Effect of Milling Conditions on the Microstructural Design in Aluminum Based Alloy Fabricated by SPS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compositions Processing
2.3. Spark Plasma Sintering (SPS)
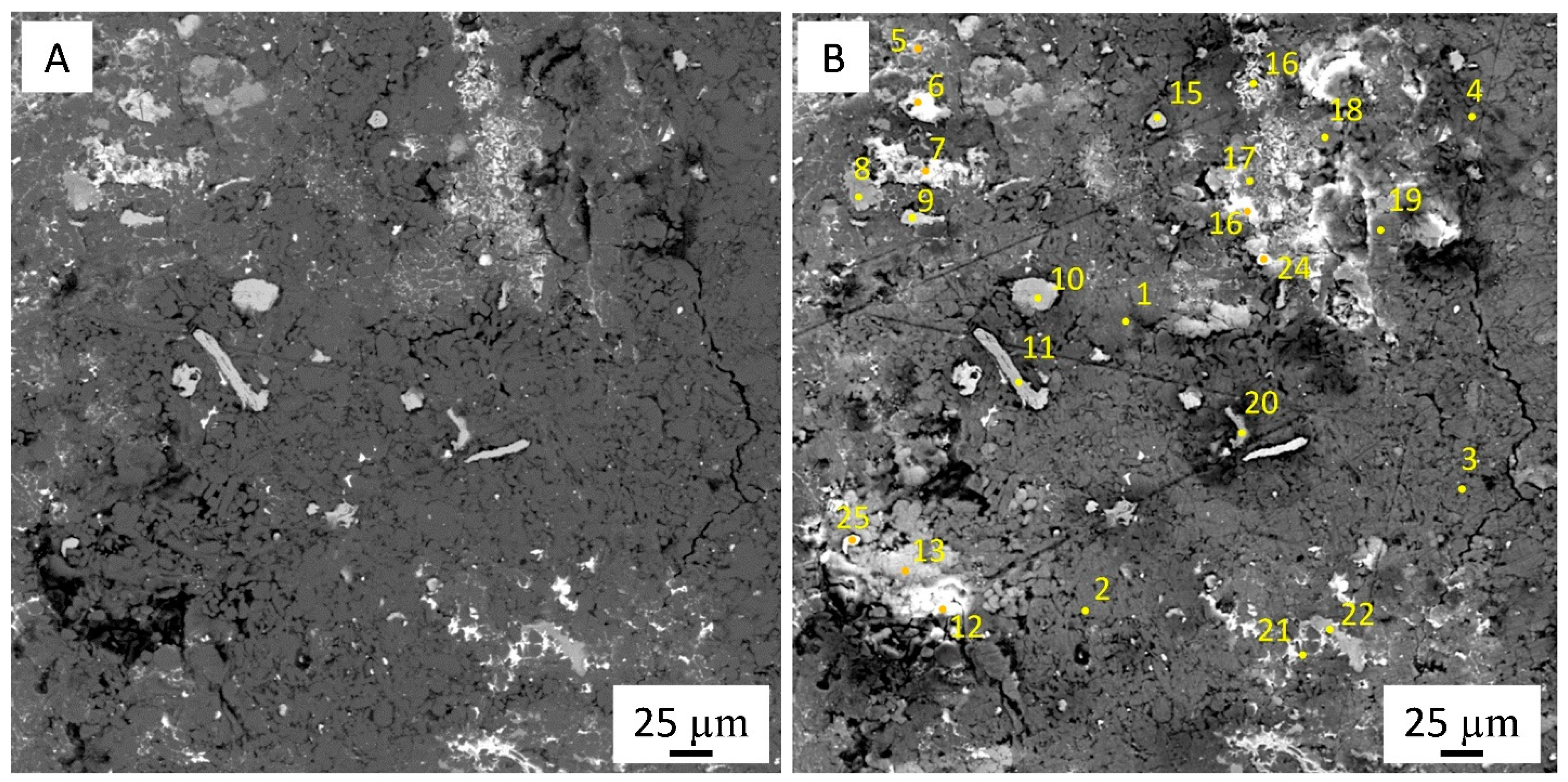
2.4. Microstructural Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pathak, J.P.; Mohan, S. Tribological behavior of conventional Al–Sn and equivalent Al–Pb alloys under lubrication. Bull. Mater. Sci. 2003, 26, 315–320. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, M.Q.; Ma, Y.; Zhu, M. Promoting the high load-carrying capability of Al-20 wt.% Sn bearing alloys through creating nanocomposite structure by mechanical alloying. Wear 2012, 294–295, 387–394. [Google Scholar] [CrossRef]
- Sercombe, T.B. Non-Conventional Sintered aluminum Powder Alloys. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 1998. [Google Scholar]
- Sangghaleh, A.; Halali, M. Effect of magnesium addition on the wetting of alumina by aluminum. Appl. Surf. Sci. 2009, 255, 8202–8206. [Google Scholar] [CrossRef]
- Riahi, A.R.; Edrisy, A.; Alpas, A.T. Effect of magnesium content on the high temperature adhesion of Al–Mg alloys to steel surfaces. Surf. Coat. Technol. 2009, 203, 2030–2035. [Google Scholar] [CrossRef]
- Marrocco, T.; Driver, L.C.; Harris, S.J.; McCartney, D.G. Microstructure and properties of thermally sprayed Al-Sn-based alloys for plain bearing applications. J. Therm. Spray Technol. 2006, 15, 634–639. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Doernberg, E.; Patel, J.B.; Fan, Z.; Schmid-Fetzer, R. Solidification of Al-Sn-Cu immiscible alloys under intense shearing. Metall. Mater. Trans. A 2009, 40, 2202–2211. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Das, A.; Doernberg, E.; Schmid-Fetzer, R. A comparative study of ternary Al-Sn-Cu immiscible alloys prepared by conventional casting and casting under high-intensity ultrasonic irradiation. Mater. Chem. Phys. 2011, 131, 241–249. [Google Scholar] [CrossRef]
