Nitrogen Solubility in Liquid Ni-V, Ni-Ta, Ni-Cr-V, and Ni-Cr-Ta Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
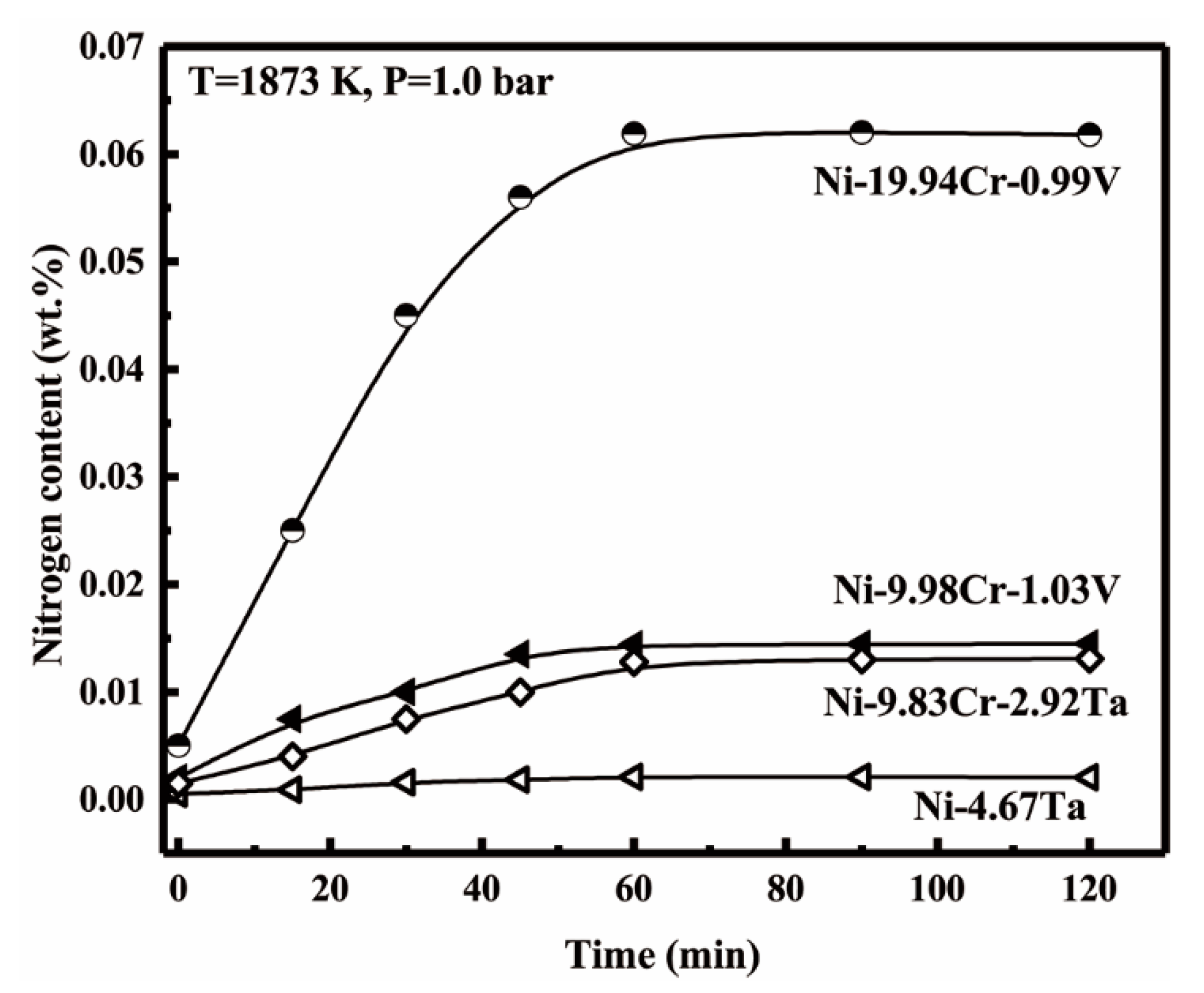
3.1. Equilibrium Time
3.2. Nitrogen Dissolution in Liquid Pure Nickel
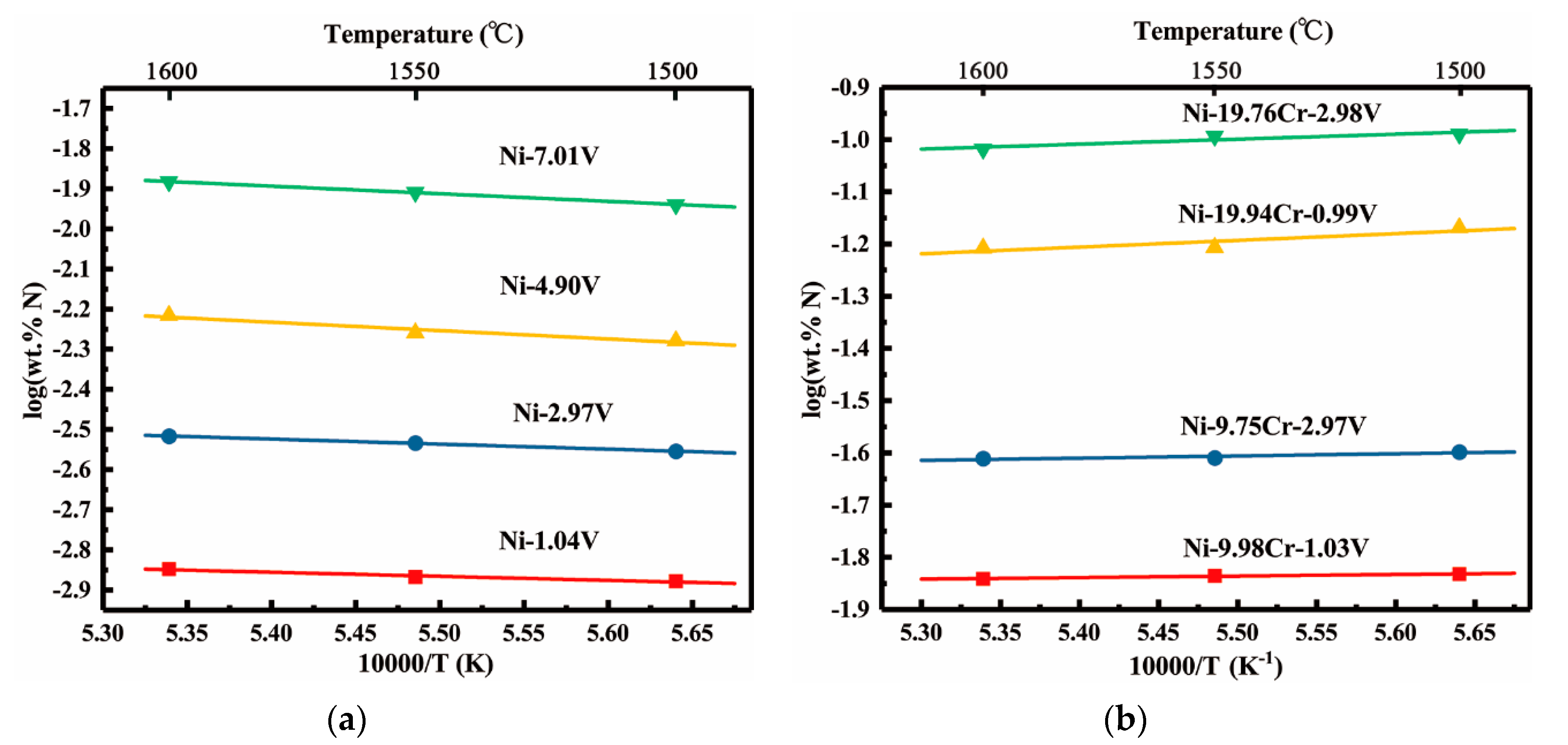
