Effects of Alloying Elements on the Stacking Fault Energies of Ni58Cr32Fe10 Alloys: A First-Principle Study
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussions
3.1. The Elastic Properties and SFEs of Ni and Ni58Cr32Fe10
3.2. SFEs at 0 K
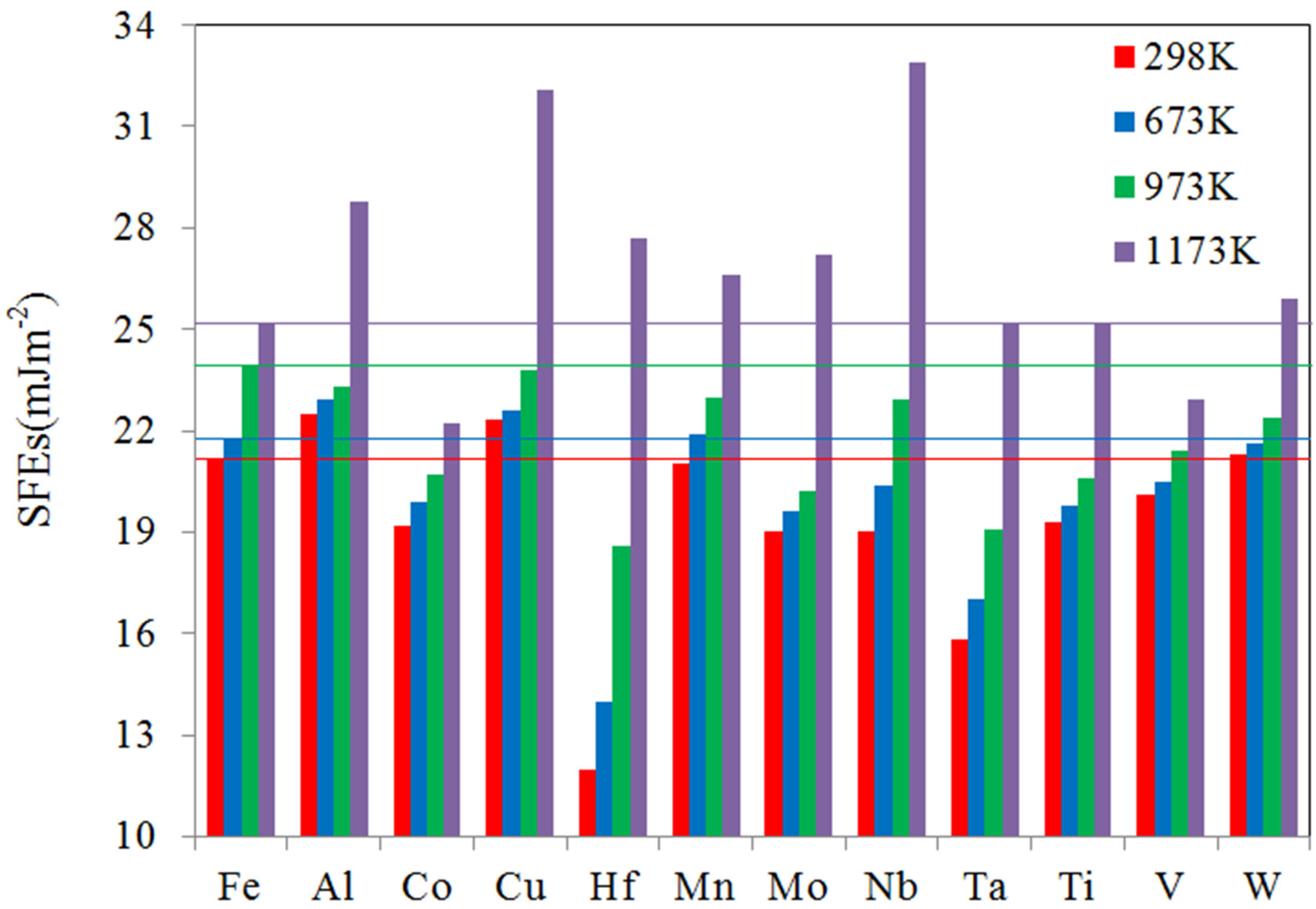
3.3. SFEs at Finite Temperature
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Li, D.Z.; Li, Y.Y.; Lu, S.P. Effect of Nb and Mo on the Microstructure, Mechanical Properties and Ductility–Dip Cracking of Ni–Cr–Fe Weld Metals. Acta Metall. Sin. 2016, 29, 928–939. [Google Scholar] [CrossRef]
- Young, G.A. The Mechanism of Ductility Dip Cracking in Nickel–Chromium Alloys. Weld. J. 2008, 87, 31S–43S. [Google Scholar]
- Nissley, N.E.; Lippold, J.C. Ductility–Dip Cracking Susceptibility of Nickel–Based Weld Metals: Part 2: Microstructural Characterization. Weld. J. 2009, 88, 131s–140s. [Google Scholar]
- Wei, X.; Xu, M.; Chen, J.; Yu, C.; Chen, J.; Lu, H.; Xu, J. Fractal Analysis of Mo and Nb Effects on Grain Boundary Character and Hot Cracking Behavior for Ni–Cr–Fe Alloys. Mater. Charact. 2018, 145, 65–76. [Google Scholar] [CrossRef]
- Nissley, N.E.; Lippold, J.C. Ductility–Dip Cracking Susceptibility of Nickel–Based Weld Metals Part 1: Strain–to–Fracture Testing. Weld. J. 2008, 87, 257s–264s. [Google Scholar]
- Ahn, H.I.; Jeong, S.H.; Cho, H.H.; Lee, H.W. Ductility–Dip Cracking Susceptibility of Inconel 690 Using Nb Content. J. Alloys Compd. 2019, 783, 263–271. [Google Scholar] [CrossRef]
- Guo, S.; Li, D.; Pen, H.; Guo, Q.; Hu, J. Hot Deformation and Processing Maps of Inconel 690 Superalloy. J. Nucl. Mater. 2011, 410, 52–58. [Google Scholar] [CrossRef]
- Blaizot, J.; Chaise, T.; Nélias, D.; Perez, M.; Cazottes, S.; Chaudet, P. Constitutive Model for Nickel Alloy 690 (Inconel 690) at Various Strain Rates and Temperatures. Int. J. Plast. 2016, 80, 139–153. [Google Scholar] [CrossRef]
- Collins, M.G.; Ramirez, A.; Lippold, J.C. An Investigation of Ductility Dip Cracking in Nickel–Based Weld Metals—Part II. Weld. J. 2003, 82, 348S–354S. [Google Scholar]
- Collins, M.G.; Lippold, J.C. An Investigation of Ductility Dip Cracking in Nickel–Based Filler Materials—Part I. Weld. J. 2003, 82, 288S–295S. [Google Scholar]
- Unfried, J.S.; Torres, E.A.; Ramirez, A.J. In Situ Observations of Ductility–Dip Cracking Mechanism in Ni–Cr–Fe Alloys. In Hot Cracking Phenomena in Welds III; Böllinghaus, T., Lippold, J., Cross, C.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 295–315. [Google Scholar]