- Pola, A.; Montesano, L.; Gelfi, M.; Roberti, R. Semisolid processing of Al-Sn-Cu alloys for bearing applications. Sol. St. Phen. 2013, 192–193, 562–568. [Google Scholar] [CrossRef]
- Zhai, W.; Hu, L.; Geng, D.L.; Wei, B. Thermodynamic properties and microstructure evolution of ternary Al–10% Cu–x % Sn immiscible alloys. J. Alloy. Compd. 2015, 627, 402–409. [Google Scholar] [CrossRef]
- Szymczak, T.; Gumienny, G.; Pacyniak, T. Effect of Vanadium and Molybdenum on the Crystallization, Microstructure and Properties of Hypoeutectic Silumin. Arch. Foundry Eng. 2015, 15, 81–86. [Google Scholar] [CrossRef]
- Pisarek, B.P. The crystallization of the aluminum bronze with additions of Si, Cr, Mo and/or W. Arch. Mat. Sci. Eng. 2007, 28, 461–466. [Google Scholar]
- Kuznetsova, E.; Gershman, I.; Mironov, A.; Podrabinnik, P.; Peretyagin, P. The effect of elements of secondary structures on the wear resistance of steel in friction against experimental aluminum alloys for monometallic journal bearings. Lubricants 2019, 7, 21. [Google Scholar] [CrossRef]
- Smirnov, A.; Peretyagin, P.; Solís, N.; Gershman, I.; Bartolomé, J.F. Wear behavior of graphene-reinforced alumina–silicon carbide whisker nanocomposite. Nanomaterials 2019, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, S.; Volosova, M.; Peretyagin, P.; Seleznev, A.; Okunkova, A.; Smirnov, A. The effect of TiC additive on mechanical and electrical properties of Al2O3 ceramic. Appl. Sci. 2018, 8, 2385. [Google Scholar] [CrossRef]
- Smirnov, A.; Seleznev, A.; Solís, N.; Pristinskiy, Y.; Peretyagin, P.; Bartolomé, J.F. The influence of wire electrical discharge machining cutting parameters on the surface roughness and flexural strength of ZrO2/TiN ceramic nanocomposites obtained by spark plasma sintering. Nanomaterials 2019, 9, 1391. [Google Scholar] [CrossRef]
- Smirnov, A.; Beltrán, J.I.; Rodriguez-Suarez, T.; Pecharromán, C.; Muñoz, M.C.; Moya, J.S.; Bartolomé, J.F. Unprecedented simultaneous enhancement in flaw tolerance and fatigue resistance of zirconia–Ta composites. Sci. Rep. 2017, 7, 44922. [Google Scholar] [CrossRef]
