Correlations between High-Temperature Oxidation Kinetics and Thermal Radiation Characteristics of Micro-Structured Nickel Surfaces Oxidized at 1173 K
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Oxidation Behavior
3.2. Thermal Radiation Properties
4. Conclusions
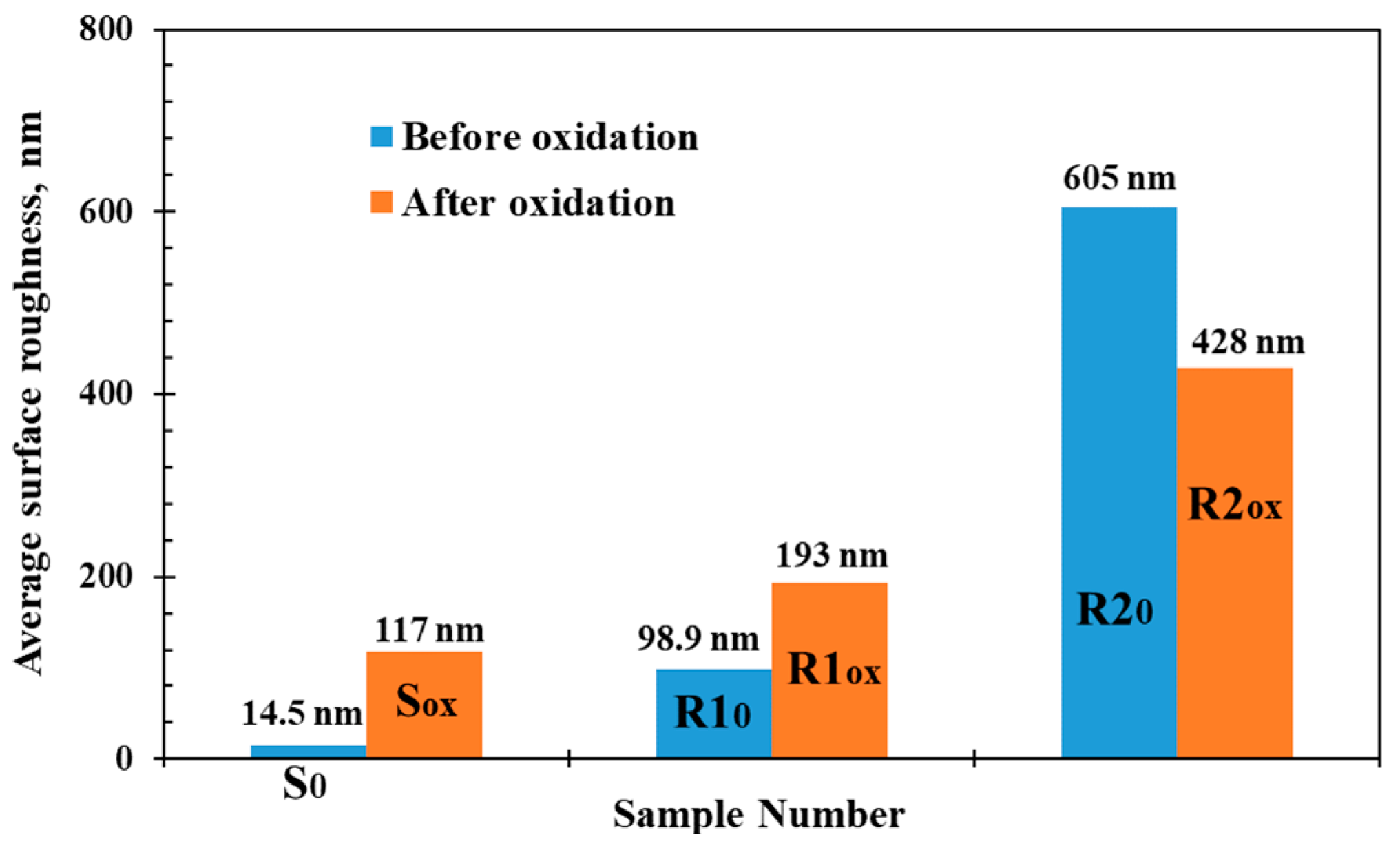
- The results show that rougher initial surfaces of the un-oxidized samples resulted in rougher sample surfaces after oxidation at high temperatures. The initial surface roughness affects the surface roughness after oxidation with the oxidation increasing the surface roughness on smooth or less rough surfaces while making the surface smoother for very rough surfaces.
