Separation of Ag and Cu from Their Aqueous Thiosulfate Complexes by UV-C Irradiation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Procedure
2.3. Analytical Methods
3. Results and Discussions
3.1. Photosensitivity of Ag Thiosulfate Complexes
3.1.1. Photolysis of Ag–Thiosulfate Complexes by UV-C Irradiation
3.1.2. The Characterization of Ag–Thiosulfate Complexes Photoproducts
3.2. Photosensitivity of Cu Thiosulfate Complexes
3.2.1. Photolysis of Cu–Thiosulfate Complexes by UV-C Irradiation
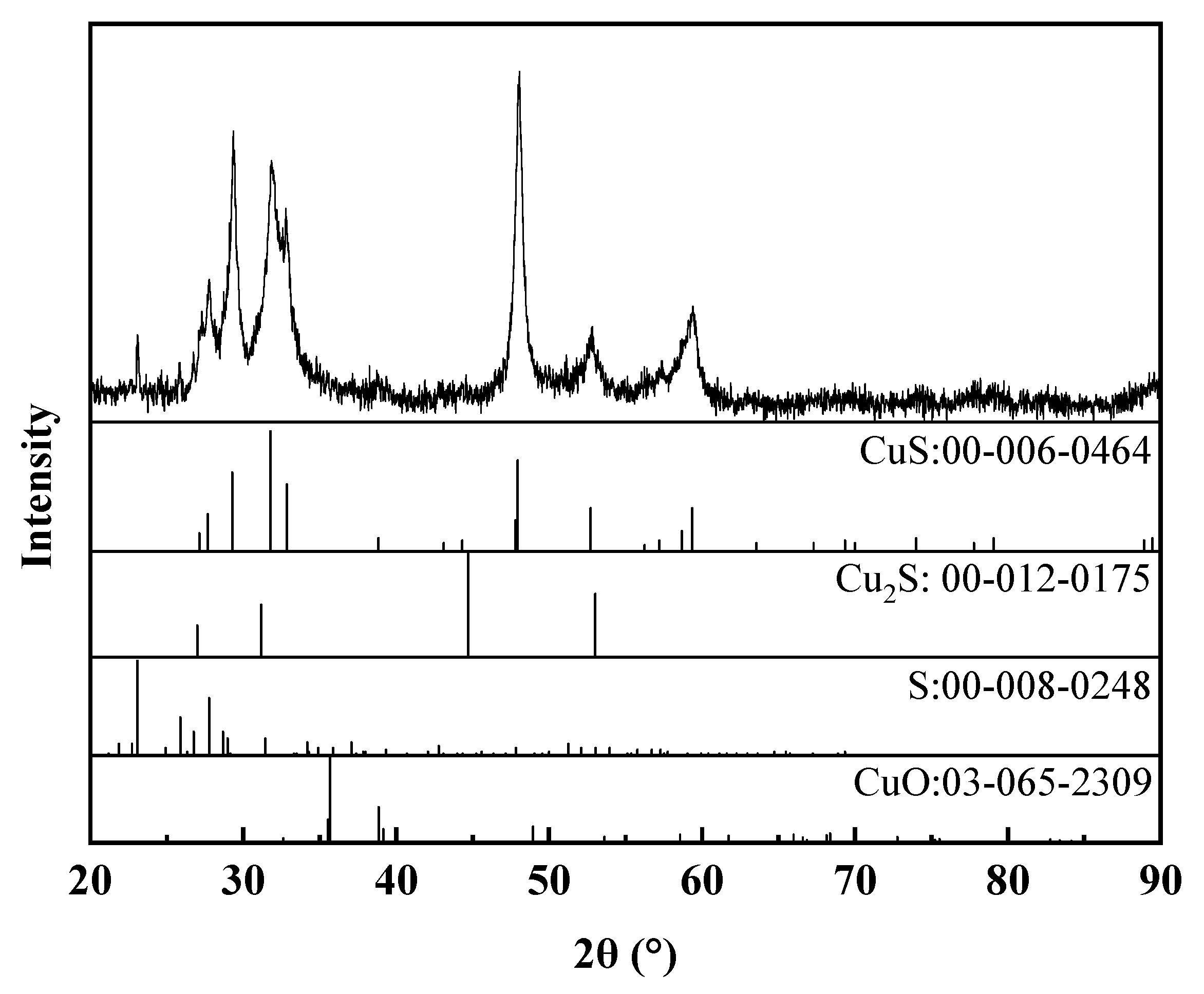
3.2.2. The Characterization of Cu–Thiosulfate Complexes Photoproducts
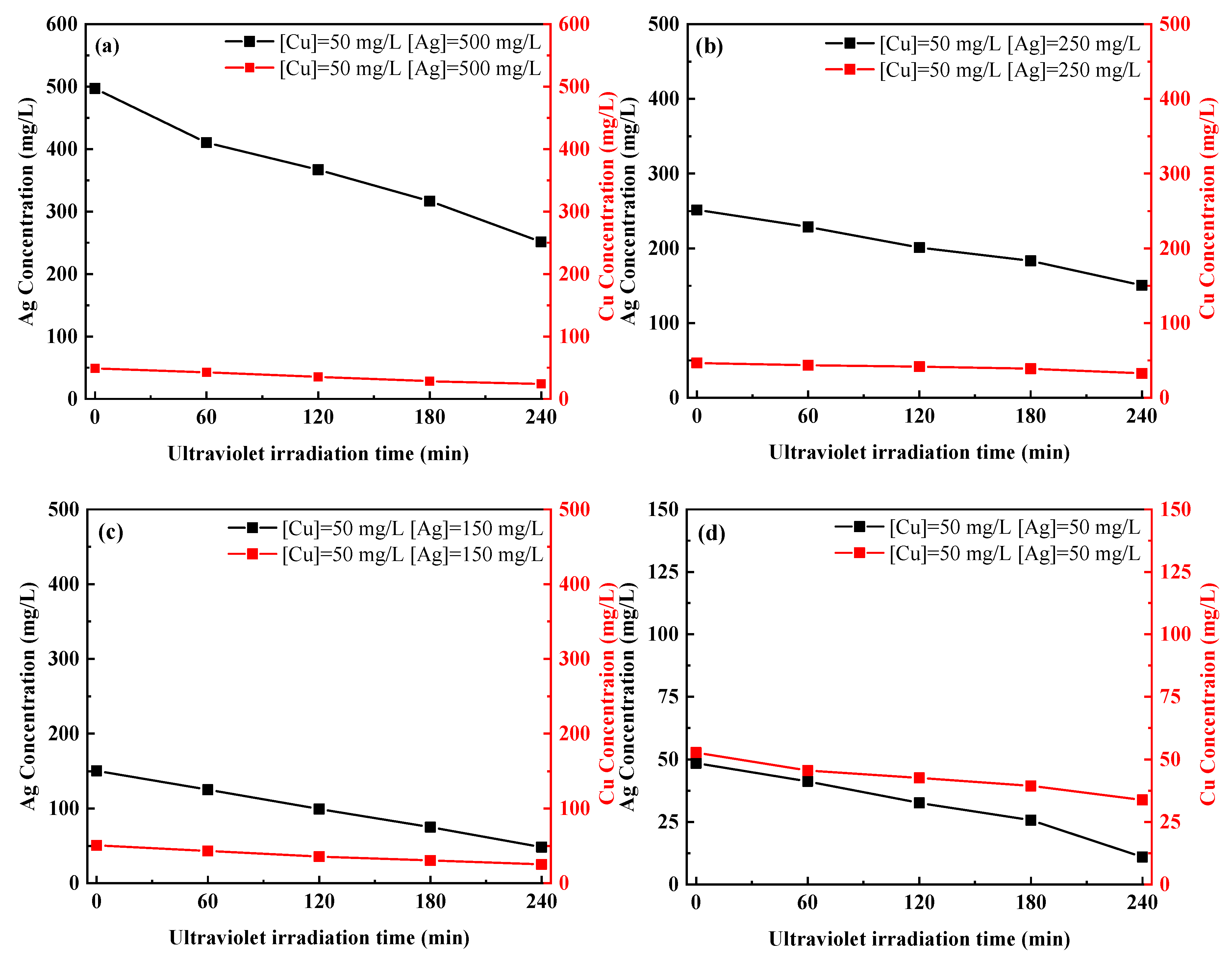
3.3. Separation of Ag and Cu by UV-C Irradiation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Briones, R.; Lapidus, G.T. The leaching of silver sulfide with the thiosulfate–ammonia–cupric ion system. Hydrometallurgy 1998, 50, 243–260. [Google Scholar] [CrossRef]
- Matti, L.; Arto, L.; Ilkka, T. Ammoniacal thiosulfate leaching of pressure oxidized sulfide gold concentrate with low reagent consumption. Hydrometallurgy 2015, 152, 120–128. [Google Scholar]
- Feng, D.; Deventer, J. Effect of thiosulphate salts on ammoniacal thiosulphate leaching of gold. Hydrometallurgy 2010, 105, 120–126. [Google Scholar] [CrossRef]
- Aylmore, M.; Muir, D. Thiosulfate leaching of gold-A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Arima, H.; Fujita, T.; Yen, W. Using nickel as a catalyst in-ammonium thiosulfate leaching for gold extraction. Mater. Trans. 2004, 45, 516–526. [Google Scholar] [CrossRef]
- Chandra, I.; Jeffrey, M. A fundamental study of ferric oxalate for dissolving gold in thiosulfate solutions. Hydrometallurgy 2005, 77, 191–201. [Google Scholar] [CrossRef]
- Deutsch, J.L.; Dreisinger, D.B. Silver sulfide leaching with thiosulfate in the presence of additives Part II: Ferric complexes and the application to silver sulfide ore. Hydrometallurgy 2013, 137, 165–172. [Google Scholar] [CrossRef]
- Cui, Y.; Tong, X.; Lopez-Valdivieso, A. Silver sulfide leaching with a copper-thiosulfate solution in the absence of ammonia. Rare Met. 2011, 30, 105. [Google Scholar] [CrossRef]