- Smirnov, A.; Peretyagin, P.; Bartolomé, J.F. Processing and mechanical properties of new hierarchical metal-graphene flakes reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 3491–3497. [Google Scholar] [CrossRef]
- Podrabinnik, P.A.; Gershman, I.S.; Mironov, A.E.; Kuznetsova, E.V.; Peretyagin, P.Y. Mechanisms involved in the formation of secondary structures on the friction surface of experimental aluminum alloys for monometallic journal bearings. Lubricants 2018, 6, 104. [Google Scholar] [CrossRef]
- Mironov, A.E.; Gershman, I.S.; Gershman, E.I.; Zheleznov, M.M. Relationship between the tribological properties of experimental aluminum alloys and their chemical composition. J. Frict. Wear 2017, 38, 87–91. [Google Scholar] [CrossRef]
- Mironov, A.E.; Gershman, I.S.; Gershman, E.I. Influence of tin on the tribotechnical properties of complex antifriction aluminum alloys. J. Frict. Wear 2018, 39, 394–399. [Google Scholar] [CrossRef]
- Podrabinnik, P.A.; Mironov, A.E.; Gershman, I.S. The influence of secondary structures on wear resistance of experimental aluminum alloys for monometallic slide bearings. Mat. Today Proceed. 2019, 11, 175–180. [Google Scholar] [CrossRef]
- Mironov, A.E.; Gershman, I.S.; Ovechkin, A.V.; Gershman, E.I. Comparison of scoring resistance of new antifriction aluminum alloys and tradition al antifriction bronze. J. Frict. Wear 2015, 36, 257–261. [Google Scholar] [CrossRef]





| Chemical Composition, wt. % | |||||||
|---|---|---|---|---|---|---|---|
| Sn | Pb | Cu | Zn | Mg | Si | Mo | Al |
| 6 | 2 | 4.3 | 1.9 | 1.7 | 0.45 | 1 | Bal. |
| Simple Mixture 1 (S1) | Simple Mixture 2 (S2) | Simple Mixture 3 (S3) | Compound Mixture | Compound Mixture Number |
|---|---|---|---|---|
| all elemental powders were milled for 24 h | M1 | |||
| Cu+Zn/24 h | Sn+Mg+Pb/24 h | Al+Si+Mo/24 h | S1+S2+S3/24 h | M2 |
| — | Sn+Mg+Pb/48 h | Al+Si+Mo/24 h | S2+S3+Cu+Zn/12 h | M3 |
| No. | Contents of Elements by Weight (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Al | Si | Cu | Zn | Mo | Sn | Pb | |