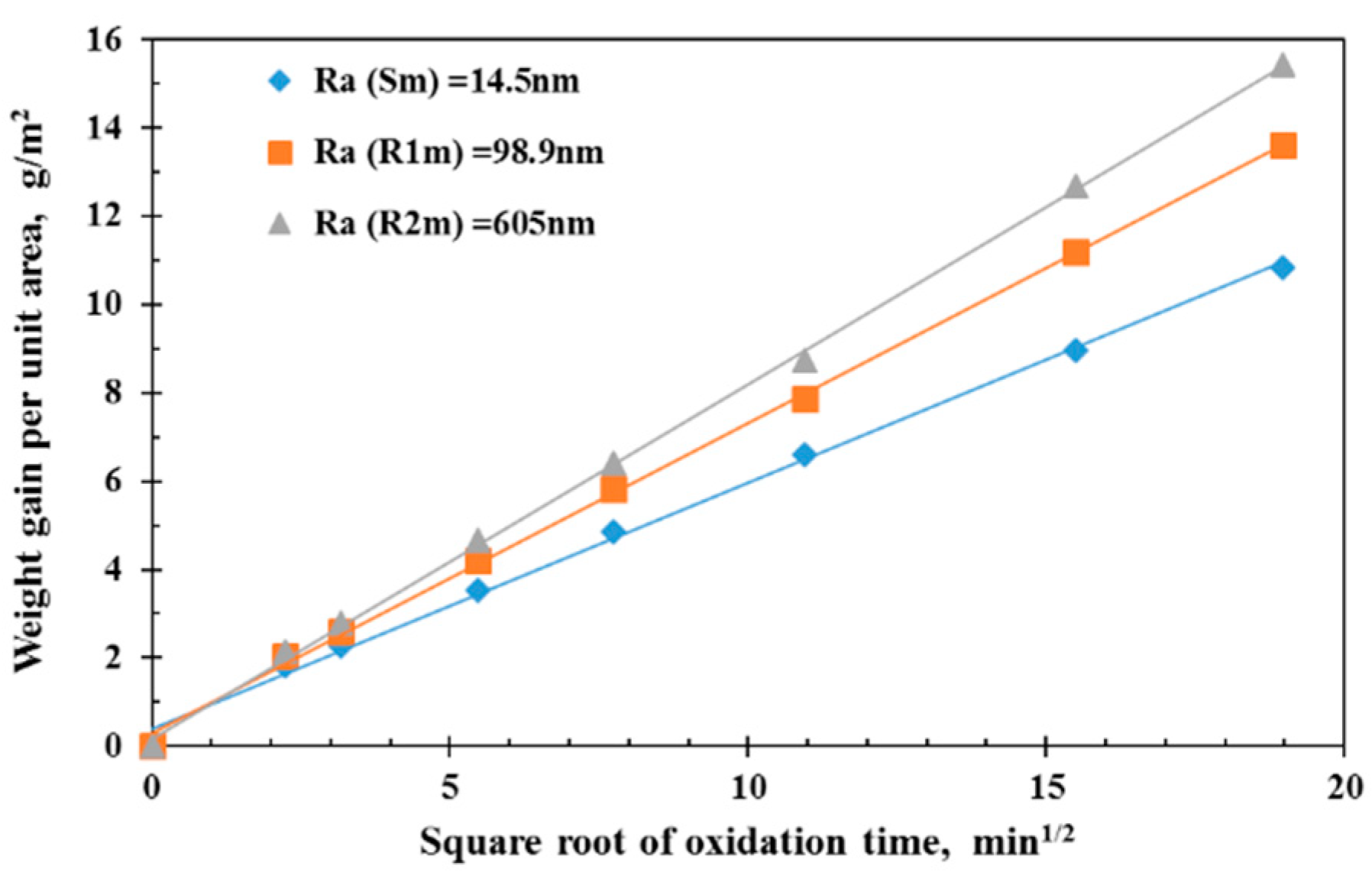
- SEM images of the oxidized samples show how the nickel oxide grains, especially the grain size, differed for different initial surface roughnesses and different oxidation times with rougher initial surfaces, which leads to larger oxide grain sizes and longer oxidation times leading to smaller grain sizes. These characteristics are consistent with the grain growth mechanism.
