Enhancing the Reduction of High-Aluminum Iron Ore by Synergistic Reducing with High-Manganese Iron Ore
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Procedures
3. Results
3.1. Effect of Reduction Temperature
3.2. Effect of Reduction Duration
3.3. Effect of Reductant Ratio
3.4. Effect of HM Ore Ratio
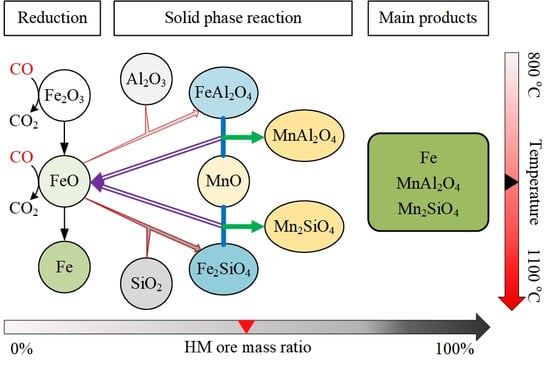
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Steel Association Economics Committee. Steel Statistical Yearbook 2018; World Steel Association: Brussels, Belgium, 2018; p. 104. [Google Scholar]
- Zhang, K.; Kleit, A.N. Mining rate optimization considering the stockpiling: A theoretical economics and real option model. Resour. Policy 2016, 47, 87–94. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Pilatos, G.; Tsakiridis, P.E.; Devlin, E.; Pissas, M. Controlled reduction of red mud by H2 followed by magnetic separation. Miner. Eng. 2017, 105, 36–43. [Google Scholar] [CrossRef]
- Chun, T.; Long, H.; Li, J. Alumina-Iron Separation of High Alumina Iron Ore by Carbothermic Reduction and Magnetic Separation. Sep. Sci. Technol. 2015, 50, 760–766. [Google Scholar] [CrossRef]
- Lu, L.; Holmes, R.J.; Manuel, J.R. Effects of alumina on sintering performance of hematite iron ores. ISIJ Int. 2007, 47, 349–358. [Google Scholar] [CrossRef]
- Raghukumar, C.; Tripathy, S.K.; Mohanan, S. Beneficiation of Indian High Alumina Iron Ore Fines—A Case Study. Int. J. Min. Eng. Miner. Proc. 2012, 1, 94–100. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud-A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Thella, J.S.; Mukherjee, A.K.; Srikakulapu, N.G. Processing of high alumina iron ore slimes using classification and flotation. Powder Technol. 2012, 217, 418–426. [Google Scholar] [CrossRef]
- Chun, T.; Li, D.; Di, Z.; Long, H.; Tang, L.; Li, F.; Li, Y. Recovery of iron from red mud by high-temperature reduction of carbon-bearing briquettes. J. S. Afr. Inst. Min. Metall. 2017, 117, 361–364. [Google Scholar] [CrossRef]
- Li, G.; Jiang, T.; Liu, M.; Zhou, T.; Fan, X.; Qiu, G. Beneficiation of High-Aluminium-Content Hematite Ore by Soda Ash Roasting. Min. Proc. Extr. Met. Rev. 2010, 31, 150–164. [Google Scholar] [CrossRef]
- Chun, T.J.; Zhu, D.Q.; Pan, J.; He, Z. Preparation of metallic iron powder from red mud by sodium salt roasting and magnetic separation. Can. Metall. Quart. 2014, 53, 183–189. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, S.C.; Sahoo, S.K.; Chakraverty, A.P.; Maharana, H.S. Progress of red mud utilization: An overview. Am. Chem. Sci. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Wang, H.; She, X.; Zhao, Q.; Xue, Q.; Wang, J. Production of iron nuggets using iron-rich red mud by direct reduction. Chin. J. Proc. Eng. 2012, 12, 816–821. [Google Scholar]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of Bauxite Residue (Red Mud) in View of Iron and Selective Rare Earths Recovery. J. Sustain. Met. 2016, 2, 28–37. [Google Scholar] [CrossRef]