| 1 | 3.66 | 5.28 | 0.12 | 89.13 | 0.52 | 0.21 | 0.54 | 0 | 0 | 0 |
| 2 | 4.54 | 6.76 | 0.12 | 86.67 | 0.55 | 0.26 | 0.75 | 0 | 0 | 0 |
| 3 | 5.39 | 6.98 | 0.21 | 85.87 | 0.63 | 0.37 | 0.56 | 0 | 0 | 0 |
| 4 | 4.64 | 5.38 | 0.13 | 88.29 | 0.63 | 0.31 | 0.62 | 0 | 0 | 0 |
| 5 | 4.27 | 7.92 | 0.11 | 85.4 | 0.42 | 0.74 | 0.72 | 0 | 0.43 | 0 |
| 6 | 2.25 | 15.36 | 0.71 | 37.76 | 5.42 | 0.22 | 0.96 | 0 | 29.74 | 7.59 |
| 7 | 4.58 | 20.24 | 2.79 | 57.91 | 0.18 | 0.38 | 0.72 | 0 | 12.54 | 0.66 |
| 8 | 2.5 | 25.76 | 0.64 | 28.04 | 0 | 0.28 | 1.6 | 0 | 26.52 | 14.66 |
| 9 | 2.69 | 18.77 | 0.48 | 58.88 | 0 | 0.3 | 0.73 | 0 | 18.15 | 0 |
| 10 | 2.93 | 16.62 | 0.39 | 64.71 | 0.2 | 0.48 | 0.73 | 0 | 13.94 | 0 |
| 11 | 6.6 | 39.21 | 2.33 | 4.06 | 0.37 | 0.21 | 0.3 | 0.29 | 25.54 | 1.1 |
| 12 | 4.85 | 25.85 | 0.95 | 48.36 | 0 | 1.09 | 0.54 | 0 | 16.99 | 1.37 |
| 13 | 6.4 | 18.37 | 1.98 | 28.55 | 0.15 | 1.32 | 0.75 | 0 | 30.58 | 11.89 |
| 14 | 8.75 | 5.93 | 1.52 | 42.1 | 0 | 32.64 | 6.05 | 0 | 3.01 | 0 |
| 15 | 26.76 | 14.56 | 0.91 | 39.21 | 0.42 | 16.96 | 0.33 | 0 | 0.72 | 0 |
| 16 | 5.49 | 21 | 0 | 40.24 | 0.18 | 0.45 | 0.59 | 0 | 28.76 | 3.3 |
| 17 | 6.5 | 28.83 | 5.08 | 1.9 | 0.18 | 0.72 | 3.08 | 0 | 51.61 | 2.1 |
| 18 | 6.43 | 3.88 | 0 | 61.91 | 0.9 | 25.85 | 0.45 | 0 | 0.58 | 0 |
| 19 | 4.2 | 6.21 | 0.3 | 53.04 | 0.63 | 21.02 | 0.36 | 0 | 14.26 | 0 |
| 20 | 16.08 | 22.84 | 0.8 | 39.31 | 0.11 | 1.36 | 0.59 | 0 | 7.35 | 11.42 |
| 21 | 8.7 | 6.86 | 0.19 | 82.05 | 0.63 | 0.67 | 0.74 | 0 | 0.17 | 0 |
| 22 | 24.54 | 28.8 | 3.8 | 4.42 | 0.6 | 0.32 | 0.23 | 0 | 34.38 | 0.75 |
| 23 | 11.42 | 32.24 | 2.31 | 8.76 | 0.64 | 0.4 | 0.36 | 0 | 41.2 | 2.68 |
| 24 | 14.69 | 16.84 | 0.64 | 61.7 | 0.26 | 2.34 | 0.71 | 0 | 2.39 | 0.43 |
| 25 | 5.06 | 19.79 | 3.02 | 2.27 | 0.22 | 0.72 | 0.53 | 0 | 60.63 | 7.75 |
| 26 | 2.9 | 28.55 | 3.47 | 26.77 | 0 | 0 | 0.53 | 0 | 12.91 | 24.87 |
| 27 | 3.2 | 8.61 | 0.56 | 0.87 | 0 | 0 | 0.58 | 0 | 80.56 | 5.61 |
| 28 | 9.4 | 14.98 | 0.4 | 69.63 | 0.26 | 0.7 | 1.03 | 0 | 3.61 | 0 |