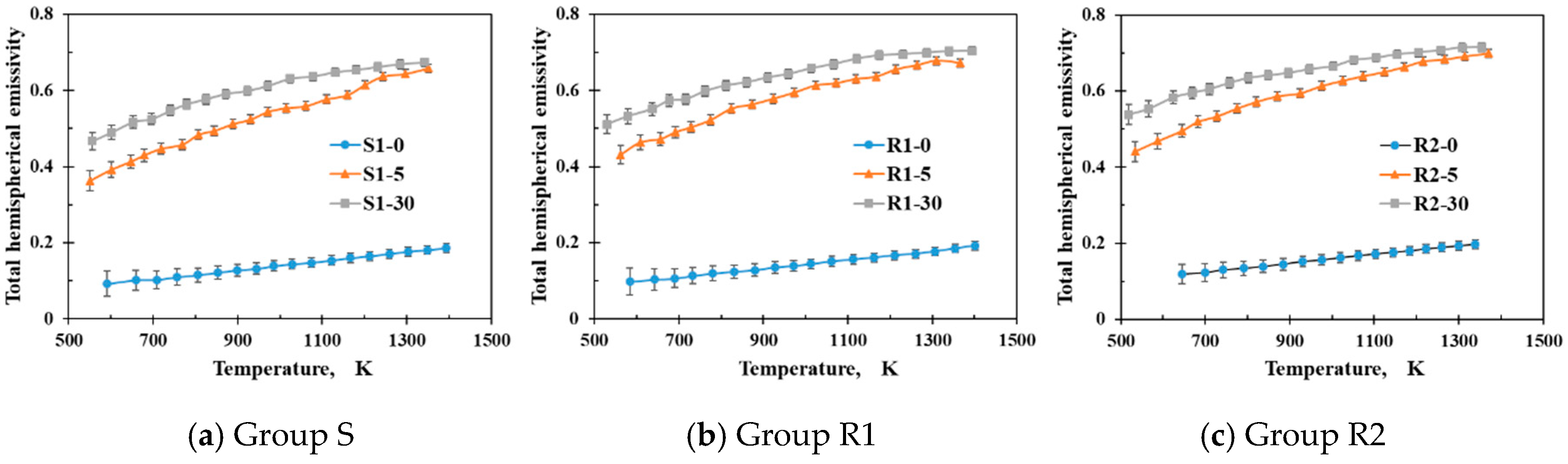
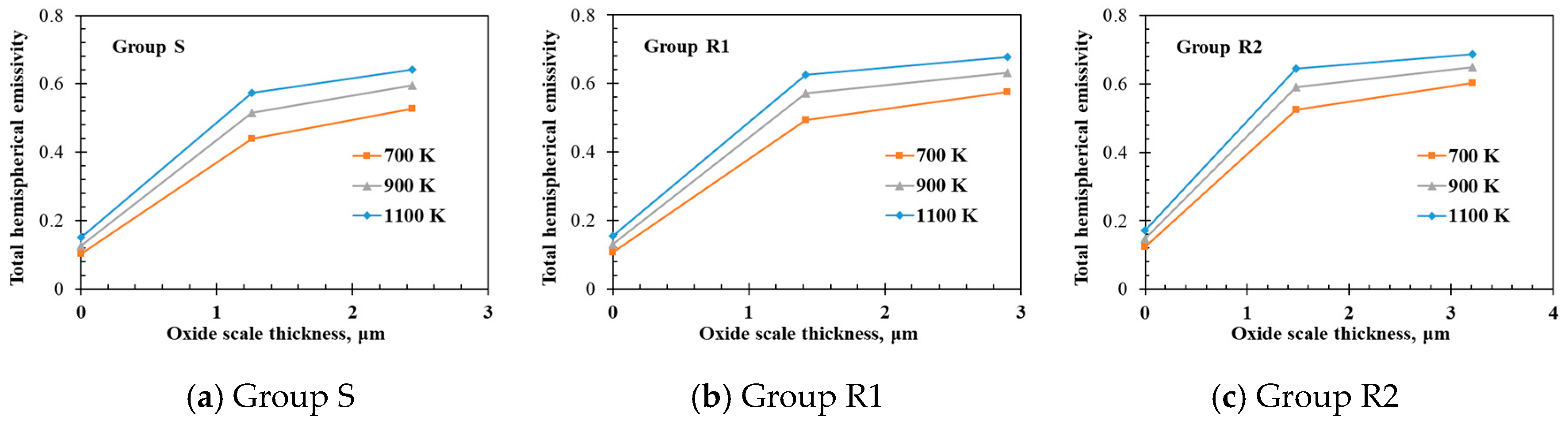
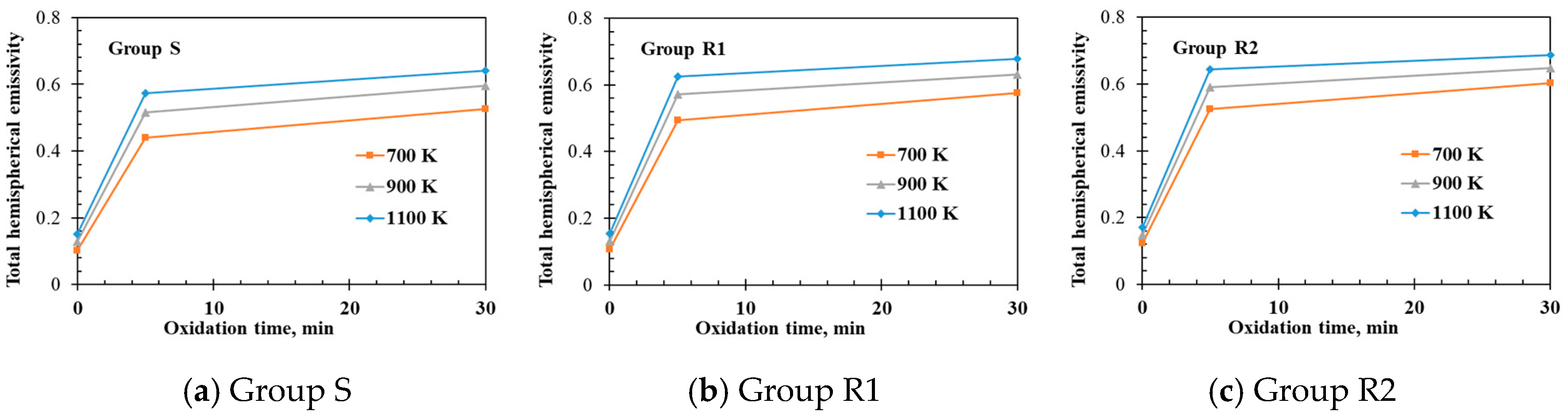
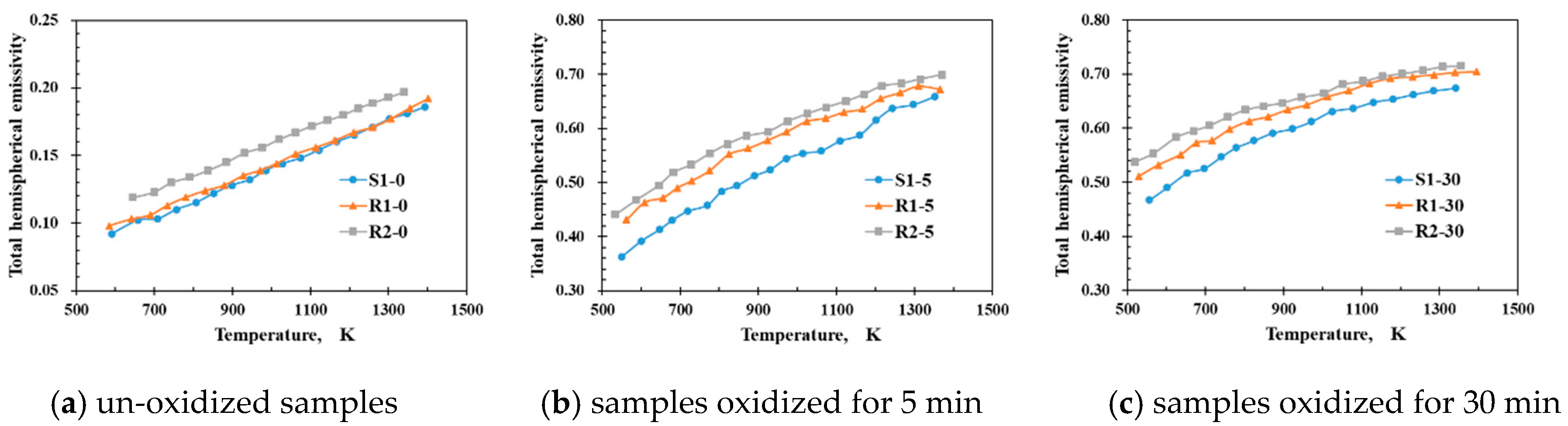
- The measured total hemispherical emissivities increased with the sample temperature (500–1400 K) and the oxide layer thickness. The larger surface roughness enhanced the emissivities for the un-oxidized samples. For the oxidized samples, the oxide layers formed on the various sample substrates had different surface microstructures and thicknesses, which affected the total hemispherical emissivity.
