Selective Extraction of Rare Earth Elements from Phosphoric Acid by Ion Exchange Resins
Abstract
:1. Introduction
- There is no risk of contamination of phosphoric acid with organic solvents, so no need for expensive post-acid treatment downstream of extraction [1].
- Resins may be sensitive to chemical degradation in concentrated phosphoric acid.
- Phenomena of swelling may lead to mechanical stresses during sorption-desorption.
- There is a risk of resin poisoning with some cationic impurities in large quantities such as iron, leading to a decrease in sorption capacity and/or selectivity.
- A high selectivity between REE and cationic impurities such as iron (Fe3+), uranium (U(IV), U(VI)) or thorium (Th(IV)), taking into account that Fe3+ is approximately 30 to 40 times more concentrated than REE in phosphoric acid solutions. Due to a similarity in extraction chemistry, Fe3+ and Th are often adsorbed onto chelating resins in preference to REE [7]. It should be noted that a first precipitation of Fe3+ is not an option since it could generate high losses in REE by co-precipitation [6].
- A good capability to bind the REE in highly concentrated H3PO4 (4 to 5 mol/L). A total ion exchange capacity greater than 2 meq/g of resin is generally sought (1 eq = 1 mole/valence). The higher the capacity the smaller the process and thus the investment cost.
- The kinetics of sorption and elution should be fast enough to limit the size of the columns.
- A mechanical, physical, and chemical stability over several cycles of extraction/elution will reduce the operating cost.
- The nature of the matrix (copolymer), the structure and the degree of crosslinking, the nature and the number of fixed ionic (functional) groups.
- The presence of competitive cationic impurities such as U, Th, Fe3+, Al3+, Ca2+, Mg2+,… In that study it should be emphasized that, no U or Th is present in the OCP’s genuine acid solutions due to a preliminary proprietary treatment.
- The nature and pH of the bulk acidic medium.
2. Choice of the Resin
3. Materials and Methods
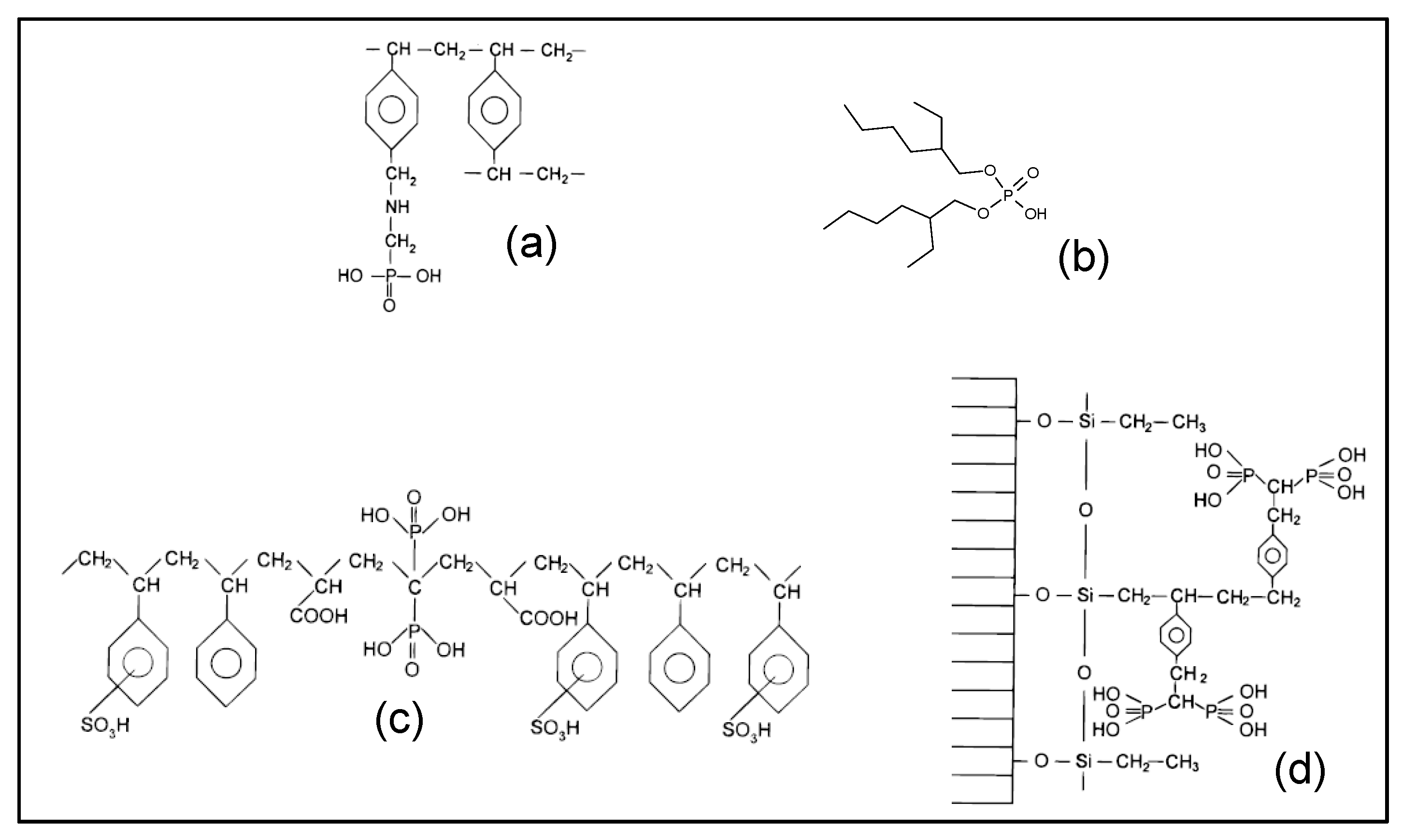
3.1. Physicochemical Properties of Resins
3.2. Composition of Aqueous Solutions
3.3. Experimental Protocol for Batch Tests (Extraction and Elution)
3.4. ICP-AES Analysis Protocol
3.5. Mineralization Protocol
- Temperature increase: gradient 10 °C/min for 20 min up to 200 °C and 100 bars at maximum power (1000 W).
- Temperature step: 10 min.
- Cooling: 30 min during which a forced convection with a fan occurs in the oven.
