Dislocations Help Initiate the α–γ Phase Transformation in Iron—An Atomistic Study
Abstract
1. Introduction
2. Simulation Method
3. Results
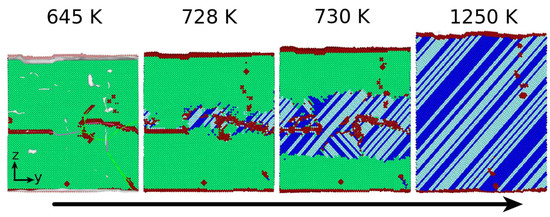
3.1. The Austenitic Transformation
3.2. The Martensitic Transformation
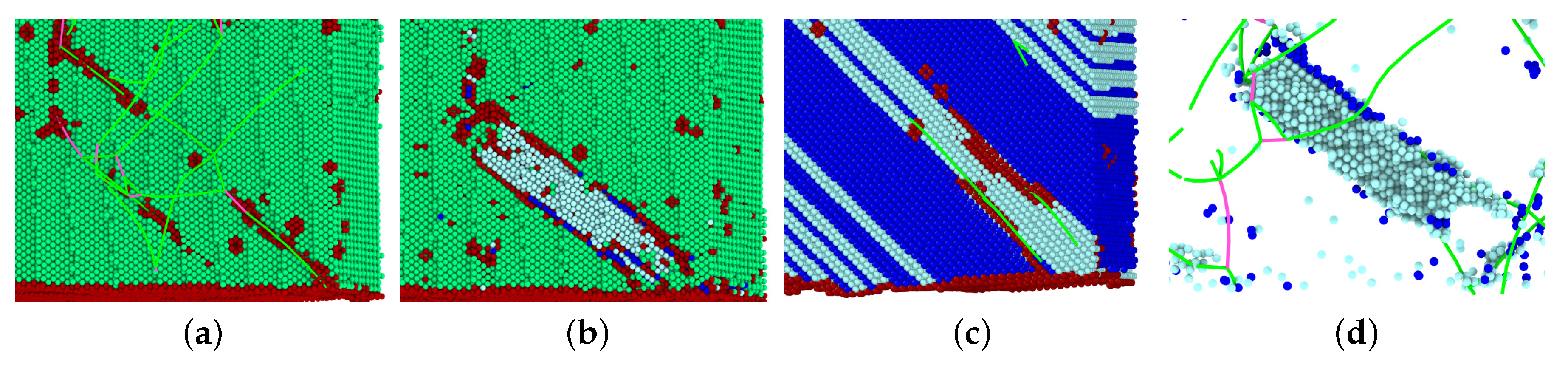
3.3. Dislocations in the Transformed Phase
4. Conclusions
- The presence of dislocations alleviates the transformation in the sense that the martensitic transformation temperature is increased and the austenitic transformation temperature is decreased. For the martensitic transformation, a dislocation-free crystal would not transform at all under the simulation conditions (system size and cooling rate); here the presence of dislocations is essential in inducing the transformation.
- For the martensitic transformation, a roughly linear dependence of the transformation temperature on the dislocation density was found. For the austenitic transformation, on the other hand, a saturation of the transformation temperature at dislocation densities above around cm was observed. These trends correlate well with the potential energy stored in the dislocations, which exhibits the same dependence on dislocation energy as the transition temperature.
- In all cases, the new phase nucleated at the dislocations. In the absence of dislocations, the new phase would nucleate at the surface (if at all). Nucleation at the dislocations is in agreement with previous MD findings for NiAl alloys [49], which show that the lattice distortion induced by the stress exerted by the dislocation configurations assists in the nucleation of the new phase, and also with other studies of defective pure Fe crystals that report phase nucleation in the vicinity of defects, such as grain boundaries [21] and phase boundaries [22]. However, Karewar et al. [23] found a more complex nucleation pattern in their study of the influence of planar defects on the martensitic transformation, depending on the resolved shear stresses in the available slip systems; depending on the configuration of the planar defects, these may increase or decrease the barrier for slip and hence for the coordinated atomic movement necessary for the martensitic transformation.