3.3. Solubility of Nitrogen in Liquid Ni-V, Ni-Cr-V Alloys
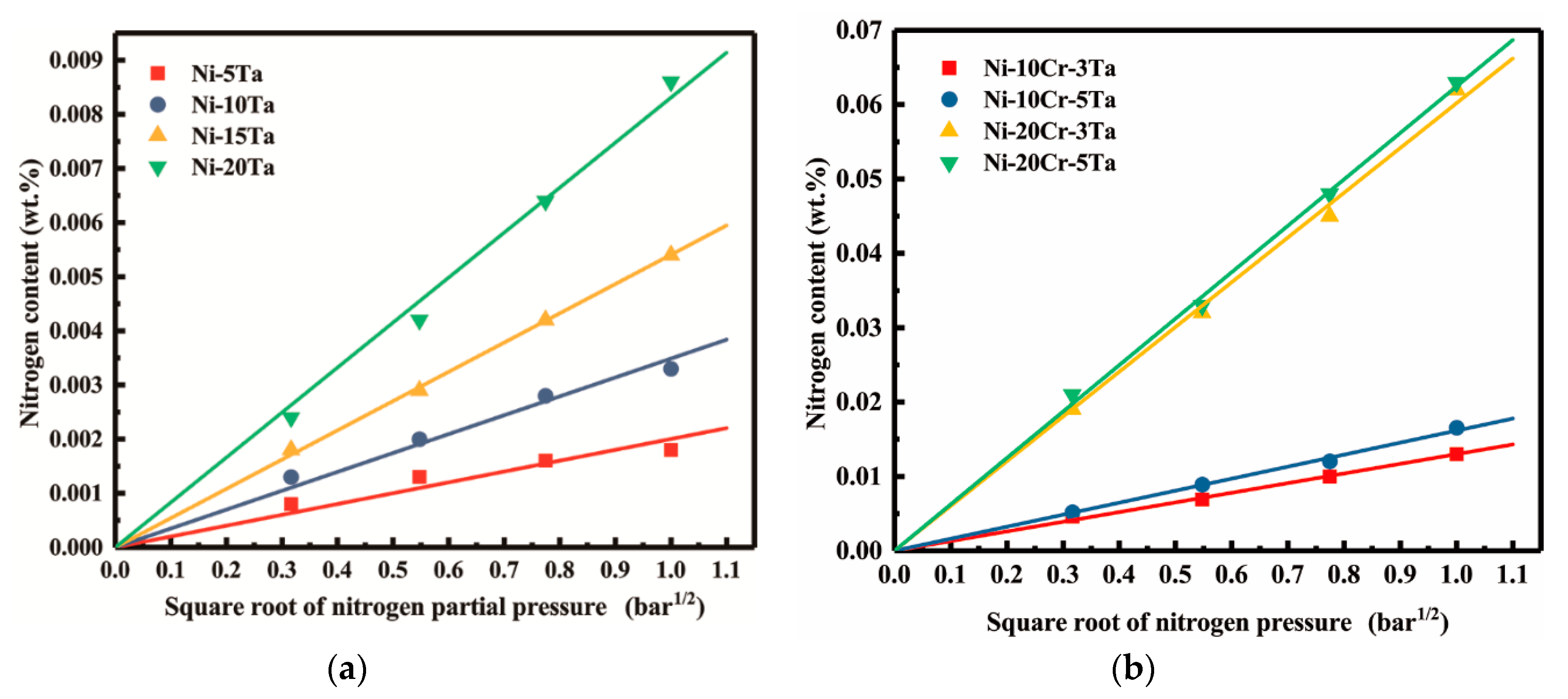
3.4. Solubility of Nitrogen in Liquid Ni-Ta, Ni-Cr-Ta Alloys
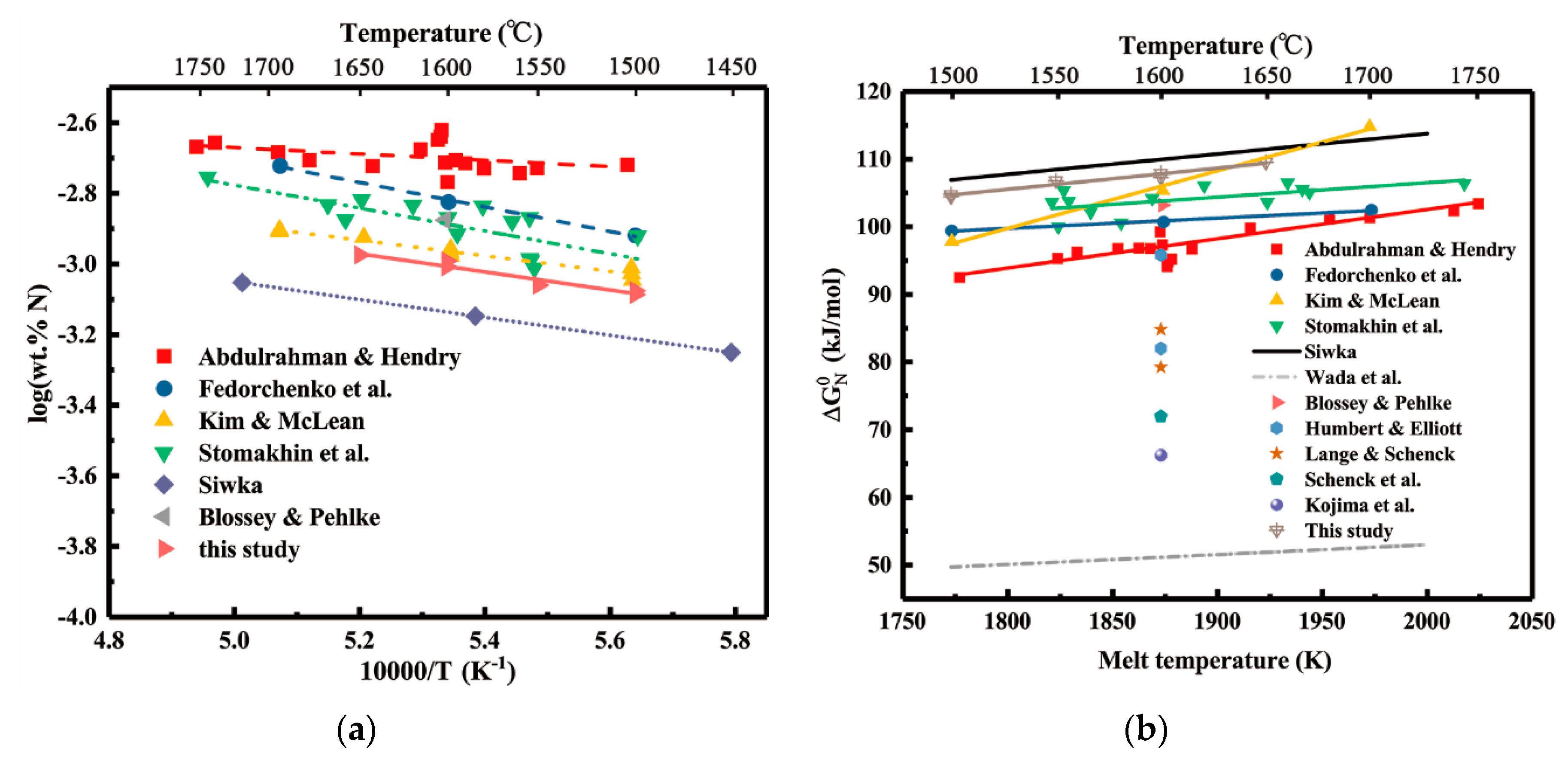
4. Discussion
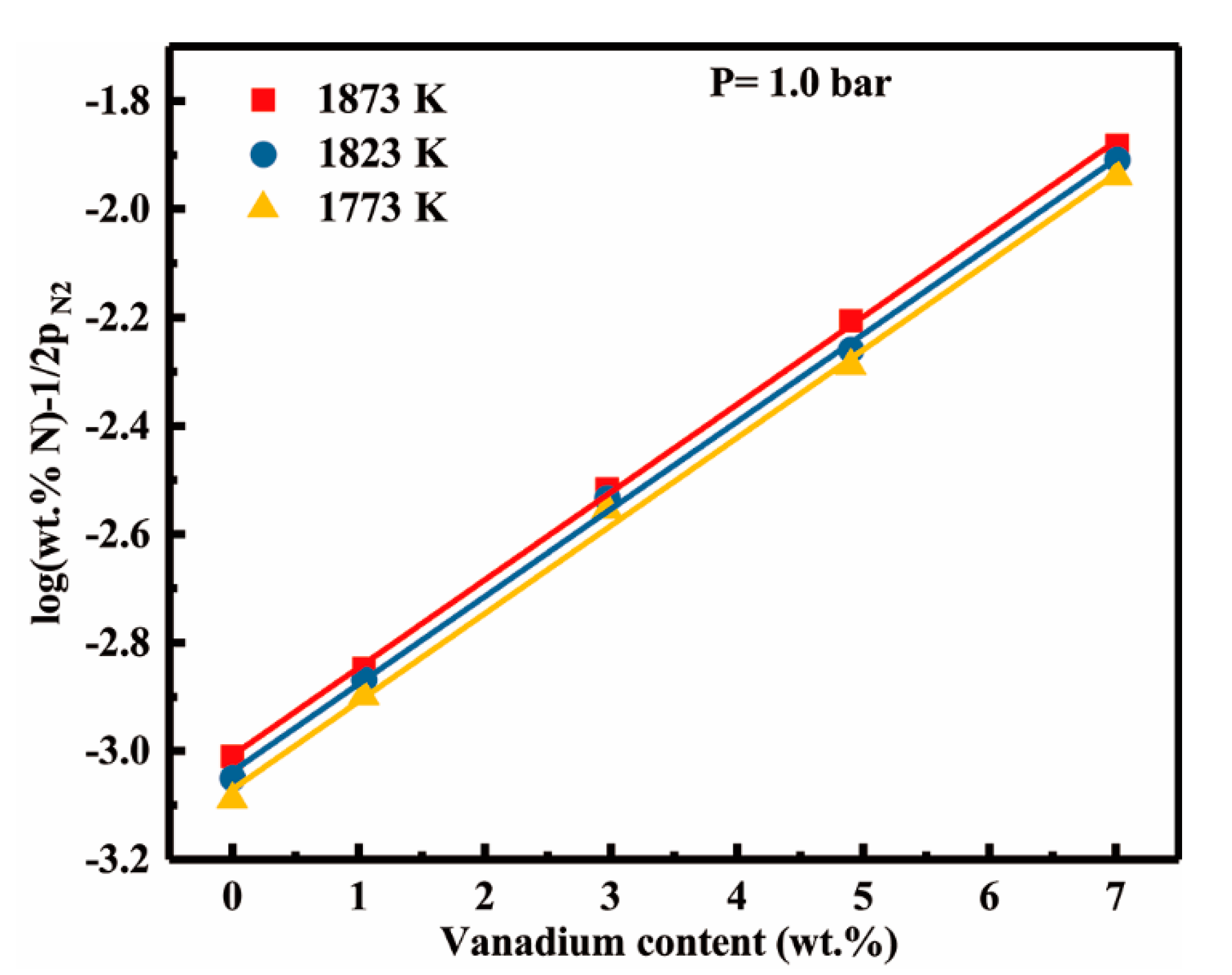
4.1. Vanadium-nitrogen Interactions in Liquid Nickel