- The surface micro-morphologies and the surface emissivities of samples R1 and R2 after oxidation were both similar. However, the surface of sample S after oxidation looked quite different from the surfaces of samples R1 and R2 with the emissivity of sample S after oxidation being lower that the emissivities of samples R1 and R2 because the oxides on samples R1 and R2 had larger grain sizes due to the initially rougher surfaces.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High-Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006; Volume 2013. [Google Scholar]
- Geng, S.; Wang, F.; Zhang, S. High temperature oxidation behavior of a sputtered pure Ni nanocrystalline coating at 700–900 °C. Surf. Coat. Technol. 2003, 167, 212–216. [Google Scholar] [CrossRef]
- Haugsrud, R. On the effects of surface coatings on the high-temperature oxidation of nickel. Corros. Sci. 2003, 45, 1289–1311. [Google Scholar] [CrossRef]
- Haugsrud, R. On the high-temperature oxidation of nickel. Corros. Sci. 2003, 45, 211–235. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, Y.B.; Ma, Y.H.; Lou, L.H.; Hu, Z.Q. Effect of Microstructure on the Oxidation Behavior of Two Nickel-Based Superalloys. Corrosion 2005, 61, 961–967. [Google Scholar] [CrossRef]
- Zhou, C.H.; Ma, H.T.; Wang, L. Comparative study of oxidation kinetics for pure nickel oxidized under tensile and compressive stress. Corros. Sci. 2010, 52, 210–215. [Google Scholar] [CrossRef]
- Honvault, C.; Peres, V.; Cassayre, L. Oxidation kinetics of a Ni–Cu based cermet at high temperature. Corros. Sci. 2013, 68, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ai, S.; Fang, D. Effect of Oxidation-Induced Material Parameter Variation on the High Temperature Oxidation Behavior of Nickel. Acta Mech. Solida Sin. 2016, 29, 337–344. [Google Scholar] [CrossRef]
- Teodorescu, G.; Jones, P.D.; Overfelt, R.A.; Guo, B.J. High temperature spectral-directional emittance of high purity nickel oxidized in air. J. Mater. Sci. 2006, 41, 7240–7246. [Google Scholar] [CrossRef]
- Fu, T.R.; Tan, P.; Pang, C.H. A steady-state measurement system for total hemispherical emissivity. Meas. Sci. Technol. 2012, 23, 025006. [Google Scholar] [CrossRef]
- Fu, T.R.; Tan, P. Transient calorimetric measurement method for total hemispherical emissivity. Trans. ASME J. Heat Transf. 2012, 134, 111601. [Google Scholar] [CrossRef]
- Fu, T.R.; Tan, P.; Zhong, M.H. Experimental research on the influence of surface conditions on the total hemispherical emissivity. Exp. Therm. Fluid Sci. 2012, 40, 159–167. [Google Scholar] [CrossRef]
- Fu, T.R.; Tan, P.; Ren, J.; Wang, H.S. Total hemispherical radiation properties of oxidized nickel at high temperatures. Corros. Sci. 2014, 83, 272–280. [Google Scholar] [CrossRef]
- Fukumoto, S.; Takahara, J.; Takahara, J.; Kobayashi, T. Narrow-band thermal radiation by resonant modes inside tungsten microcavities. In Proceedings of the International Quantum Electronics Conference (IQEC), San Francisco, CA, USA, 21 May 2004; pp. 427–429. [Google Scholar]
- Huang, J.G.; Xuan, Y.M.; Li, Q. Investigation on emissive properties of peroskite-type oxide LSMO with grating surface. Sci. China Technol. Sci. 2011, 54, 220–225. [Google Scholar] [CrossRef]