3.6. Sorption Yields YM and RM of a Cation M
- By balancing the concentrations (in mg/L) in aqueous phase before (CM-ini) and after extraction (CM-end):YM (extraction) = (CM-ini − CM-end)/CM-ini
- By balancing the masses of cation M in the aqueous phase before extraction and fixed onto the resin. This last mass is determined by the concentration of the mineralization solution, CM-miner (mg/L), and by the knowledge of the total masses and volumes used for the extraction (mext (mg), Vext(L)) and the mineralization (mminer (mg), Vminer (L)):RM (extraction) = (CM-minerVminer)/(CM-iniVext)(mext/mminer)The errors on YM and RM are estimated to be ±15%.
3.7. Mass of Adsorbed Cation Per Gram of Dry Resin (XM)
- Taking into account initial and post-extraction concentrations in the aqueous phase:XM = (CM-ini − CM-end)Vext/mextThe error on XM is estimated to be ±15%.
- By direct mineralization of the resin trapping the cation:XM-fixed = CM-minerVminer/mminerThe error on XM based on that calculation is estimated to be ±10%.It is worth mentioning that XM is often referred to as the resin adsorption capacity for cation M.
3.8. Experimental Protocol for the Kinetic Studies
- Washing the resin with demineralized water.
- Conditioning the resin under H+ ionic form using sulfuric acid.
- Washing the resin to remove the remaining sulfuric acid.
3.9. Mass of Eluted Cations Per Gram of Dry Resin (XM-eluted)
4. Results and Discussion
4.1. Extraction of REE from Synthetic Solutions REH and REOCP
- The behavior of the aminophosphonic resins IRC-747, S940, CH-93, and TP-260 is similar: extraction decreases when Z/IR increases from the scandium up to a plateau constituted by the group of La, Nd, Gd, and then increases with Z/IR from Dy to Yb. It is worth noting that the efficiency of extraction (see Figure 4) decreases with IR similarly to that observed with CH-93 resins [12,13,17] or with phosphorus extractants [18], for example dialkylphosphinic acid CYANEX 272 [19] or di(2-ethylhexyl)phosphoric acid D2EHPA [20]).
- Without impurities in the phosphoric acid solution, the VP-OC-1026 resin on which the D2EHPA is adsorbed behaves similarly with the aminophosphonic resins but with a lower sorption efficiency.
- Without impurities, the extraction efficiency of Actinide Resin-B is not clearly related to Z/IR or to IR (see Figure 4). The presence of impurities flattens the extraction efficiency, which becomes nearly constant between 10% and 15% for all of the REE.