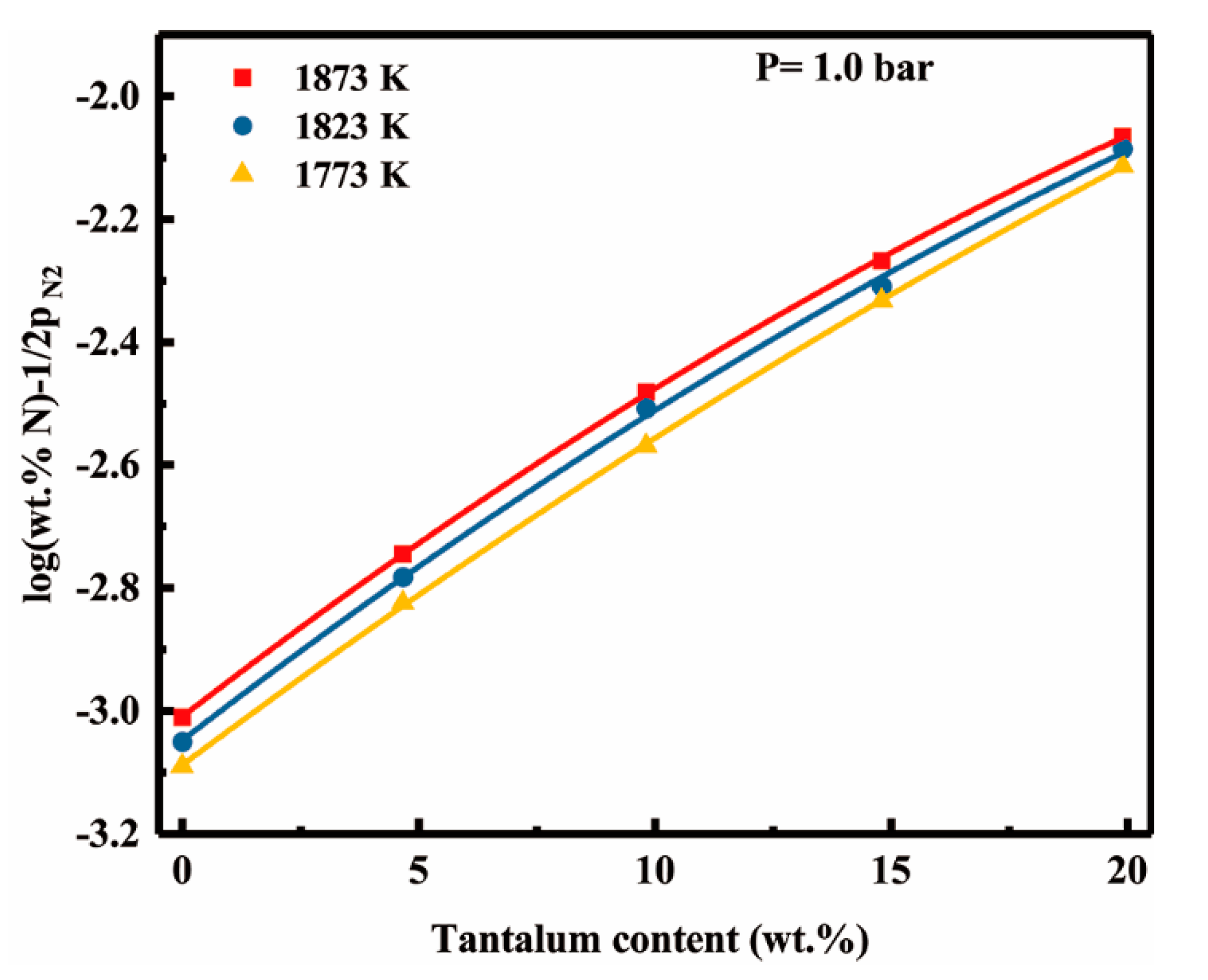
4.2. Tantalum-Nitrogen Interactions in Liquid Nickel
4.3. The Second Order Cross-Interaction Parameters
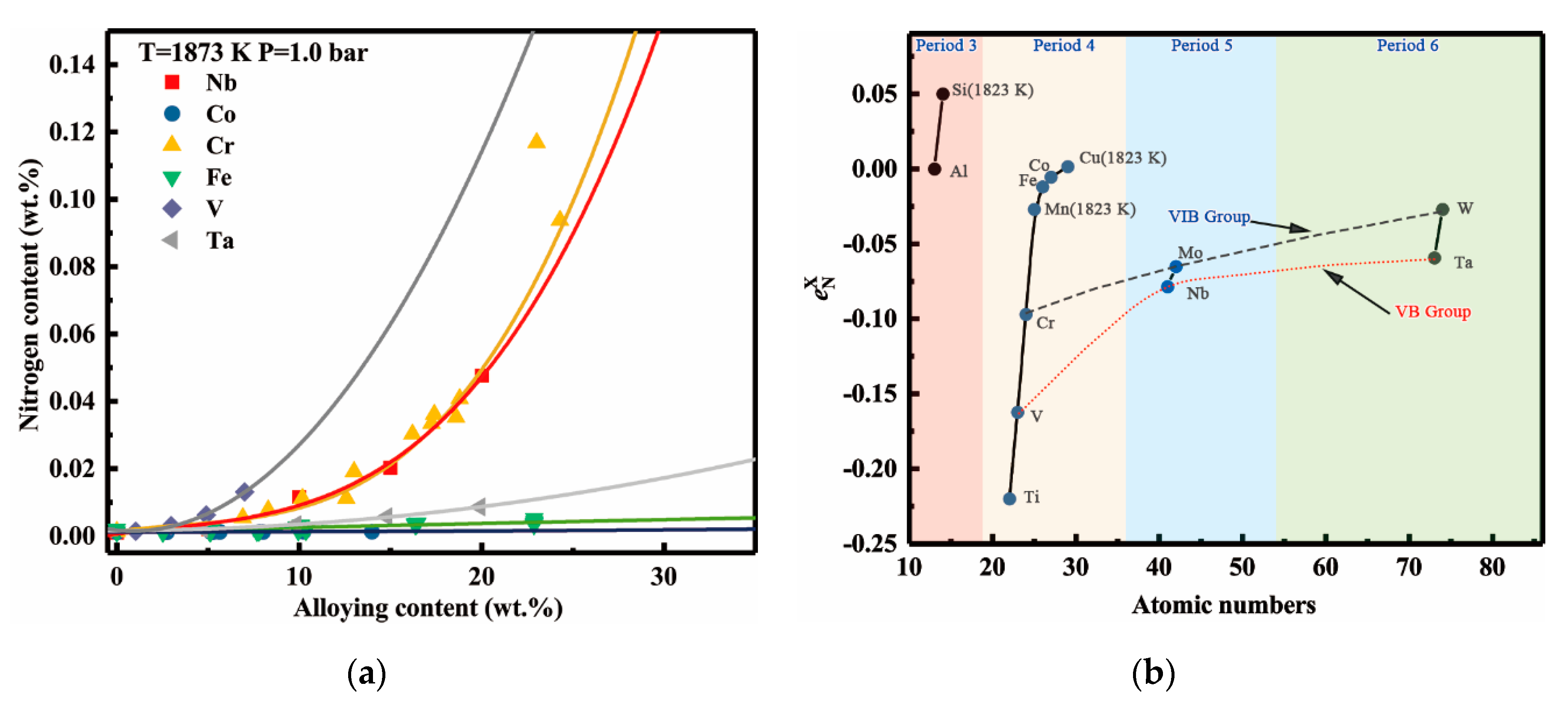
4.4. Discussion of the Effect of Alloying Elements on the Nitrogen Solubility
5. Conclusions
- (1)
- Both vanadium and tantalum increase the nitrogen solubility in liquid nickel-based alloys, and vanadium has larger effects on nitrogen solubility than tantalum.
- (2)
- It was confirmed that nitrogen dissolution obeys Sieverts’ law in each alloys at 1873 K and partial pressures of nitrogen in range from 0.1 to 1.0 bar.