| 29 | 9.14 | 38.6 | 1.11 | 41.48 | 0.31 | 0.25 | 0.54 | 0 | 7.33 | 0.66 |
| 30 | 8.92 | 28.02 | 4.16 | 1.84 | 0.42 | 3.65 | 2.24 | 0 | 47.14 | 3.62 |
| 31 | 7.34 | 15.09 | 2.65 | 21.92 | 0.2 | 9.8 | 0.38 | 0.33 | 39.99 | 2.62 |
| 32 | 4.65 | 21.78 | 1.31 | 45.06 | 0.09 | 0.3 | 0.55 | 0 | 21.95 | 4.32 |
| 33 | 7.54 | 8.38 | 0.36 | 80.15 | 0.62 | 1.59 | 0.64 | 0 | 0.42 | 0 |
| 34 | 5.45 | 9.87 | 0.15 | 82.87 | 0.37 | 0.59 | 0.71 | 0 | 0 | 0 |
| 35 | 7.62 | 47.03 | 5.03 | 12.7 | 0.18 | 0.18 | 0.29 | 0 | 25.7 | 1.12 |
| 36 | 8.37 | 40.69 | 2.05 | 35.55 | 0.41 | 0.22 | 0.73 | 0 | 10.67 | 0.83 |
| 37 | 10.23 | 23.48 | 0.44 | 58.33 | 0.21 | 0.41 | 0.75 | 0 | 5.37 | 0.65 |
| 38 | 7.62 | 4.83 | 0.33 | 60.91 | 0.77 | 25.16 | 0.38 | 0 | 0 | 0 |
| 39 | 6.76 | 26.99 | 3.93 | 13.07 | 0.11 | 0.3 | 0.75 | 0 | 43.79 | 4.01 |
| No. | Contents of Elements by Weight (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Al | Si | Fe | Cu | Zn | Mo | Sn | Pb | |
| 1 | 2.62 | 7.83 | 0 | 85.03 | 0.16 | 0 | 0.48 | 0.77 | 0.26 | 1.84 | 1.02 |
| 2 | 3.19 | 7.36 | 0 | 89.06 | 0 | 0.17 | 0.21 | 0 | 0 | 0 | 0 |
| 3 | 3 | 16.16 | 0.13 | 79.09 | 0 | 0 | 0 | 0.33 | 0.62 | 0 | 0.66 |
| 4 | 5.77 | 16.44 | 0 | 77.79 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 4.29 | 34.98 | 0.77 | 20.05 | 0.14 | 0 | 1.63 | 0.84 | 0 | 30.19 | 7.11 |
| 6 | 6.62 | 29.6 | 1.27 | 6.29 | 0.49 | 0 | 1.44 | 0.4 | 0 | 51.51 | 2.38 |
| 7 | 3.52 | 24.07 | 0.64 | 15.55 | 0.33 | 0 | 1.17 | 0.6 | 0.31 | 52.02 | 1.8 |
| 8 | 3.45 | 5.63 | 2.64 | 31.14 | 0.26 | 0 | 49.53 | 0.96 | 0 | 6.39 | 0 |
| 9 | 3.65 | 1.73 | 0.26 | 0.59 | 0 | 0 | 71.91 | 21.48 | 0 | 0.38 | 0 |
| 10 | 7.1 | 4.71 | 0.6 | 1.2 | 0 | 0 | 66.83 | 19.56 | 0 | 0 | 0 |
| 11 | 8.6 | 6.13 | 0.32 | 0.94 | 0 | 0 | 50.28 | 33.02 | 0 | 0 | 0.71 |
| 12 | 16.29 | 45.16 | 2.79 | 30.51 | 0.85 | 0 | 0.26 | 0.43 | 1.1 | 1.47 | 1.13 |
| 13 | 2.77 | 11.04 | 0 | 86.19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 4.03 | 28.73 | 6.27 | 20.62 | 0.38 | 0 | 3.02 | 1.33 | 0 | 29.62 | 6 |