- The presence of a sulfonic group together with an alkylphosphonic function in the Diphonix and Monophos resins results in an inverted behavior compared to the above resins: the extraction efficiency increases as Z/IR increases from the scandium to the neodymium and then decreases for the highest Z/IR although it continuously increases with IR (see Figure 2, Figure 3 and Figure 4). Despite their low specific surface area compared to the tested aminophosphonic resins (see Table 1) the Diphonix and Monophos resins extract REE in the same order of magnitude (from 10 to 20% except for scandium and ytterbium for the Diphonix). It is then probable that the number of accessible sites of complexing functions per gram of resin is higher for Diphonix and Monophos than for the other resins.
4.2. Extraction of REE from JSYNT and J1 OCP Phosphoric Acid Solutions
4.3. Adsorption Isotherm of Er
4.4. Extraction Kinetics with IRC-747 and TP-260
- The diffusion of ions from the solution to the resin surface whose rate R can be described by:R = −ln (1 − F) = kt
- The diffusion of ions within the solid resin whose rate R can be described by:R = −ln (1 − F2) = kt
- And the chemical reaction between ions and functional groups (of the resin).
4.5. Elution Conditions in Carbonate or Sulfuric Media
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soldenhoff, K.; Tran, M.T.; Griffith, C. Recovery of Uranium from Phosphoric by Ion Exchange. In Proceedings of the 2009 IAEA Technical Meeting—Uranium from Unconventional Resources, Vienna, Austria, 22–26 June 2009. [Google Scholar]
- Kim, H.; Eggert, R.G.; Carlsen, B.W.; Dixon, B.W. Potential uranium supply from phosphoric acid A U.S. analysis comparing solvent extraction and Ion exchange recovery. Resour. Policy 2016, 49, 222–231. [Google Scholar] [CrossRef]
- Michel, P. Les Techniques de L’industrie Minérale; Société de L’industrie Minérale: Paris, France, 2006; Volume 32, pp. 95–102. [Google Scholar]
- Cote, D.B.G.; Mokhtari, H.; Courtaud, B.; Moyer, B.A.; Chagnes, A. Recovery of Uranium from Wet Phosphoric Acid by Solvent Extraction Processes. Chem. Rev. 2014, 114, 12002–12023. [Google Scholar]
- Volkman, Y. Recovery of Uranium from Phosphoric Acid by Ion Exchange; Report IAEA-TECDOC-533; IAEA: Vienna, Austria, 1987; pp. 59–68. [Google Scholar]
- Mashkovtsev, M.; Botalov, M.; Smyshlyaev, D.; Pajarre, R.; Kangas, P.; Rychkov, V.; Koukkari, P. Pilot-scale recovery of rare earths and scandium from phosphogypsum and uranium leachates. In Proceedings of the 2016 Mineral Engineering Conference, Swieradow-Zdroj, Poland, 25–28 September 2016. [Google Scholar]
- Page, M.J.; Soldenhoff, K.; Ogden, M.D. Comparative Study of the Application of Chelating Resins for Rare Earth Recovery. Hydrometallurgy 2017, 169, 275–281. [Google Scholar] [CrossRef]
- Helfferich, F. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- Reddy, B.R.; Kumar, J.R. Rare Earths Extraction, Separation, and Recovery from Phosphoric Acid Media. Solvent Extr. Ion Exch. 2016, 34, 226–240. [Google Scholar] [CrossRef]
- Quinn, J.E.; Soldenhoff, K.H.; Stevens, G.W.; Lengkeek, N.A. Solvent extraction of rare earth elements using phosphonic/phosphinic acid mixtures. Hydrometallurgy 2015, 157, 298–305. [Google Scholar] [CrossRef]