- (3)
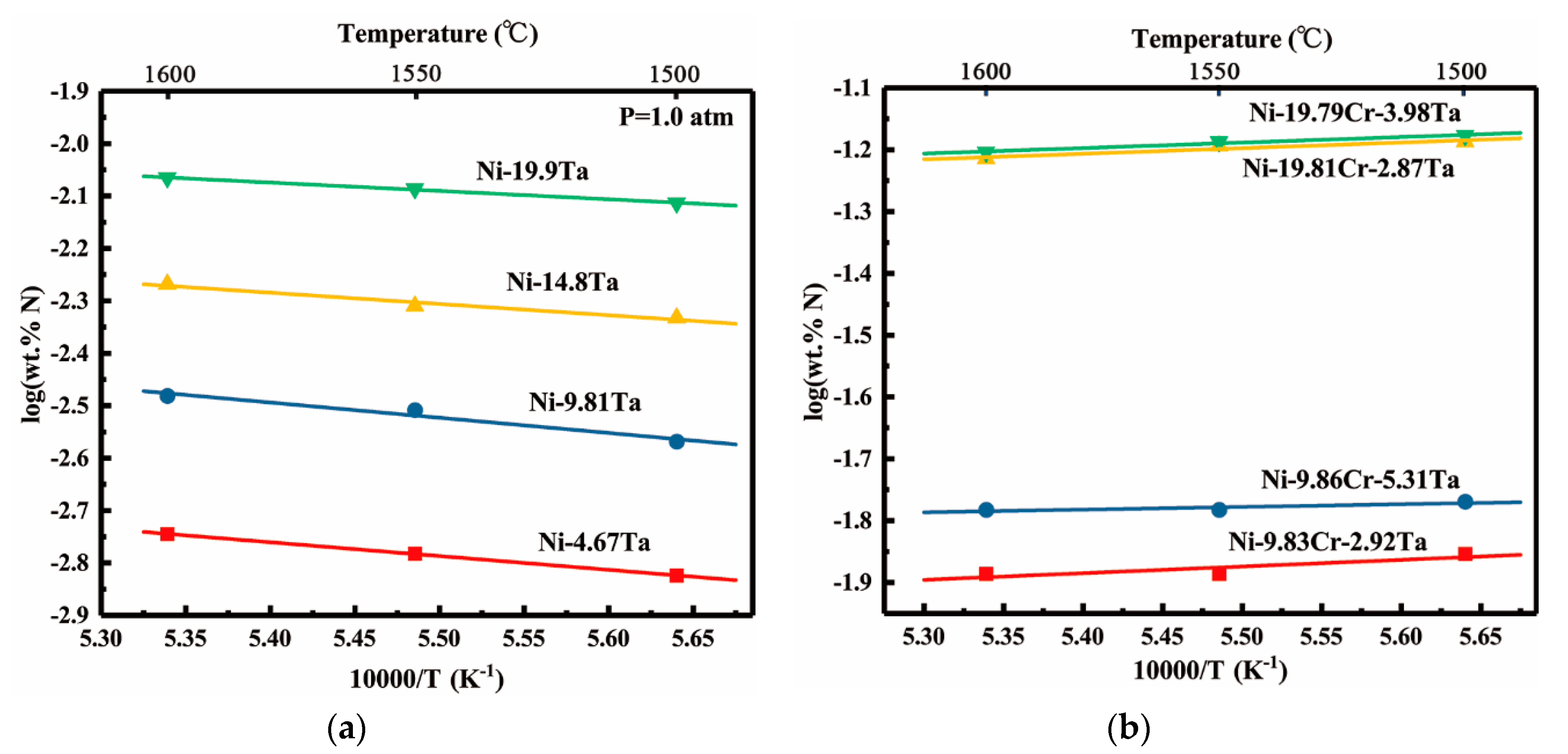
- Nitrogen solubility in liquid Ni-V and Ni-Ta alloys increases with increasing temperature, this fact can be attributed to the endothermic reaction of nitrogen dissolution. However, the rising of temperature decreases the solubility of nitrogen in liquid Ni-Cr-Ta and Ni-Cr-V alloys in which the temperature dependency of the nitrogen solubility shows a transfer from positive to negative with the addition of chromium. Moreover, the enthalpies and entropies of dissolution of nitrogen in liquid pure Ni, Ni-V, Ni-Ta, Ni-Cr-V, and Ni-Cr-Ta alloys were determined at 1873 K.
- (4)
- The effect of vanadium content up to 7.01 wt pct on dissolution behavior of nitrogen can be described by the first order interaction parameters, 30/T − 0.1783. In addition, we have determined the second order cross-interaction parameters between nitrogen, and chromium with vanadium at 1873 K as −0.0017 ± 0.0016.
- (5)
- The effect of tantalum addition (up to 19.9 wt pct) on the nitrogen solubility in liquid nickel can be expressed in terms of the interaction parameters. The first and second order interaction parameters, and , have been determined as a function of temperature, 66.7/T − 0.0952 and = 6.6/T − 0.0042. The second order cross-interaction parameters between nitrogen, and chromium with tantalum was also determined at 1873 K as −0.0022 ± 0.0007.
- (6)
- Vanadium addition apparently increases the nitrogen solubility and its effect is only lower than that of titanium, but clearly stronger than that of tantalum. In the binary Ni-X systems (X = Ti, V, Cr, Nb, Ta, Mo, W, Fe, Co), the sequence of the increasing effects on the nitrogen solubility can be specified as Ti > V > Cr > Nb > Ta > Mo > W > Fe > Co.
- (7)
- The results obtained in this paper do not only enrich the thermodynamic data of nitrogen in liquid nickel-based alloys. The similar approach can also be extended to chromium-based alloys, which would provide a basis for chromium-based alloys as structural materials used at high temperatures.