- The orientation relationships governing the transformation in the nuclei at the dislocations are governed by the Burgers path and the Kurdjumov–Sachs and Nishiyama–Wassermann paths; these pathways have also been identified to dominate the transformation behavior of pure iron in other simulational studies [50]. However, when, after growth and coalescence of the nuclei, the entire sample has transformed, a simple microstructure results. After the martensitic transformation, the bcc-phase is characterized by a homogeneous phase consisting of only few twinned grains separated by twin boundaries; the austenitic phase, on the other hand, is single-crystalline, containing planar defects such as stacking-fault planes and plates of hcp material. This simple microstructure is the consequence of the free surfaces of the thin film, which tend to form conserved planes under the transformation [39]. As a consequence, the final orientation relationships of the transformed sample are characterized by the Bain and the Pitsch pathway.
- While the new phase nucleates earlier when dislocations are present, the duration of the transformation is slowed down, as multiple nuclei compete in their growth.
- The transformed crystal contains abundant dislocations. The dislocation density becomes reduced in the case of the martensitic transformation but may even increase during the austenitic transformation. A detailed analysis demonstrates that the dislocations in the novel structure are ‘inherited’ from the original phase.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 2nd ed.; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Pepperhoff, W.; Acet, M. Constitution and Magnetism of Iron and Its Alloys; Springer: Berlin, Germany, 2001. [Google Scholar]
- Pereloma, E.; Edmonds, D.V. (Eds.) Phase Transformations in Steels; Vol. 2: Diffusionless Transformations, High Strength Steels, Modelling and Advanced Analytical Techniques; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar]
- Yang, Z.; Johnson, R.A. An EAM simulation of the α-γ iron interface. Model. Simul. Mater. Sci. Eng. 1993, 1, 707. [Google Scholar] [CrossRef]
- Bos, C.; Sietsma, J.; Thijsse, B.J. Molecular dynamics simulation of interface dynamics during the fcc-bcc transformation of a martensitic nature. Phys. Rev. B 2006, 73, 104117. [Google Scholar] [CrossRef]
- Urbassek, H.M.; Sandoval, L. Molecular dynamics modeling of martensitic transformations in steels. In Phase Transformations in Steels; Pereloma, E., Edmonds, D.V., Eds.; Vol. 2: Diffusionless Transformations, High Strength Steels, Modelling and Advanced Analytical Techniques; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 433–463. [Google Scholar]
- Biglari, M.; Mittemeijer, E.J. Energetics of nucleation at the austenite-ferrite interface: The effect of applied stress. Model. Simul. Mater. Sci. Eng. 2012, 20, 075010. [Google Scholar] [CrossRef]
- Wang, B.; Urbassek, H.M. Computer simulation of strain-induced phase transformations in thin Fe films. Model. Simul. Mater. Sci. Eng. 2013, 21, 085007. [Google Scholar] [CrossRef]
- Sak-Saracino, E.; Urbassek, H.M. Effect of uni- and biaxial strain on phase transformations in Fe thin films. Int. J. Comput. Mater. Sci. Eng. 2016, 5, 1650001. [Google Scholar] [CrossRef]
- Maresca, F.; Curtin, W.A. The austenite/lath martensite interface in steels: Structure, athermal motion, and in-situ transformation strain revealed by simulation and theory. Acta Mater. 2017, 134, 302–323. [Google Scholar] [CrossRef]
- Sandoval, L.; Urbassek, H.M.; Entel, P. Solid-solid phase transitions and phonon softening in an embedded-atom method model for iron. Phys. Rev. B 2009, 80, 214108. [Google Scholar] [CrossRef]