- Unfried Silgado, J.; Ramirez, A.J. Modeling and Characterization of as–Welded Microstructure of Solid Solution Strengthened Ni–Cr–Fe Alloys Resistant to Ductility–Dip Cracking Part II: Microstructure Characterization. Met. Mater. Int. 2014, 20, 307–315. [Google Scholar] [CrossRef]
- Unfried–Silgado, J.; Wu, L.; Ferreira, F.F.; Garzón, C.M.; Ramírez, A.J. Stacking Fault Energy Measurements in Solid Solution Strengthened Ni–Cr–Fe Alloys Using Synchrotron Radiation. Mater. Sci. Eng. A 2012, 558, 70–75. [Google Scholar] [CrossRef]
- Tian, C.; Han, G.; Cui, C.; Sun, X. Effects of Stacking Fault Energy on the Creep Behaviors of Ni–Base Superalloy. Mater. Des. 2014, 64, 316–323. [Google Scholar] [CrossRef]
- Andric, P.; Yin, B.; Curtin, W.A. Stress–Dependence of Generalized Stacking Fault Energies. J. Mech. Phys. Solids 2019, 122, 262–279. [Google Scholar] [CrossRef]
- Shang, S.L.; Zacherl, C.L.; Fang, H.Z.; Wang, Y.; Du, Y.; Liu, Z.K. Effects of Alloying Element and Temperature on the Stacking Fault Energies of Dilute Ni–Base Superalloys. J. Phys. Condens. Matter 2012, 24, 505403. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Wang, Y.; Du, Y.; Tschopp, M.A.; Liu, Z.K. Integrating Computational Modeling and First–Principles Calculations to Predict Stacking Fault Energy of Dilute Multicomponent Ni–Base Alloys. Comput. Mater. Sci. 2014, 91, 50–55. [Google Scholar] [CrossRef]
- Kumar, K.; Sankarasubramanian, R.; Waghmare, U.V. Influence of Dilute Solute Substitutions in Ni on Its Generalized Stacking Fault Energies and Ductility. Comput. Mater. Sci. 2018, 150, 424–431. [Google Scholar] [CrossRef]
- Lu, J.; Hultman, L.; Holmström, E.; Antonsson, K.H.; Grehk, M.; Li, W.; Vitos, L.; Golpayegani, A. Stacking Fault Energies in Austenitic Stainless Steels. Acta Mater. 2016, 111, 39–46. [Google Scholar] [CrossRef]
- Lu, S.; Hu, Q.M.; Johansson, B.; Vitos, L. Stacking Fault Energies of Mn, Co and Nb Alloyed Austenitic Stainless Steels. Acta Mater. 2011, 59, 5728–5734. [Google Scholar] [CrossRef]
- Vitos, L.; Nilsson, J.O.; Johansson, B. Alloying Effects on the Stacking Fault Energy in Austenitic Stainless Steels from First–Principles Theory. Acta Mater. 2006, 54, 3821–3826. [Google Scholar] [CrossRef]
- Selke, W. The Annni Model—Theoretical Analysis and Experimental Application. Phys. Rep. 1988, 170, 213–264. [Google Scholar] [CrossRef]
- Lu, S.; Hu, M.Q.; Delczeg–Czirjak, E.K.; Johansson, B.; Vitos, L. Determining the minimum grain size in severe plastic deformation process via first-principles calculations. Acta Mater. 2012, 60, 4506–4513. [Google Scholar] [CrossRef]