Author Contributions
Funding
Conflicts of Interest
References
- Mitchell, A. Progress in understanding clean metal production for IN718. Superalloys 1994, 718, 109–123. [Google Scholar]
- Cockcroft, S.L.; Degawa, T.; Mitchell, A.; Tripp, D.W.; Schmalz, A. Inclusion precipitation in superalloys. Superalloys 1992, 1992, 577–586. [Google Scholar]
- Huang, X.; Zhang, Y.; Hu, Z. Effect of small amounts of nitrogen on properties of a Ni-based superalloy. Metall. Mater. Trans. A 1999, 30, 1755–1761. [Google Scholar] [CrossRef]
- Guo, X.L.; Yu, J.B.; Li, X.F.; Hou, Y.; Ren, Z.M. Effect of nitrogen content on the microstructure and mechanical properties of a cast nickel-base superalloy. Ironmak. Steelmak. 2018, 45, 215–223. [Google Scholar] [CrossRef]
- Harris, K.; Wahl, J.B. Developments in superalloy castability and new applications for advanced superalloys. Mater. Sci. Tech. 2009, 25, 147–153. [Google Scholar] [CrossRef]
- Shahapurkar, D.S.; Small, W.M. Effect of titanium on the nitrogen solubility in complex liquid Fe−Cr−Ni alloys. Metall. Trans. B 1987, 182, 31–235. [Google Scholar] [CrossRef]
- Wei, S. Thermodynamic study of interaction coefficients in multicomponent metallic solutions by the solubility method. Steel Res. 1992, 63, 159–165. [Google Scholar] [CrossRef]
- Suzuki, K.; Ban-Ya, S.; Hino, M. Deoxidation equilibrium of chromium stainless steel with Si at the temperatures from 1823 to 1923 K. ISIJ Int. 2001, 41, 813–817. [Google Scholar] [CrossRef]
- Turnock, P.H.; Pehlke, R.D. The solubility of nitrogen in multicomponent liquid iron alloys. TMS-AIME 1966, 236, 1540–1547. [Google Scholar]
- Rašev, Z.; Ivanov, R. Die Stickstofflöslichkeit in eisen-chrom-mangan-schmelzen bei 1600 °C und Gasdrücken bis zu 25 bar. Arch. Eisenhütten. 1979, 50, 369–371. [Google Scholar] [CrossRef]
- Chang, Y.; Fitzner, K.; Zhang, M.X. The solubility of gases in liquid metals and alloys. Pro. Mater. Sci. 1988, 32, 97–259. [Google Scholar] [CrossRef]
- Mukai, K. Interaction Parameters in ternary liquid alloys. Trans. Jpn. Ins. Met. 1975, 16, 141–150. [Google Scholar] [CrossRef]
- Ishll, F.; Fuwa, T. Effect of alloying element on the solubility of nitrogen in liquid iron. Tetsu-to-Hagané 1982, 68, 1560–1568. [Google Scholar]
- Abdulrahman, R.F.; Hendry, A. The solubility of nitrogen in liquid pure nickel. Metall. Mater. Trans. B 2001, 32, 1095–1101. [Google Scholar] [CrossRef]
- Siwka, J. Experimental measurements of nitrogen solubility in liquid nickel and cobalt. In Proceedings of the International Conference on High Nitrogen Steels, Jiuzhaigou Valley, China, 29–31 August 2006; Metallurgy Industry Press: Beijing, China. [Google Scholar]
- Kowanda, C.; Speidel, M.O. Solubility of nitrogen in liquid nickel and binary Ni–Xi alloys (Xi = Cr, Mo, W, Mn, Fe, Co) under elevated pressure. Scr. Mater. 2003, 48, 1073–1078. [Google Scholar] [CrossRef]
- Schenck, H.; Frohberg, M.G.; Graf, H. Untersuchung über die beeinflussung der gleichgewichte von stickstoff mit flüssigen eisenlösungen durch den zusatz weiterer Elemente (I). Arch. Eisenhütten. 1958, 29, 673–676. [Google Scholar] [CrossRef]
- Abdulrahman, R.F.; Hendry, A. Solubility of nitrogen in liquid nickel-based alloys. Metall. Mater. Trans. B. 2001, 32, 1103–1112. [Google Scholar] [CrossRef]
- Kowanda, C. Stickstofflöslichkeit in nickel und nickelbasislegierungen im schmelzflüssigen zustand unter erhöhtem Stickstoffpartialdruck. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, September 2000. [Google Scholar]
- Herrera-Trejo, M.; Ablitzer, D. Dissolution of nitrogen in liquid Ni–Cr superalloys. Can. Metall. Quart. 1997, 36, 341–345. [Google Scholar] [CrossRef]
- Gu, Y.F.; Harada, H.; Ro, Y. Chromium and chromium-based alloys: Problems and possibilities for high-temperature service. JOM 2004, 56, 28–33. [Google Scholar] [CrossRef]
- Soleimani-Dorcheh, A.; Galetz, M.C. Oxidation and nitridation behavior of Cr–Si alloys in air at 1473 K. Oxid. Met. 2015, 84, 73–90. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Galetz, M.C. Challenges in developing oxidation-resistant chromium-based alloys for applications above 900 °C. JOM 2016, 68, 2793–2802. [Google Scholar] [CrossRef]