| 15 | 4.05 | 3.64 | 0.39 | 2.42 | 0 | 0 | 85.32 | 3.34 | 0.25 | 0 | 0.6 |
| 16 | 3.7 | 30.29 | 3.68 | 5.15 | 0.24 | 0 | 0.53 | 0.25 | 0 | 48.44 | 7.72 |
| 17 | 3.25 | 20.69 | 0.84 | 63.02 | 0.15 | 0 | 0.74 | 0.97 | 0.39 | 7.39 | 2.54 |
| 18 | 2.87 | 14.96 | 0.29 | 75.98 | 0.14 | 0 | 0.74 | 1.06 | 0.35 | 2.18 | 1.44 |
| 19 | 3.65 | 4.71 | 0.08 | 90.44 | 0.12 | 0 | 0.3 | 0.7 | 0 | 0 | 0 |
| 20 | 5.27 | 24.73 | 0.39 | 20.18 | 0.14 | 0 | 37.15 | 10.53 | 0.34 | 0.5 | 0.77 |
| 21 | 3.06 | 17.72 | 0.25 | 60.18 | 0.15 | 0 | 1.09 | 0.82 | 0.35 | 12.3 | 4.07 |
| 22 | 3.29 | 8.11 | 0.25 | 45.95 | 0.19 | 0 | 34.41 | 0.96 | 0 | 6.37 | 0.46 |
| 23 | 2.25 | 15.32 | 0.72 | 12.48 | 0 | 0 | 0.35 | 2.12 | 0 | 57.3 | 9.47 |
| 24 | 9.16 | 28.06 | 1.56 | 3.77 | 0.16 | 1.24 | 0 | 0.25 | 44.54 | 7.76 | 3.5 |
| 25 | 3.52 | 1.12 | 0 | 0.58 | 0 | 0 | 77.12 | 17.66 | 0 | 0 | 0 |
| No. | Contents of Elements by Weight (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Al | Si | Cu | Zn | Mo | Sn | Pb | |
| 1 | 2.06 | 12.09 | 0.36 | 77.62 | 0.16 | 1.04 | 1.97 | 0.24 | 3.5 | 0.94 |
| 2 | 1.61 | 10.98 | 0.38 | 78.11 | 0.19 | 1.09 | 1.93 | 0 | 4.59 | 1.11 |
| 3 | 1.89 | 8.51 | 0.66 | 81.75 | 0.44 | 0.99 | 2.11 | 0 | 2.53 | 1.12 |
| 4 | 1.7 | 5 | 0.12 | 88.81 | 0.3 | 1.57 | 2.21 | 0 | 0.29 | 0 |
| 5 | 2.69 | 13.31 | 1.12 | 69.18 | 0.21 | 1.03 | 1.88 | 0 | 4.35 | 6.23 |
| 6 | 1.97 | 13.95 | 0.23 | 78.63 | 0.19 | 1.49 | 1.86 | 0.44 | 0.84 | 0.4 |
| 7 | 2.16 | 5.92 | 0.17 | 85.36 | 0.24 | 1.88 | 2 | 0.35 | 1.45 | 0.47 |
| 8 | 6.88 | 5.02 | 0.09 | 81.64 | 0.2 | 2.43 | 2.16 | 0 | 1.57 | 0 |
| 9 | 2.1 | 6.64 | 0.23 | 80.11 | 0.24 | 5.42 | 1.91 | 0 | 2.48 | 0.87 |
| 10 | 1.84 | 4.81 | 0.28 | 87.57 | 0.26 | 1.06 | 2.1 | 0 | 1.02 | 1.06 |
| 11 | 1.07 | 1.99 | 0.46 | 42.29 | 0.3 | 43.91 | 0.71 | 0 | 7.45 | 1.82 |
| 12 | 1.91 | 0.95 | 0.4 | 27.71 | 0 | 63.26 | 2.98 | 0 | 2.08 | 0.71 |
| 13 | 1.52 | 2.57 | 1.02 | 42.9 | 0.34 | 46.31 | 0.71 | 0 | 3.46 | 1.17 |
| 14 | 4.11 | 20.02 | 5.24 | 3.14 | 0.3 | 3.58 | 6.66 | 0 | 39.02 | 17.32 |
| 15 | 1.82 | 1.71 | 0.17 | 45.65 | 0.29 | 48.47 | 0.44 | 0 | 1.02 | 0.42 |
