bonding donor property at the rear side of the ligand. N. J. Chem. 2003, 27, 983–988. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Martinez, A.; Déchamps, I.; Dupont, L. Use of dicyanamide ionic liquids for metal ions extraction. RSC Adv. 2016, 6, 107894–107904. [Google Scholar] [CrossRef]
- Zhou, Y.; Boudesocque, S.; Mohamadou, A.; Dupont, L. Extraction of metal ions with Task-specific ionic liquids : Influence of a coordinating anion extraction. Sep. Sci. Technol. 2015, 50, 38–44. [Google Scholar] [CrossRef]
- De Gaetano, Y.; Mohamadou, A.; Boudesocque, S.; Hubert, J.; Plantier-Royon, R.; Dupont, L. Ionic liquids derived from esters of Glycine Betaine: Synthesis and characterization. J. Mol. Liq. 2016, 207, 60–66. [Google Scholar] [CrossRef]
- Powell, K.J. SC Query SC-database, data version 4.44, soft version 5.3 46.
- Mimoun, M.; Pointud, Y.; Juillard, J.; Juillard, J. Interactions in methanol of divalent metal cations with bacterial iono-phores, lasalocid and monensin-Thermodynamical aspects. Bull. Soc. Chim. Fr. 1994, 131, 13158–13165. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: New York, NY, USA, 1986. [Google Scholar]
- Skopenko, V.V.; Samoilenko, V.M.; Movchan, O.G. Study of cadmium halide and pseudohalide complexes in dimethylacetamide. Zh. Neorg. Khim. 1981, 26, 1319–1323. [Google Scholar]
- Skopenko, V.V.; Samoilenko, V.M.; Garbous, S. Potentiometric study of lead halide and pseudohalide complexes in dimethylacetamide. Zh. Neorg. Khim. 1982, 27, 665–668. [Google Scholar]
- Valle-Bourrouet, G.; Pineda L, W.; Falvello L, R.; Lusar, R.; Weyhermueller, T. Synthesis, structure and spectroscopic characterization of Ni(II), Co(II), Cu(II) and Zn(II) complexes with saccharinate and pyrazole. Polyhedron 2007, 26, 4440–4478. [Google Scholar] [CrossRef]
- Ali, M.A.; Mirza, A.H.; Ting, W.Y.; Hamid, M.H.S.A.; Bernhardt, P.V.; Butcher, R.J. Mixed-ligand nickel(II) and copper(II) complexes of tridentate ONS and NNS ligands derived from S-alkyldithiocarbazates with the saccharinate ion as a co-ligand. Polyhedron 2012, 48, 167–173. [Google Scholar] [CrossRef]


| Ionic Liquid | Density (g·mL−1) |
|---|---|
| BuNC2OC4-Sac | 1.11 |
| BuNC2OC8-Sac | 1.04 |
| BuNC2OC4-ClSal | 1.10 |
| BuNC2OC8-ClSal | 1.02 |
| BuNC2OC4-Dca | 0.96 |
| BuNC2OC8-Dca | 0.96 |
| Ionic Liquid | Cu(II) | Cd(II) | Pb(II) | Ni(II) | Co.(II) |
|---|---|---|---|---|---|
| BuNC2OC4-Sac | 1.5 | 120.0 | 0.8 | 0.1 | 0.2 |
| BuNC2OC8-Sac | 0.4 | 25.4 | 0.1 | - | - |
| BuNC2OC4-ClSal | 1301.4 | 439.6 | 735.6 | 40.0 | 29.7 |
| BuNC2OC8-ClSal | 21.30 | 12.8 | 59.9 | 4.5 | 3.7 |
| BuNC2OC4-Dca | 192.5 | 178.5 | 0.1 | 24.1 | 18.1 |
| BuNC2OC8-Dca | 13.7 | 1.8 | 0.1 | 0.3 | 3.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diabate, P.D.; Dupont, L.; Boudesocque, S.; Mohamadou, A. Novel Task Specific Ionic Liquids to Remove Heavy Metals from Aqueous Effluents. Metals 2018, 8, 412. https://doi.org/10.3390/met8060412
Diabate PD, Dupont L, Boudesocque S, Mohamadou A. Novel Task Specific Ionic Liquids to Remove Heavy Metals from Aqueous Effluents. Metals. 2018; 8(6):412. https://doi.org/10.3390/met8060412
Chicago/Turabian StyleDiabate, Pape Diaba, Laurent Dupont, Stéphanie Boudesocque, and Aminou Mohamadou. 2018. "Novel Task Specific Ionic Liquids to Remove Heavy Metals from Aqueous Effluents" Metals 8, no. 6: 412. https://doi.org/10.3390/met8060412
APA StyleDiabate, P. D., Dupont, L., Boudesocque, S., & Mohamadou, A. (2018). Novel Task Specific Ionic Liquids to Remove Heavy Metals from Aqueous Effluents. Metals, 8(6), 412. https://doi.org/10.3390/met8060412