- Deng, Z.; Oraby, E.A.; Eksteen, J.J. The sulfide precipitation behaviour of Cu and Au from their aqueous alkaline glycinate and cyanide complexes. Sep. Purif. Technol. 2019, 218, 181–190. [Google Scholar] [CrossRef]
- Xing, D.W.; Lee, M.S.; Senanayake, G. Recovery of metals from chloride leach solutions of anode slimes by solvent extraction. Part II: Recovery of silver and copper with LIX 63 and Alamine 336. Hydrometallurgy 2018, 180, 49–57. [Google Scholar] [CrossRef]
- Kononova, O.N.; Shatnykh, K.A.; Prikhod’Ko, K.V.; Kashirin, D.M. Ion-Exchange Recovery of Gold(I) and Silver(I) from Thiosulfate Solutions. Russ. J. Phys. Chem. A 2009, 83, 2340–2345. [Google Scholar] [CrossRef]
- Norasikin, O.; Hanapi, M.; Masahiro, G. Separation of silver from photographic wastes by emulsion liquid membrane system. J. Membr. Sci. 2006, 282, 171–177. [Google Scholar]
- Egorov, N.B. Investigation of Lead Thiosulfate Photolysis in Aqueous Solutions. High. Energy Chem. 2013, 48, 5. [Google Scholar] [CrossRef]
- Egorov, N.B.; Eremin, L.P.; Larionov, A.M.; Usov, V.F.; Tsepenko, E.A.; D’yachenko, A.S. Products of Photolysis of Cadmium Thiosulfate Aquepus Solutions. High. Energy Chem. 2008, 42, 119–122. [Google Scholar] [CrossRef]
- Wei, W.; Chao, H.; Feng, X. Efficient mercury recovery from mercuric-thiosulfate solutions by ultraviolet photolysis. Environ. Chem. Lett. 2018, 16, 1049–1054. [Google Scholar]
- Liu, Y.; Jordan, R.G.; Qiu, S.L. Electronic structures of ordered Ag-Mg alloys. Phys. Rev. B Condens. Matter. 1994, 49, 4478–4484. [Google Scholar] [CrossRef]
- Kaushik, V.K. XPS core level spectra and Auger parameters for some silver compounds. J. Electron. Spectrosc. 1991, 56, 273–277. [Google Scholar] [CrossRef]
- Yu, X.R.; Liu, F.; Wang, Z.Y.; Chen, Y. Auger parameters for sulfur-containing compounds using a mixed aluminum-silver excitation source. J. Electron. Spectrosc. 1990, 50, 159–166. [Google Scholar] [CrossRef]