- Li, W.; Lu, S.; Hu, Q.; Johansson, B.; Kwon, S.K.; Grehk, M.; Johnsson, J.Y.; Vitos, L. Generalized Stacking Fault Energy of Γ–Fe. Philos. Mag. 2016, 96, 524–541. [Google Scholar] [CrossRef]
- Grimvall, G. Spin Disorder in Paramagnetic Fcc Iron. Phys. Rev. B 1989, 39, 12300–12301. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Khmelevskyi, S.; Mohn, P.; Johansson, B. Temperature–Induced Longitudinal Spin Fluctuations in Fe and Ni. Phys. Rev. B 2007, 75, 054402. [Google Scholar] [CrossRef]
- Kirubaharan, A.M.; Kuppusami, P.; Chakravarty, S.; Ramachandran, D.; Singh, A. Thermal Expansion and Residual Stress Behaviour of Electron Beam Evaporated Yttria Stabilized Zirconia Films on Inconel–690 Substrates. J. Alloys Compd. 2017, 722, 585–592. [Google Scholar] [CrossRef]
- Vitos, L. Computational Quantum Mechanics for Materials Engineers the EMTO Method and Applications; Springer: London, UK, 2007. [Google Scholar]
- Vitos, L. Total–Energy Method Based on the Exact Muffin–Tin Orbitals Theory. Phys. Rev. B 2001, 64, 014107. [Google Scholar] [CrossRef]
- Soven, P. Coherent–Potential Model of Substitutional Disordered Alloys. Phys. Rev. 1967, 156, 809–813. [Google Scholar] [CrossRef]
- Vitos, L.; Abrikosov, I.A.; Johansson, B. Anisotropic Lattice Distortions in Random Alloys from First–Principles Theory. Phys. Rev. Lett. 2001, 87, 156401. [Google Scholar] [CrossRef]
- Gyorffy, B.L.; Pindor, A.J.; Staunton, J.; Stocks, G.M.; Winter, H. A First–Principles Theory of Ferromagnetic Phase Transitions in Metals. J. Phys. F Met. Phys. 1985, 15, 1337. [Google Scholar] [CrossRef]
- Ceperley, D.M.; Alder, B.J. Ground State of the Electron Gas by a Stochastic Method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Lemire, R.J.; McRae, G.A. The corrosion of Alloy 690 in high-temperature aqueous media—Thermodynamic considerations. J. Nucl. Mater. 2001, 294, 141–147. [Google Scholar] [CrossRef]
- Vitos, L.; Kollár, J.; Skriver, H.L. Full charge-density scheme with a kinetic-energy correction: Application to ground-state properties of the 4d metals. Phys. Rev. B 1997, 55, 13521–13527. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Dou, Y.; Luo, H.; Zhang, J. Elastic Properties of Fecr20ni8xn (X = Mo, Nb, Ta, Ti, V, W and Zr) Austenitic Stainless Steels: A First Principles Study. Metals 2019, 9, 145. [Google Scholar] [CrossRef]