- Sandoval, L.; Urbassek, H.M. Transformation pathways in the solid-solid phase transitions of iron nanowires. Appl. Phys. Lett. 2009, 95, 191909. [Google Scholar] [CrossRef]
- Sandoval, L.; Urbassek, H.M.; Entel, P. The Bain versus Nishiyama-Wassermann path in the martensitic transformation of Fe. New J. Phys. 2009, 11, 103027. [Google Scholar] [CrossRef]
- Meyer, R.; Entel, P. Martensite-austenite transition and phonon dispersion curves of Fe1−xNix studied by molecular-dynamics simulations. Phys. Rev. B 1998, 57, 5140. [Google Scholar] [CrossRef]
- Wang, B.; Sak-Saracino, E.; Gunkelmann, N.; Urbassek, H.M. Molecular-dynamics study of the α ↔ γ phase transition in Fe-C. Comput. Mater. Sci. 2014, 82, 399–404. [Google Scholar] [CrossRef]
- Wang, B.; Sak-Saracino, E.; Sandoval, L.; Urbassek, H.M. Martensitic and austenitic phase transformations in Fe-C nanowires. Model. Simul. Mater. Sci. Eng. 2014, 22, 045003. [Google Scholar] [CrossRef]
- Sak-Saracino, E.; Urbassek, H.M. Temperature-induced phase transformation of Fe1−xNix alloys: Molecular-dynamics approach. Eur. Phys. J. B 2015, 88, 169. [Google Scholar] [CrossRef]
- Sandoval, L.; Urbassek, H.M. Finite-size effects in Fe-nanowire solid-solid phase transitions: A molecular dynamics approach. Nano Lett. 2009, 9, 2290–2294. [Google Scholar] [CrossRef]
- Sandoval, L.; Urbassek, H.M. Solid-solid phase transitions in Fe nanowires induced by axial strain. Nanotechnology 2009, 20, 325704. [Google Scholar] [CrossRef]
- Wang, B.; Urbassek, H.M. Phase transitions in an Fe system containing a bcc/fcc phase boundary: An atomistic study. Phys. Rev. B 2013, 87, 104108. [Google Scholar] [CrossRef]
- Meiser, J.; Urbassek, H.M. Martensitic transformation of pure iron at a grain boundary: Atomistic evidence for a two-step Kurdjumov-Sachs-Pitsch pathway. AIP Adv. 2016, 6, 085017. [Google Scholar] [CrossRef]
- Meiser, J.; Urbassek, H.M. Ferrite-to-Austenite and Austenite-to-Martensite Phase Transformations in the Vicinity of a Cementite Particle: A Molecular Dynamics Approach. Metals 2018, 8, 837. [Google Scholar] [CrossRef]
- Karewar, S.; Sietsma, J.; Santofimia, M.J. Effect of pre-existing defects in the parent fcc phase on atomistic mechanisms during the martensitic transformation in pure Fe: A molecular dynamics study. Acta Mater. 2018, 142, 71–81. [Google Scholar] [CrossRef]
- Cahn, J.W. Nucleation on dislocations. Acta Metall. 1957, 5, 169–172. [Google Scholar] [CrossRef]
- Dollins, C.C. Nucleation on dislocations. Acta Metall. 1970, 18, 1209–1215. [Google Scholar] [CrossRef]
- Malygin, G.A. Heterogeneous Nucleation of Martensite at Dislocations and the Martensitic-Transformation Kinetics in Shape Memory Alloys. Phys. Solid State 2003, 45, 345–351. [Google Scholar] [CrossRef]
- Sharma, H.; Sietsma, J.; Offerman, S.E. Preferential Nucleation during Polymorphic Transformations. Sci. Rep. 2016, 6, 30860. [Google Scholar] [CrossRef]
- Engin, C.; Sandoval, L.; Urbassek, H.M. Characterization of Fe potentials with respect to the stability of the bcc and fcc phase. Model. Simul. Mater. Sci. Eng. 2008, 16, 035005. [Google Scholar] [CrossRef]
- Ou, X. Molecular dynamics simulations of fcc-to-bcc transformation in pure iron: A review. Mater. Sci. Technol. 2017, 33, 822–835. [Google Scholar] [CrossRef]
- Hirel, P. Atomsk: A tool for manipulating and converting atomic data files. Comput. Phys. Commun. 2015, 197, 212–219. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Nose, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distribution. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Faken, D.; Jonsson, H. Systematic analysis of local atomic structure combined with 3D computer graphics. Comput. Mater. Sci. 1994, 2, 279–286. [Google Scholar] [CrossRef]