- Peisert, H.; Chassé, T.; Streubel, P.; Meisel, A.; Szargan, R. Relaxation energies in XPS and XAES of solid sulfur compounds. J. Electron. Spectrosc. 1994, 68, 321–328. [Google Scholar] [CrossRef]
- Manocha, A.S.; Park, R.L. Flotation related ESCA studies on PbS surfaces. Appl. Surf. Sci. 1977, 1, 129–141. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Cook, J.M. Interactions of SO2 with sodium deposited on CaO. J. Colloid Interface Sci. 1986, 114, 525–535. [Google Scholar] [CrossRef]
- Schulze, P.D.; Shaffer, S.L.; Hance, R.L.; Utley, D.L. Adsorption of water on rhenium studied by XPS. J. Vac. Sci. Technol. A 1983, 1, 97. [Google Scholar] [CrossRef]
- Wagner, C.D.; Zatko, D.A.; Raymond, R.H. Use of the oxygen KLL Auger lines in identification of surface chemical states by electron spectroscopy for chemical analysis. Anal. Chem. 1980, 52, 1445. [Google Scholar] [CrossRef]
- Perry, D.; Taylor, J. X-ray photoelectron and Auger spectroscopic studies of Cu2S and CuS. J. Mater. Sci. Lett. 1986, 5, 384–386. [Google Scholar] [CrossRef]
- Strohmeier, B.; Levden, D.; Field, R.; Hercules, D. Surface spectroscopic characterization of CuAl2O3 catalysts. J. Catal. 1985, 94, 514–530. [Google Scholar] [CrossRef]
- Hussain, Z.; Salim, M.A.; Khan, M.A.; Khawaja, E.E. X-ray photoelectron and auger spectroscopy study of copper-sodium-germanate glasses. J. Non-Cryst. Solids 1989, 110, 44–52. [Google Scholar] [CrossRef]
- Nakai, I.; Sugitani, Y.; Nagashima, K.; Niwa, Y. X-ray photoelectron spectroscopic study of copper minerals. J. Inorg. Nucl. Chem. 1978, 40, 789–791. [Google Scholar] [CrossRef]
- Schoen, G. High resolution Auger electron spectroscopy of metallic copper. J. Electron. Spectrosc. 1972, 1, 377–387. [Google Scholar] [CrossRef]
- Brion, D. Etude par spectroscopie de photoelectrons de la degradation superficielle de FeS2, CuFeS2, ZnS et PbS a l’air et dans l’eau. Appl. Surf. Sci. 1980, 5, 133–152. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Gati, D.; Dzhurinskii, B.F.; Sergushin, N.P.; Salyn, Y.V. X-Ray electron spectrum study of oxides. Zh. Neorgan. Khim. 1975, 20, 2307. (In Russian) [Google Scholar]