- Shang, S.L.; Kim, D.E.; Zacherl, C.L.; Wang, Y.; Du, Y.; Liu, Z.K. Effects of Alloying Elements and Temperature on the Elastic Properties of Dilute Ni–Base Superalloys from First–Principles Calculations. J. Appl. Phys. 2012, 112, 053515. [Google Scholar] [CrossRef]
- Simmons, G.; Wang, H. Single Crystal Elastic Constants and Calculated Aggregate Properties; Mit: Cambridge, MA, USA, 1971. [Google Scholar]
- Carter, C.B.; Holmes, S.M. The stacking-fault energy of nickel. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1977, 35, 1161–1172. [Google Scholar] [CrossRef]


| Alloy | V | B | G | SFE |
|---|---|---|---|---|
| Ni | 10.97 | 198.4 | 102.7 | 140.9 |
| 10.982 a | 196.5 a | 103.2 a | 131.5 b | |
| 10.95 c | 187.6 c | 101.1 c | 120–130 d | |
| Ni58Cr32Fe10 | 11.59 | 180.1 | 82.1 | 20.5 21.2 e |
| Alloy 690 | 79.3 f | |||
| Ni59.3Cr30.7Fe10 | 11.37 f | 61.0 g |
| Alloy | V | SFEs0 | SFEsmag (298 K) | SFEsmag (673 K) | SFEsmag (973 K) | SFEsmag (1173 K) |
|---|---|---|---|---|---|---|
| Ni58Cr32Fe10 | 11.60 | 20.5 | 0.7 | 1.3 | 3.4 | 4.7 |
| Ni56Cr32Fe10Al2 | 11.66 | 21.9 | 0.6 | 1.0 | 1.4 | 6.9 |
| Ni56Cr32Fe10Co2 | 11.59 | 18.6 | 0.6 | 1.3 | 2.1 | 3.6 |
| Ni56Cr32Fe10Cu2 | 11.63 | 21.8 | 0.5 | 0.8 | 2.0 | 10.3 |
| Ni56Cr32Fe10Hf2 | 11.93 | 11.5 | 0.5 | 2.5 | 7.1 | 16.2 |
| Ni56Cr32Fe10Mn2 | 11.63 | 20.4 | 0.6 | 1.5 | 2.6 | 6.2 |
| Ni56Cr32Fe10Mo2 | 11.76 | 18.5 | 0.5 | 1.1 | 1.7 | 8.7 |
| Ni56Cr32Fe10Nb2 | 11.83 | 18.5 | 0.5 | 1.9 | 4.4 | 14.4 |
| Ni56Cr32Fe10Ta2 | 11.80 | 15.3 | 0.5 | 1.7 | 3.8 | 9.9 |
| Ni56Cr32Fe10Ti2 | 11.79 | 18.9 | 0.4 | 0.9 | 1.7 | 6.3 |
| Ni56Cr32Fe10V2 | 11.66 | 19.6 | 0.5 | 0.9 | 1.8 | 3.3 |
| Ni56Cr32Fe10W2 | 11.74 | 20.7 | 0.6 | 0.9 | 1.7 | 5.2 |
| Alloy | μfcc | μhcp | μdhcp | SFEmag |
|---|---|---|---|---|
| Ni58Cr32Fe10 | 2.04 | 1.94 | 2.01 | 0.7 |
| Fe70.5Cr17.5Ni12 a | 1.62 | 0.00 | – | 36.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Luo, H.; Jiang, Y.; Tang, X. Effects of Alloying Elements on the Stacking Fault Energies of Ni58Cr32Fe10 Alloys: A First-Principle Study. Metals 2019, 9, 1163. https://doi.org/10.3390/met9111163
Dou Y, Luo H, Jiang Y, Tang X. Effects of Alloying Elements on the Stacking Fault Energies of Ni58Cr32Fe10 Alloys: A First-Principle Study. Metals. 2019; 9(11):1163. https://doi.org/10.3390/met9111163
Chicago/Turabian StyleDou, Yuchen, Hong Luo, Yong Jiang, and Xiaohua Tang. 2019. "Effects of Alloying Elements on the Stacking Fault Energies of Ni58Cr32Fe10 Alloys: A First-Principle Study" Metals 9, no. 11: 1163. https://doi.org/10.3390/met9111163