- Honeycutt, J.D.; Andersen, H.C. Molecular dynamics study of melting and freezing of small Lennard-Jones clusters. J. Phys. Chem. 1987, 91, 4950–4963. [Google Scholar] [CrossRef]
- Stukowski, A.; Albe, K. Extracting dislocations and non-dislocation crystal defects from atomistic simulation data. Model. Simul. Mater. Sci. Eng. 2010, 18, 085001. [Google Scholar] [CrossRef]
- Fujita, F.E. (Ed.) Structural Phase Transformation. In Physics of New Materials, 2nd ed.; Springer Series in Materials Science; Springer: Berlin, Germany, 1998; Volume 27, Chapter 6; p. 141. [Google Scholar]
- Meiser, J.; Urbassek, H.M. Influence of the Crystal Surface on the Austenitic and Martensitic Phase Transition in Pure Iron. Crystals 2018, 8, 469. [Google Scholar] [CrossRef]
- Freitas, R.; Asta, M.; de Koning, M. Nonequilibrium free-energy calculation of solids using LAMMPS. Comput. Mater. Sci. 2016, 112, 333–341. [Google Scholar] [CrossRef]
- Wang, B.; Urbassek, H.M. Role of the Surface in Solid–Solid Phase Transitions: Molecular Dynamics Study of the α-γ Transition in Fe. Metall. Mater. Trans. A 2016, 47, 2471–2480. [Google Scholar] [CrossRef]
- Burgers, W.G. On the process of transition of the cubic-body-centered modification into the hexagonal-close-packed modification of zirconium. Physica 1934, 1, 561–586. [Google Scholar] [CrossRef]
- Kurdjumov, G.V.; Sachs, G. Über den Mechanismus der Stahlhärtung. Z. Phys. 1930, 64, 325–343. [Google Scholar] [CrossRef]
- Bain, E.C. The nature of martensite. Trans. AIME 1924, 70, 25–47. [Google Scholar]
- Wang, B.; Urbassek, H.M. Atomistic dynamics of the bcc ↔ fcc phase transition in iron: Competition of homo- and heterogeneous phase growth. Comput. Mater. Sci. 2014, 81, 170–177. [Google Scholar] [CrossRef]
- Wassermann, G. Einfluß der α-γ-Umwandlung eines irreversiblen Nickelstahls auf Kristallorientierung und Zugfestigkeit. Arch. Eisenhüttenwes. 1933, 6, 347–351. [Google Scholar] [CrossRef]
- Nishiyama, Z. Mechanism of transformation from face-centred to body-centred cubic lattice. Sci. Rep. Tohoku Imp. Univ. 1934, 23, 637. [Google Scholar]
- Pitsch, W. The martensite transformation in thin foils of iron-nitrogen alloys. Philos. Mag. 1959, 4, 577–584. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.M.; Clapp, P.C.; Rifkin, J.A. Molecular dynamics simulations of the effects of defects on martensite nucleation. J. Appl. Phys. 2004, 95, 1698–1705. [Google Scholar] [CrossRef]
- Song, H.; Hoyt, J.J. An atomistic simulation study of the crystallographic orientation relationships during the austenite to ferrite transformation in pure Fe. Model. Simul. Mater. Sci. Eng. 2015, 23, 085012. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meiser, J.; Urbassek, H.M. Dislocations Help Initiate the α–γ Phase Transformation in Iron—An Atomistic Study. Metals 2019, 9, 90. https://doi.org/10.3390/met9010090
Meiser J, Urbassek HM. Dislocations Help Initiate the α–γ Phase Transformation in Iron—An Atomistic Study. Metals. 2019; 9(1):90. https://doi.org/10.3390/met9010090
Chicago/Turabian StyleMeiser, Jerome, and Herbert M. Urbassek. 2019. "Dislocations Help Initiate the α–γ Phase Transformation in Iron—An Atomistic Study" Metals 9, no. 1: 90. https://doi.org/10.3390/met9010090
APA StyleMeiser, J., & Urbassek, H. M. (2019). Dislocations Help Initiate the α–γ Phase Transformation in Iron—An Atomistic Study. Metals, 9(1), 90. https://doi.org/10.3390/met9010090