| 16 | 1.72 | 6.78 | 0.36 | 32.33 | 2.89 | 38.27 | 1.36 | 0 | 14.05 | 2.24 |
| 17 | 5.08 | 11.73 | 0.8 | 2.01 | 0.28 | 12.07 | 0.84 | 0 | 65.11 | 2.09 |
| 18 | 8.16 | 15.46 | 2.46 | 4.43 | 0.18 | 5.26 | 1.22 | 19.14 | 41.02 | 2.66 |
| 19 | 2.69 | 6 | 1.62 | 30.73 | 0.26 | 36.71 | 2.05 | 0 | 11.74 | 8.2 |
| 20 | 2.03 | 2.76 | 0.16 | 44.87 | 0.31 | 46.61 | 0.62 | 0 | 1.78 | 0.87 |
| 21 | 1.88 | 1.56 | 0.31 | 45.08 | 0.33 | 49 | 0.66 | 0 | 1.18 | 0 |
| 22 | 2.55 | 3.36 | 0.14 | 35.45 | 0.35 | 39.2 | 0.72 | 0 | 7.41 | 10.82 |
| 23 | 2.05 | 9.31 | 0.16 | 39.4 | 0.17 | 0.98 | 1.28 | 42.86 | 2.08 | 1.7 |
| 24 | 2.44 | 16.23 | 0.5 | 55.04 | 0.18 | 7.92 | 1.51 | 12.11 | 2.63 | 1.46 |
| 25 | 2.58 | 16.66 | 0.21 | 73.41 | 0.16 | 1.63 | 1.96 | 1.56 | 1.32 | 0.49 |
| 26 | 1.86 | 12.44 | 0.17 | 73.35 | 0.19 | 1.49 | 1.71 | 4.77 | 2.04 | 1.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnov, A.; Kuznetsova, E.; Pristinskiy, Y.; Podrabinnik, P.; Mironov, A.; Gershman, I.; Peretyagin, P. Effect of Milling Conditions on the Microstructural Design in Aluminum Based Alloy Fabricated by SPS. Metals 2019, 9, 1164. https://doi.org/10.3390/met9111164
Smirnov A, Kuznetsova E, Pristinskiy Y, Podrabinnik P, Mironov A, Gershman I, Peretyagin P. Effect of Milling Conditions on the Microstructural Design in Aluminum Based Alloy Fabricated by SPS. Metals. 2019; 9(11):1164. https://doi.org/10.3390/met9111164
Chicago/Turabian StyleSmirnov, Anton, Ekaterina Kuznetsova, Yuri Pristinskiy, Pavel Podrabinnik, Alexander Mironov, Iosif Gershman, and Pavel Peretyagin. 2019. "Effect of Milling Conditions on the Microstructural Design in Aluminum Based Alloy Fabricated by SPS" Metals 9, no. 11: 1164. https://doi.org/10.3390/met9111164
APA StyleSmirnov, A., Kuznetsova, E., Pristinskiy, Y., Podrabinnik, P., Mironov, A., Gershman, I., & Peretyagin, P. (2019). Effect of Milling Conditions on the Microstructural Design in Aluminum Based Alloy Fabricated by SPS. Metals, 9(11), 1164. https://doi.org/10.3390/met9111164