- Wagner, C.D.; Zatko, D.A.; Raymond, R.H. Surface studies of the interaction of cesium hydroxide vapor with 304 stainless steel. Oxid. Met. 1987, 28, 33–59. [Google Scholar]








| Methods | Research Target | Results | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Solvent extraction (extractant: LIX 63 and Alamine 336; stripping agent: HCl) | Chloride leach solution of anode slimes | Above 99% of Cu(II) and most of Ag(I) extracted | Highly selective and easy to industrialize | Limited to cation system, needs stripping | [11] |
| Ion exchange removal (ion exchange synthesized by Purolite UK) | Thiosulfate complex solution | Purolite A530 has the best properties towards Ag and Au | With regeneration capacity and simple device | Limited to purified exchange capacity | [12] |
| Emulsion liquid membrane system (Cyanex 302) | Liquid photographic waste | Almost complete recovery of silver | High selectivity, simultaneous extraction and separation | Liquid membrane instability | [13] |
| Silver Concentration/mg/L | k/min−1 | Goodness of Fit |
|---|---|---|
| 100 | 0.00210 | 0.99305 |
| 300 | 0.00176 | 0.99864 |
| 500 | 0.00141 | 0.99679 |
| Copper Concentration/mg/L | k/min−1 | Goodness of Fit |
|---|---|---|
| 50 | 0.00149 | 0.985 |
| 75 | 0.00130 | 0.997 |
| 100 | 0.00101 | 0.986 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Wang, G.; Zou, M.; Shi, C. Separation of Ag and Cu from Their Aqueous Thiosulfate Complexes by UV-C Irradiation. Metals 2019, 9, 1178. https://doi.org/10.3390/met9111178
Han C, Wang G, Zou M, Shi C. Separation of Ag and Cu from Their Aqueous Thiosulfate Complexes by UV-C Irradiation. Metals. 2019; 9(11):1178. https://doi.org/10.3390/met9111178
Chicago/Turabian StyleHan, Chao, Guangxin Wang, Mingjin Zou, and Chaoyang Shi. 2019. "Separation of Ag and Cu from Their Aqueous Thiosulfate Complexes by UV-C Irradiation" Metals 9, no. 11: 1178. https://doi.org/10.3390/met9111178
APA StyleHan, C., Wang, G., Zou, M., & Shi, C. (2019). Separation of Ag and Cu from Their Aqueous Thiosulfate Complexes by UV-C Irradiation. Metals, 9(11), 1178. https://doi.org/10.3390/met9111178