- Fryburg, G.C.; Stearns, C.A.; Kohl, F.J. mechanism of beneficial effect of tantalum in hot corrosion of nickel-base superalloys. J. Electrochem. Soc. 1977, 124, 1147–1148. [Google Scholar] [CrossRef]
- Luo, L.; Ru, Y.; Qin, L.; Pei, Y.; Ma, Y.; Li, S.; Zhao, X.; Gong, S. Effects of alloyed aluminum and tantalum on the topological inversion behavior of Ni-based single crystal superalloys at high temperature. Adv. Eng. Mater. 2019, 21, 1800793. [Google Scholar] [CrossRef]
- Allen, A.T.; Douglass, D.L. Internal-nitriding behavior of Ni-V and Ni-3Nb alloys. Oxid. Met. 1999, 51, 1–22. [Google Scholar] [CrossRef]
- Morita, Z.I.; Iwanaga, Y.; Hamada, S.; Adachi, A. Solubility of nitrogen and equilibrium of Ta-nitride forming reaction in liquid Fe-Ta alloys. Tetsu-to-Hagané 1973, 59, 214–221. [Google Scholar] [CrossRef]
- Kunze, H.D.; Schürmann, E.; Parlee, N.A.D. Influence of temperature and equivalent effect of added elements on the solubility, activity, and activity coefficient of nitrogen in liquid iron. Metall. Tran. 1970, 1, 281–290. [Google Scholar]
- Rowers, J.C.; Gokcen, N.A. Nitrogen concentration in liquid Fe-Mn alloys at high pressure. J. Phase Equilib. 1993, 14, 179–183. [Google Scholar] [CrossRef]
- Roskosz, M.; Bouhifd, M.A.; Jephcoat, A.P.; Marty, B.; Mysen, B.O. Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochim. Cosmochim. Acta 2013, 121, 15–28. [Google Scholar] [CrossRef]
- Gomersall, D.W. Solubility of nitrogen in liquid iron alloys. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, September 1967. [Google Scholar]
- Fedorchenko, V.I.; Averin, V.V.; Samarin, A.M. Solubility of nitrogen in liquid nickel and in nickel-chromium, nickel-molybdenum, and nickel-tungsten melts. Dokl. Akad. Nauk SSSR 1968, 4, 894–896. [Google Scholar]
- Kim, C.K.; McLean, A.; Ishii, F.; Ban-Ya, S. Thermodynamic of nitrogen dissolution in liquid nickel alloys. ISS Trans. 1987, 8, 29–35. [Google Scholar]
- Stomakhin, A.Y.; Bayer, P.; Polyakov, A.Y. Solubility of nitrogen in liquid nickel and in Ni-Cr, Ni-Mo and Ni-W alloys. Lzvest Akad Nauk.SSSR Metall. 1965, 4, 37–45. [Google Scholar]
- Blossey, R.G.; Pehlke, R.D. The solubility of nitrogen in liquid Fe-Ni-Co alloys. TMS-AIME 1966, 236, 566–569. [Google Scholar]
- Kojima, Y.; Inouye, M.; Yamada, Y. Solubility and diffusivity of nitrogen in liquid iron-nickel and iron-cobalt alloys at 1600 °C. Trans. Iron Steel Inst. Jpn. 1975, 15, 599–605. [Google Scholar]
- Lange, K.W.; Schenck, H. Gas solubility measurements and the derivation of inherent thermodynamic information. Metall. Trans. 1970, 1, 2036–2038. [Google Scholar] [CrossRef]
- Wada, H.; Gunji, K.; Wada, T. Solubility of nitrogen in molten Fe-Ni and Fe-Cr alloys. Trans. Iron Steel Inst. Jpn. 1968, 8, 329–336. [Google Scholar]
- Humbert, J.C.; Elliott, J.F. The solubility of nitrogen in liquid Fe-Cr-Ni alloys. TMS-AIME 1960, 218, 1076–1087. [Google Scholar]
- Wada, H.; Pehlke, R.D. Nitrogen solution and titanium nitride precipitation in liquid Fe-Cr-Ni alloys. Metall. Trans. B 1977, 8, 443–450. [Google Scholar] [CrossRef]
- Wada, H.; Gunji, K.; Wada, T. The solubility of nitrogen in liquid Fe-Ni and Fe-Cr alloys. J. Jpn Ins. Met. 1968, 32, 933–938. [Google Scholar] [CrossRef]
- Wagner, C. The activity coefficient of oxygen and other nonmetallic elements in binary liquid alloys as a function of alloy composition. Acta Metall. 1973, 21, 1297–1303. [Google Scholar] [CrossRef]
- Hu, D.C.; Chang, Y.A. The activity coefficient of nonmetallic solutes in ternary and higher order liquid alloys. Metall. Trans. B 1980, 11, 172–175. [Google Scholar] [CrossRef]
- Wada, H.; Pehlke, R.D. Solubility of nitrogen in liquid Fe-Cr-Ni alloys containing manganese and molybdenum. Metall. Trans. B 1977, 8, 675–682. [Google Scholar] [CrossRef]
- Wada, H.; Pehlke, R.D. Nitrogen solubility and aluminum nitride precipitation in liquid iron, Fe-Cr, Fe-Cr-Ni, and Fe-Cr-Ni-Mo alloys. Metall. Trans. B 1978, 9, 441–448. [Google Scholar] [CrossRef]
- Sokolov, V.M.; Fedorenko, I.V. Estimation of hydrogen solubility in liquid alloys of iron, nickel and copper. Int. J. Hydrogen Energ. 1996, 21, 931–934. [Google Scholar] [CrossRef]