- Yin, H.X.; Zhu, C.R.; Shen, Y.; Yang, H.F.; Liu, Z.; Gu, C.Z.; Liu, B.L.; Xu, X.G. Fabrication of large-scale spherical-cap structure and the application on n-GaN based light-emitting diodes. J. Vacuum Sci. Technol. B Microelectron. Nanometer Struct. 2014, 32, 031208. [Google Scholar] [CrossRef]
- Lin, C.; Povinelli, M.L. Optical absorption enhancement in silicon nanowire arrays with a large lattice constant for photovoltaic applications. Opt. Express 2009, 17, 19371–19381. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Huang, Y.F.; Jen, Y.J.; Ganguly, A.; Chen, K.H.; Chen, L.C. Anti-reflecting and photonic nanostructures. Mater. Sci. Eng. R Rep. 2010, 69, 1–35. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Yang, B. Antireflective surfaces based on biomimetic nanopillared arrays. Nano Today 2010, 5, 117–127. [Google Scholar] [CrossRef]
- Lamikiz, A.; de Lacalle, L.N.L.; Sanchez, J.A.; Bravo, U. Calculation of the specific cutting coefficients and geometrical aspects in sculptured surface machining. Mach. Sci. Technol. 2005, 9, 411–436. [Google Scholar]
- Arizmendi, M.; Campa, F.J.; Fernández, J.; de Lacalle, L.N.L.; Gil, A.; Bilbao, E.; Veiga, F.; Lamikiz, A. Model for surface topography prediction in peripheral milling considering tool vibration. CIRP Ann. Manuf. Technol. 2009, 58, 93–96. [Google Scholar] [CrossRef]
- De Lacalle, L.N.L.; Rodríguez, A.; Lamikiz, A.; Celaya, A.; Alberdi, R. Five-Axis Machining and Burnishing of Complex Parts for the Improvement of Surface Roughness. Mater. Manuf. Process. 2011, 26, 997–1003. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Shores, D.A. Cracking and Spalling of Oxide Scale from 304 Stainless-Steel at High-Temperatures. J. Electrochem. Soc. 1994, 141, 1255–1260. [Google Scholar] [CrossRef]
- Huntz, A.M.; Lefevre, B.; Cassino, F. Roughness and oxidation: Application to NiO growth on Ni at 800 °C. Mater. Sci. Eng. A 2000, 290, 190–197. [Google Scholar] [CrossRef]
- Haugsrud, R. High-temperature oxidation of Cu-10 wt% Ni and Cu-15 wt% Ni at 900–1050 °C. Corros. Sci. 2000, 42, 383–399. [Google Scholar] [CrossRef]
- Evans, J.L. Effect of Surface Roughness on the Oxidation Behavior of the Ni-Base Superalloy ME3. J. Mater. Eng. Perform. 2010, 19, 1001–1004. [Google Scholar] [CrossRef]
- Akhiani, H.; Szpunar, J.A. Effect of surface roughness on the texture and oxidation behavior of Zircaloy-4 cladding tube. Appl. Surf. Sci. 2013, 285, 832–839. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Chen, C.; Wan, K. High-temperature oxidation behavior of Ni-based superalloys with Nb and Y and the interface characteristics of oxidation scales. Surf. Interface Anal. 2014, 47, 362–370. [Google Scholar] [CrossRef]
- Li, W.; Lei, Z.; Shi, X.; Wang, S.; Zhang, Y.; Fan, D. Effect of microgroove surface on surface properties and cytocompatibility of silicone rubber. J. Third Milit. Med. Univ. 2015, 8, 736–740. [Google Scholar]