- Nesterenko, P.N.; Zhukova, O.S.; Shpigun, O.A.; Jones, P. Synthesis and Ion-Exchange Properties of Silica Chemically Modified with Aminophosphonic Acid. J. Chromatogr. A 1998, 813, 47–53. [Google Scholar] [CrossRef]
- Radhika, S.; Nagaraju, V.; Kumar, B.N.; Kantam, M.L.; Reddy, B.R. Solid-liquid extraction of Gd(III) and separation possibilities of rare earths from phosphoric acid solutions using Tulsion CH-93 and Tulsion CH-90 resins. J. Rare Earths 2012, 30, 1270–1275. [Google Scholar] [CrossRef]
- Kumar, B.N.; Radhika, S.; Reddy, B.R. Solid–liquid extraction of heavy rare-earths from phosphoric acid solutions using Tulsion CH-96 and T-PAR resins. Chem. Eng. J. 2010, 160, 138–144. [Google Scholar] [CrossRef]
- Cheira, M.F. Characteristics of uranium recovery from phosphoric acid by an aminophosphonic resin and application to wet process phosphoric acid. Eur. J. Chem. 2015, 6, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Kabay, N.; Demircioglu, M.; Yayh, S.; Gunay, E.; Yuksel, M.; Saglam, M.; Streat, M. Recovery of Uranium from Phosphoric Acid Solutions Using Chelating Ion-Exchange Resins. Ind. Eng. Chem. Res. 1998, 37, 1983–1990. [Google Scholar] [CrossRef]
- Flahaut, J. Les éléments des Terres Rares; Masson: Paris, France, 1969. [Google Scholar]
- Reddy, B.R.; Kumar, B.N.; Radhika, S. Solid Liquid Extraction of Terbium from Phosphoric Acid Medium using Bifunctional Phosphinic Acid Resin Tulsion CH 96. Solvent Extr. Ion Exch. 2009, 27, 695–711. [Google Scholar] [CrossRef]
- Bunus, F.; Dumitrescu, R. Simultaneous extraction of rare earth elements and uranium from phosphoric acid. Hydrometallurgy 1992, 28, 331–338. [Google Scholar] [CrossRef]
- Li, D. A review on yttrium solvent extraction chemistry and separation process. J. Rare Earths 2017, 35, 107–119. [Google Scholar] [CrossRef]
- Ochsentihn-Petropulu, M.; Lyberopulu, T.; Parissakis, G. Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal. Chim. Acta 1995, 315, 231–237. [Google Scholar] [CrossRef]
- Bao, S.; Hawker, W.; Vaughan, J. Scandium Loading on Chelating and Solvent Impregnated Resin from Sulfate Solution. Solvent Extr. Ion Exch. 2018, 36, 100–113. [Google Scholar] [CrossRef]
- Alguacil, F.J. A kinetic study of cadmium(II) adsorption on Lewatit TP260 resin. J. Chem. Res. 2003, 3, 144–146. [Google Scholar] [CrossRef]
- Rey, J.; Atak, S.; Dourdain, S.; Arrachart, G.; Berthon, L.; Pellet-Rostaing, S. Synergistic Extraction of Rare Earth Elements from Phosphoric Acid Medium using a Mixture of Surfactant AOT and DEHCNPB. Solvent Extr. Ion Exch. 2017, 35, 321–331. [Google Scholar] [CrossRef]









| Functional Group | Name of Resin | Ionic Form | Bead Size from Suppliers (min 95%) (mm) | Specific Surface Area (BET) m2/g | Pore Size (BJH Desorption) (nm) | Densities |
|---|---|---|---|---|---|---|
| Amino phosphonic | Tulsion CH-93 | Na+ | 0.3–1.2 | 28 | 40–50 | 0.612 |
| Purolite S940 | Na+ | 0.43–0.85 | 21 | 40–50 | 0.469 | |
| Amberlite IRC-747 | Na+ | 0.52–0.66 | 21 | 40–50 | 0.530 | |
| Lewatit TP-260 | Na+ | 0.58–0.68 | 15 | 40–50 | 0.726 | |
| DIPEX Extractant | Actinide Resin-B | Na+ | 0.1–0.15 | 76 | 20 | 0.416 |