- Lupis, C.H.P.; Elliott, J.F. Relationship between interaction coefficients epsilon and e. TMS-AIME 1965, 233, 257–258. [Google Scholar]
- Avercn, B.B. Nitrogen in Metals; Chinese Metallurgical Industry Press: Beijing, China, 1981; pp. 73–81. [Google Scholar]
- Schürmann, E.; Sittard, M.; Völker, R. Systematic of the equivalent influence of alloying elements on the nitrogen and hydrogen solubility in iron, cobalt, and nickel base alloys. Steel Res. 1986, 57, 546–551. [Google Scholar] [CrossRef]
- Pettifor, D.G. Theoretical predictions of structure and related properties of intermetallics. Mater. Sci. Tech. 1992, 8, 345–349. [Google Scholar] [CrossRef]
- Morinaga, M.; Yukawa, N.; Adachi, H. Alloying effect on the electronic structure of Ni3Al. J. Phys. Soc. Jpn. 1984, 53, 653–663. [Google Scholar] [CrossRef]
- Pettifor, D.G. Structure maps for pseudobinary and ternary phases. Mater.Sci. Tech. 1988, 4, 675–691. [Google Scholar] [CrossRef]
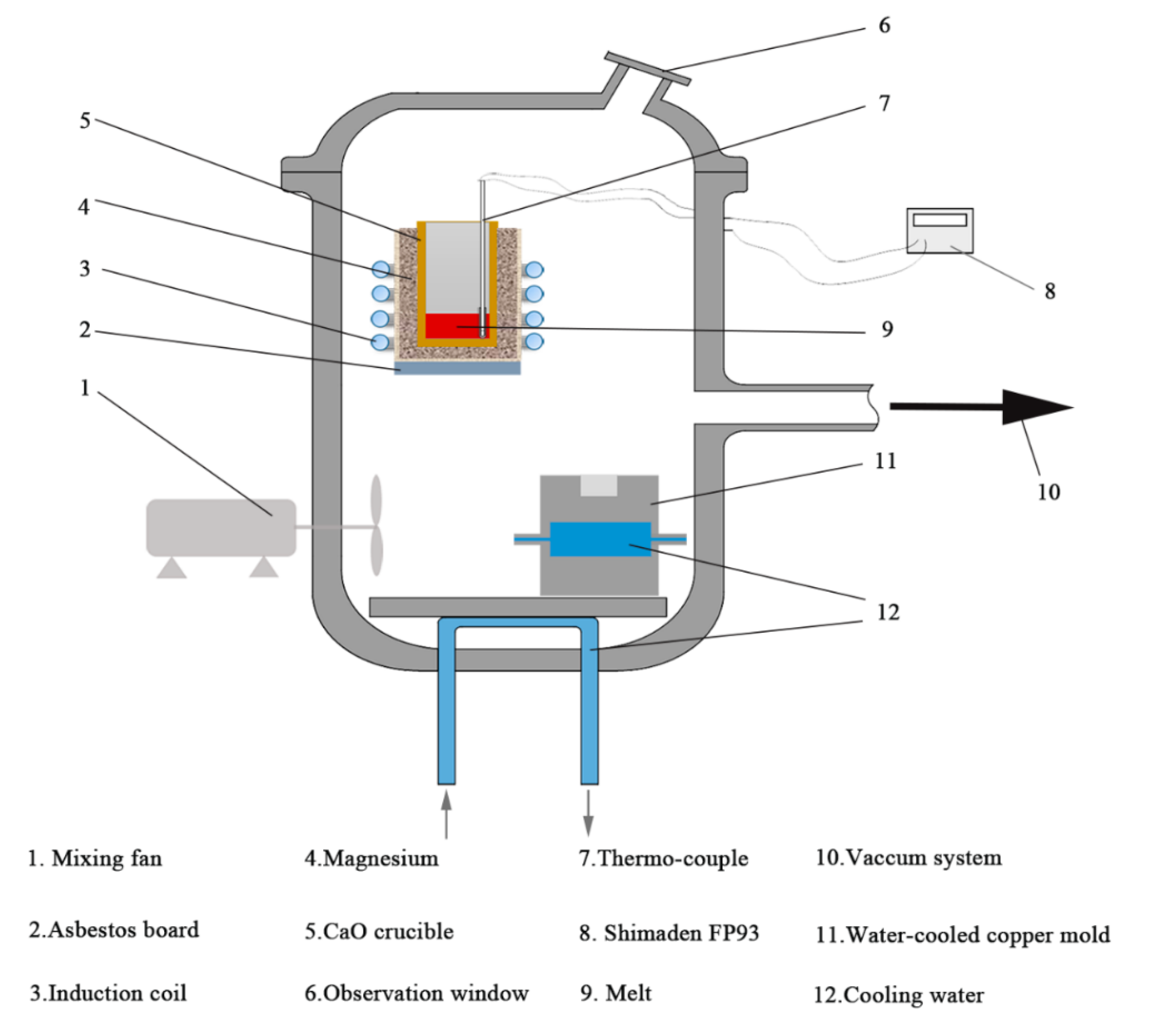

| Material | Purity (wt.%) |
|---|---|
| Electrolytic nickel bead | 99.99 |
| Electrolytic chromium | 99.99 |
| Electrolytic vanadium | 99.95 |
| Electron beam tantalum | 99.99 |
| Ni-V | Ni-Ta | Ni-Cr-V | Ni-Cr-Ta |
|---|---|---|---|
| Ni-1.04V | Ni-4.67Ta | Ni-9.98Cr-1.03V | Ni-9.83Cr-2.92Ta |
| Ni-2.97V | Ni-9.81Ta | Ni-9.75Cr-2.97V | Ni-9.86Cr-5.31Ta |
| Ni-4.90V | Ni-14.80Ta | Ni-19.94Cr-0.99V | Ni-19.81Cr-2.87Ta |
| Ni-7.01V | Ni-19.9Ta | Ni-19.76Cr-2.98V | Ni-19.79Cr-3.98Ta |
| Year | Investigators | N × 10−4 (wt.%) | Experimental Method | Ref. |
|---|---|---|---|---|
| 1959 | Schenck et al. | 25 | CPSM | [17] |
| 1960 | Humbert and Elliott | 5 to 12.1 | CPSM | [39] |
| 1965 | Stomakhin et al. | 12.6 | CPSM | [34] |
| 1966 | Blossey and Pehlke | 13 | CPSM | [35] |
| 1968 | Wada et al. | 13 | LMT | [38] |
| 1968 | Fedorchenko et al. | 15 | CPSM | [32] |
| 1970 | Lange and Schenck | 14.4 | SM | [37] |
| 1975 | Kojima et al. | 13 to 35 | SM | [36] |
| 1977 | Wada and Pehlke | 5.3 | SM | [40] |
| 1987 | Kim and McLean | 11.4 | SM | [33] |
| 2001 | Abdulrahman et al. | 20 | CPSM | [14] |
| 2003 | Kowanda et al. | 12 | SM | [16] |
| 2006 | Siwka | 9 | SM | [15] |
| This study | 10 | SM |
| Alloy | Ks | r2 | Alloy | Ks | r2 |
|---|---|---|---|---|---|
| Ni-1.04V | 0.0022 | 0.976 | Ni-9.98Cr-1.03V | 0.0147 | 0.999 |
| Ni-2.97V | 0.0042 | 0.982 | Ni-9.75Cr-2.97V | 0.0245 | 0.999 |
| Ni-4.90V | 0.0084 | 0.992 | Ni-19.94Cr-0.99V | 0.0647 | 0.997 |
| Ni-7.01V | 0.0147 | 0.999 | Ni-19.76Cr-2.98V | 0.0962 | 0.999 |
| Ni-4.67Ta | 0.0020 | 0.993 | Ni-9.83Cr-2.92Ta | 0.0130 | 0.999 |
| Ni-9.81Ta | 0.0035 | 0.998 | Ni-9.86Cr-5.31Ta | 0.0162 | 0.999 |
| Ni-14.80Ta | 0.0054 | 0.999 | Ni-19.81Cr-2.87Ta | 0.0602 | 0.999 |
| Ni-19.9Ta | 0.0083 | 0.999 | Ni-19.79Cr-3.98Ta | 0.0625 | 0.999 |
| Alloy System | −log fN,1873k | (J/mol) | (J/mol) | |
|---|---|---|---|---|
| Ni-V | Ni-1.04V | 0.1524 | −3666 | 52.56 |
| Ni-2.97V | 0.4833 | −4535 | 45.76 | |
| Ni-4.9V | 0.7943 | −6837 | 38.58 | |
| Ni-7.01V | 1.1173 | −7528 | 32.02 | |
| Ni-Cr-V | Ni-9.98Cr-1.03V | 1.1591 | 1070 | 35.81 |
| Ni-9.75Cr-2.97V | 1.3892 | 1530 | 31.65 | |
| Ni-19.94Cr-0.99V | 1.7924 | 4659 | 25.61 | |
| Ni-19.76Cr-2.98V | 1.9818 | 3443 | 21.33 | |
| Ni-Ta | Ni-4.67Ta | 0.2553 | −9496 | 47.47 |
| Ni-9.81Ta | 0.5185 | −10484 | 41.91 | |
| Ni-14.8Ta | 0.7324 | −7764 | 39.27 | |
| Ni-19.9Ta | 0.9345 | −5762 | 36.46 | |
| Ni-Cr-Ta | Ni-9.83Cr-2.92Ta | 1.1461 | 3893 | 37.57 |
| Ni-9.86Cr-5.31Ta | 1.2305 | 1569 | 34.71 | |
| Ni-19.81Cr-2.87Ta | 1.8129 | 3291 | 24.48 | |
| Ni-19.79Cr-3.98Ta | 1.8228 | 3222 | 24.26 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, K.; Chen, B.; Shu, L.; Liu, K. Nitrogen Solubility in Liquid Ni-V, Ni-Ta, Ni-Cr-V, and Ni-Cr-Ta Alloys. Metals 2019, 9, 1184. https://doi.org/10.3390/met9111184
Qian K, Chen B, Shu L, Liu K. Nitrogen Solubility in Liquid Ni-V, Ni-Ta, Ni-Cr-V, and Ni-Cr-Ta Alloys. Metals. 2019; 9(11):1184. https://doi.org/10.3390/met9111184
Chicago/Turabian StyleQian, Kun, Bo Chen, Lei Shu, and Kui Liu. 2019. "Nitrogen Solubility in Liquid Ni-V, Ni-Ta, Ni-Cr-V, and Ni-Cr-Ta Alloys" Metals 9, no. 11: 1184. https://doi.org/10.3390/met9111184