- Wang, L.; Jiang, W.G.; Li, X.W.; Dong, J.; Zheng, W.; Feng, H.; Lou, L. Effect of Surface Roughness on the Oxidation Behavior of a Directionally Solidified Ni-Based Superalloy at 1100 °C. Acta Metall. Sin. 2015, 28, 381–385. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Hou, P.Y.; Gesmundo, F.; Niu, F. Effect of surface roughness on the development of protective Al2O3 on Fe–10Al (at.%) alloys containing 0–10 at.% Cr. Appl. Surf. Sci. 2006, 253, 881–888. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, L.H.; Hsu, P.F. Oxide-Film Effect on Infrared Radiative Properties of Grating Structures of Aluminum. J. Thermophys. Heat Transf. 2011, 25, 80–86. [Google Scholar] [CrossRef]
- Peraldi, R.; Monceau, D.; Pieraggi, B. Correlations between growth kinetics and microstructure for scales formed by high-temperature oxidation of pure nickel. I. morphologies and microstructures. Oxid. Met. 2002, 58, 249–273. [Google Scholar] [CrossRef]


| Sample No. | Dimensions (mm × mm × mm) | Oxidation Time (min) | Measurement | |
|---|---|---|---|---|
| S | S0 | 70 × 10 × 0.2 | 0 | Surface roughness and morphology |
| Sox | 70 × 10 × 0.2 | 60 | Surface roughness and morphology | |
| Sm | 39 × 10 × 0.2 | 5–360 | Oxidation weight gain | |
| S-0 | 270 × 10 × 0.2 | 0 | SEM image and thermal surface radiation property measurements | |
| S-5 | 270 × 10 × 0.2 | 5 | ||
| S-30 | 270 × 10 × 0.2 | 30 | ||
| R1 | R10 | 70 × 10 × 0.2 | 0 | Surface roughness and morphology |
| R1ox | 70 × 10 × 0.2 | 60 | Surface roughness and morphology | |
| R1m | 44 × 10 × 0.2 | 5–360 | Oxidation weight gain | |
| R1-0 | 270 × 10 × 0.2 | 0 | SEM image and thermal surface radiation property measurements | |
| R1-5 | 270 × 10 × 0.2 | 5 | ||
| R1-30 | 270 × 10 × 0.2 | 30 | ||
| R2 | R20 | 70 × 10 × 0.2 | 0 | Surface roughness and morphology |
| R2ox | 70 × 10 × 0.2 | 60 | Surface roughness and morphology | |
| R2m | 39 × 10 × 0.2 | 5–360 | Oxidation weight gain | |
| R2-0 | 270 × 10 × 0.2 | 0 | SEM image and thermal surface radiation property measurements | |
| R2-5 | 270 × 10 × 0.2 | 5 | ||
| R2-30 | 270 × 10 × 0.2 | 30 | ||
| Sample No | Oxidation Time/min | Initial Surface Roughness/nm | Oxide Scale Thickness/μm | |
|---|---|---|---|---|
| S | S-0 | 0 | 14.5 | 0 |
| S-5 | 5 | 14.5 | 1.26 | |
| S-30 | 30 | 14.5 | 2.44 | |
| R1 | R1-0 | 0 | 98.9 | 0 |
| R1-5 | 5 | 98.9 | 1.42 | |
| R1-30 | 30 | 98.9 | 2.90 | |
| R2 | R2-0 | 0 | 605 | 0 |
| R2-5 | 5 | 605 | 1.48 | |
| R2-30 | 30 | 605 | 3.21 | |
| Group No. | S | R1 | R2 |
|---|---|---|---|
| Total hemispherical emissivity of un-oxidized substrates | 0.160 | 0.163 | 0.179 |
| Initial surface roughness/nm | 14.5 | 98.9 | 605 |
| Oxide scale thickness/μm | 2.0 | 2.0 | 2.0 |
| Total hemispherical emissivity of oxidized samples | 0.566 | 0.595 | 0.608 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Fu, T.; Shi, C. Correlations between High-Temperature Oxidation Kinetics and Thermal Radiation Characteristics of Micro-Structured Nickel Surfaces Oxidized at 1173 K. Metals 2019, 9, 17. https://doi.org/10.3390/met9010017
Li B, Fu T, Shi C. Correlations between High-Temperature Oxidation Kinetics and Thermal Radiation Characteristics of Micro-Structured Nickel Surfaces Oxidized at 1173 K. Metals. 2019; 9(1):17. https://doi.org/10.3390/met9010017
Chicago/Turabian StyleLi, Biying, Tairan Fu, and Congling Shi. 2019. "Correlations between High-Temperature Oxidation Kinetics and Thermal Radiation Characteristics of Micro-Structured Nickel Surfaces Oxidized at 1173 K" Metals 9, no. 1: 17. https://doi.org/10.3390/met9010017