- He, P.; Ju, D.; Shen, P.; Jin, H. Experimental research on comprehensive utilization of red mud based on direct reduction and melting by RHF iron bead technology. Energ. Metall. Ind. 2017, 36, 57–60. [Google Scholar]
- Stjernberg, J.; Olivas-Ogaz, M.A.; Antti, M.L.; Ion, J.C.; Lindblom, B. Laboratory scale study of the degradation of mullite/corundum refractories by reaction with alkali-doped deposit materials. Ceram. Int. 2013, 39, 791–800. [Google Scholar] [CrossRef]
- Gan, L.; Xu, J.; Zhang, Z.; Liang, Y.; Wei, M.; Wen, C. Red Mud Pellets and Its Preparation Method. CN103602805A, 26 February 2014. [Google Scholar]
- Shankar, A. Studies on High Alumina Blast Furnace Slags. Ph.D. Thesis, Royal Institute of Technolog, Stockholm, Sweden, 15 June 2007. [Google Scholar]
- Zhou, X.; Zhu, D.; Pan, J.; Luo, Y.; Liu, X. Upgrading of High-Aluminum Hematite-Limonite Ore by High Temperature Reduction-Wet Magnetic Separation Process. Metals 2016, 6, 57. [Google Scholar] [CrossRef]
- Donskoi, E.; Olivares, R.I.; McElwain, D.L.S.; Wibberley, L.J. Experimental study of coal based direct reduction in iron ore/coal composite pellets in a one layer bed under nonisothermal, asymmetric heating. Ironmak. Steelmak. 2006, 33, 24–28. [Google Scholar] [CrossRef]
- Zhu, D.; Luo, Y.; Pan, J.; Zhou, X. Reaction Mechanism of Siderite Lump in Coal-Based Direct Reduction. High Temp. Mater. Procees. 2016, 35, 185–194. [Google Scholar] [CrossRef]















| Ores | Fetotal | Mntotal | Al2O3 | SiO2 | CaO | MgO | Pb | Zn | P | S | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| High-aluminum iron ore (HA ore) | 41.92 | 1.24 | 13.74 | 13.96 | 0.13 | 0.88 | 0.64 | 0.21 | 0.130 | 0.014 | 7.20 |
| High-manganese iron ore (HM ore) | 42.32 | 9.24 | 6.60 | 4.22 | 0.20 | 0.20 | 1.86 | 0.98 | 0.065 | 0.018 | 11.05 |
| Series No. | High-Aluminum Iron Ore: High-Maganese Iron Ore (HA:HM) Ratio | C/Fe Mass Ratio | Temperature | Reduction Duration |
|---|---|---|---|---|
| 1 | 100:0 | 1.5 | 800 °C, 850 °C, 900 °C, 950 °C, 1000 °C, 1050 °C, 1100 °C | 60 min |
| 2 | 100:0 | 1.5 | 1050 °C | 15 min, 30 min, 45 min, 60 min, 75 min, 90 min, 120 min |
| 3 | 100:0 | 0.5, 1.0, 1.5, 2.0 | 1050 °C | 90 min |
| 4 | 100:0, 80:20, 60:40, 40:60, 20:80, 0:100 | 1.5 | 1050 °C | 90 min |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Luo, Y.; Chen, T.; Zhu, D. Enhancing the Reduction of High-Aluminum Iron Ore by Synergistic Reducing with High-Manganese Iron Ore. Metals 2019, 9, 15. https://doi.org/10.3390/met9010015
Zhou X, Luo Y, Chen T, Zhu D. Enhancing the Reduction of High-Aluminum Iron Ore by Synergistic Reducing with High-Manganese Iron Ore. Metals. 2019; 9(1):15. https://doi.org/10.3390/met9010015
Chicago/Turabian StyleZhou, Xianlin, Yanhong Luo, Tiejun Chen, and Deqing Zhu. 2019. "Enhancing the Reduction of High-Aluminum Iron Ore by Synergistic Reducing with High-Manganese Iron Ore" Metals 9, no. 1: 15. https://doi.org/10.3390/met9010015
APA StyleZhou, X., Luo, Y., Chen, T., & Zhu, D. (2019). Enhancing the Reduction of High-Aluminum Iron Ore by Synergistic Reducing with High-Manganese Iron Ore. Metals, 9(1), 15. https://doi.org/10.3390/met9010015