| D2EHPA | Lewatit VP OC 1026 | H+ | 0.31–1.6 | 5.5 | 50 | 0.607 |
| Monophosphonic + sulfonic | Monophos | H+ | 0.3–1.2 | 0.66 | 30–40 | - |
| Diphosphonic + sulfonic | Diphonix | H+ | 0.3–1.2 | 0.14 | Not measurable | - |
| CM (mg/L) or (g/L) | J0 | J1 | REH REOCP | JSYNT | Ionic Radius [16] (A) | Z | Z/IR (A−1) |
|---|---|---|---|---|---|---|---|
| Sc | 6.5 | 10 | 72 | 6.7 | 0.68 | 21 | 31 |
| Y | 24.6 | 37 | 72 | 6.8 | 0.89 | 39 | 44 |
| La | 4.5 | 8.8 | 53 | 7.5 | 1.06 | 57 | 54 |
| Pr | 1.2 | 1.3 | - | - | 1.01 | 59 | 58 |
| Nd | 3.1 | 5.8 | 54 | 5.0 | 1.00 | 60 | 60 |
| Sm | 0.9 | 1.5 | - | - | 0.96 | 62 | 65 |
| Eu | 0.3 | 0.4 | - | - | 0.95 | 63 | 66 |
| Gd | 1.7 | 2.7 | 72 | 6.4 | 0.94 | 64 | 68 |
| Tb | 0.4 | 0.4 | - | - | 0.92 | 65 | 71 |
| Dy | 1.9 | 3.0 | 74 | 6.8 | 0.91 | 66 | 73 |
| Ho | 0.7 | 0.8 | - | - | 0.89 | 67 | 75 |
| Er | 1.9 | 2.8 | 73 | 6.7 | 0.88 | 68 | 77 |
| Tm | 0.5 | 0.5 | - | - | 0.87 | 69 | 79 |
| Yb | 2.8 | 4.1 | 74 | 6.8 | 0.86 | 70 | 81 |
| Lu | 0.8 | 0.8 | - | - | 0.85 | 71 | 84 |
| Al | 0.9 | 1.1 | 0.8 | - | - | - | |
| Ca | 0.8 | 0.8 | 1.0 | - | - | - | |
| Fe | 1.7 | 1.4 | 1.4 | 1.3 | - | - | - |
| Mg | 3.9 | 4.3 | 4.8 | - | - | - | - |
| V | 0.12 | 0.11 | 0.1 | - | - | - | - |
| Zn | 0.22 | 0.21 | 0.30 | - | - | - | - |
| [H3PO4] (mol/L) | 3.9 | 3.8 | 4.2 | 4.2 | - | - | - |
| Name of Resin | IRC-747 | IRC-747 | TP-260 | TP-260 | |||
|---|---|---|---|---|---|---|---|
| Eluting Solution | H2SO4 9M | Na2CO3 1M | Na2CO3 1M | H2SO4 18M | |||
| YM,RM Elution (%) | Aq | Min | Aq | Min | Aq | Min | Aq |
| La | 39 | 34 | 25 | 30 | 20 | 15 | 72 |
| Nd | 36 | 31 | 47 | 47 | 45 | 41 | 68 |
| Gd | 44 | 36 | 60 | 46 | 51 | 46 | 79 |
| Dy | 36 | 34 | 58 | 51 | 57 | 43 | 80 |
| Er | 32 | 28 | 68 | 55 | 70 | 56 | 79 |
| Yb | 29 | 26 | 66 | 56 | 63 | 53 | 78 |
| Y | 30 | 29 | 65 | 45 | 61 | 43 | 78 |
| Sc | 0 | 0 | 50 | 43 | 36 | 32 | 34 |
| Al | 9 | 9 | 30 | 25 | 31 | 29 | 0 |
| Ca | 48 | 68 | 65 | - | 75 | - | 3 |
| Fe | 4 | 3 | 9 | 7 | 11 | 8 | 8 |
| Mg | 80 | 15 | 14 | 2 | 19 | 18 | 16 |
| V | 35 | 26 | 100 | 81 | 106 | 92 | 0 |
| Zn | 83 | - | - | - | - | - | 79 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hérès, X.; Blet, V.; Di Natale, P.; Ouaattou, A.; Mazouz, H.; Dhiba, D.; Cuer, F. Selective Extraction of Rare Earth Elements from Phosphoric Acid by Ion Exchange Resins. Metals 2018, 8, 682. https://doi.org/10.3390/met8090682
Hérès X, Blet V, Di Natale P, Ouaattou A, Mazouz H, Dhiba D, Cuer F. Selective Extraction of Rare Earth Elements from Phosphoric Acid by Ion Exchange Resins. Metals. 2018; 8(9):682. https://doi.org/10.3390/met8090682
Chicago/Turabian StyleHérès, Xavier, Vincent Blet, Patricia Di Natale, Abla Ouaattou, Hamid Mazouz, Driss Dhiba, and Frederic Cuer. 2018. "Selective Extraction of Rare Earth Elements from Phosphoric Acid by Ion Exchange Resins" Metals 8, no. 9: 682. https://doi.org/10.3390/met8090682
APA StyleHérès, X., Blet, V., Di Natale, P., Ouaattou, A., Mazouz, H., Dhiba, D., & Cuer, F. (2018). Selective Extraction of Rare Earth Elements from Phosphoric Acid by Ion Exchange Resins. Metals, 8(9), 682. https://doi.org/10.3390/met8090682





