Metallothermic Al-Sc Co-Reduction by Vacuum Induction Melting Using Ca
Abstract
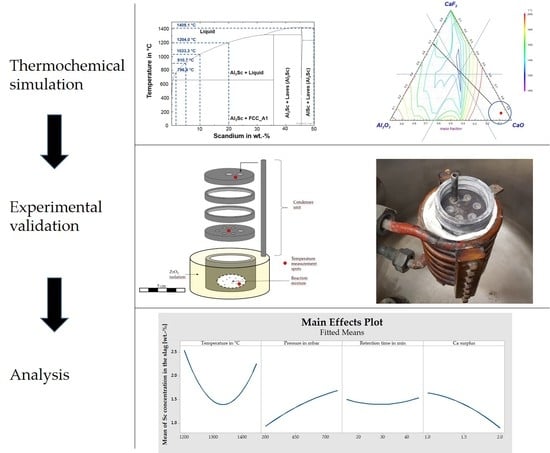
:1. Introduction
2. Materials and Methods
2.1. Thermochemical Considerations
2.1.1. Al-Sc Alloys
2.1.2. Slag Design
2.2. Materials
- Sc extraction yield is at 100%,
- The resulting slag phase has a liquidus temperature of <1400 C,
- Minimum Ca contents are expected in the metallic matrix.
2.3. Experimental Procedure
3. Results
3.1. Thermogravimetric Analysis
3.2. Al-Sc Synthesis via VIM
3.3. Interdependencies of Parameters
4. Discussion
- The co-reduction of AlO and ScF with Ca results in the phase separation of a slag and a metal. Tantalum crucibles are not practicable due to the strong attack by the resulting metal phase.
- SEM-EDS analysis of the slag and metal samples confirms the synthesis of an oxyfluoridic slag; Al-Sc with 32.5 wt.-% Sc is found in fragments.
- In the chosen experimental setting, the contamination of the metal sample with Ta and Ca is too excessive to be used directly for alloying processes. However, the Sc concentration without impurities amounts to 4.68 wt.-% on average, which is above the concentration for commercial Al-Sc master alloys (2 wt.-% ). A refining step needs to be investigated in order to remove the impurities by distillation/sublimation.
- Temperature, pressure, and retention time strongly affect the Sc content remaining in the slag.
- Gas–solid reactions between the evolving Ca and the feedstock are found to be the major reaction mechanism. This assumption is supported by the results from TG analysis, as well as the preference of low pressures (200 mbar) within the system.
- The low concentration of Al in the metal phase, as well as the F concentration that was found to be much smaller compared to the thermochemical calculations, which might indicate an evaporation of AlF that was not taken into account within the thermochemical considerations.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectometry |
| IC | Ion Chromatography |
| SEM-EDS | Scanning Electron Microscopy-Energy Dispersive X-Ray Spectroscopy |
| FCC | Fray–Farthing–Chen–Cambridge |
| VIM | Vacuum Induction Melting |
| TG | Thermogravimetric |
References
- Lathabai, S.; Lloyd, P.G. The effect of scandium on the microstructure, mechanical properties and weldability of a cast Al-Mg alloy. Acta Mater. 2002, 50, 4275–4292. [Google Scholar] [CrossRef]
- Parker, B.A.; Zhou, Z.F.; Nolle, P. The effect of small additions of scandium on the properties of aluminium alloys. J. Mater. Sci. 1995, 30, 452–458. [Google Scholar] [CrossRef]
- Røyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Zakharov, V.V.; Fisenko, I.A. Alloying Aluminum Alloys with Scandium. Metal Sci. Heat Treat. 2017, 59. [Google Scholar] [CrossRef]
- Hfijek, B.; Karen, P.; Bro, V. Studies on Hydrolyzable Carbides. XXII: The Carbothermal Reduction of Scandium Oxide Sc2O3. Monatshefte für Chemie 1986, 1278, 1271–1278. [Google Scholar]
- Halmann, M.; Steinfeld, A.; Epstein, M.; Vishnevetsky, I. Vacuum carbothermic reduction of alumina. Miner. Process. Extr. Metall. Rev. 2014, 35, 126–135. [Google Scholar] [CrossRef]
- Fischer, W.; Brünger, K.; Grieneisen, H. Über das metallische Scandium. Zeitschrift fur Anorganische und Allgemeine Chemie 1937, 231, 54–62. [Google Scholar] [CrossRef]
- Spedding, F.H.; Daane, A.H.; Wakefield, G.; Dennison, D.H. Preparation and Properties of High Purity Scandium Metal. Trans. Metall. Soc. AIME 1960, 218, 608–611. [Google Scholar]
- Xu, C.; Liu, X.; Ma, F.; Wang, Z.; Wang, W.; Ma, C. Preparation Of Al-Sc Master Alloy By Aluminothermic Reaction With Special Molten Salt. In Proceedings of the 13th International Conference on Aluminum Alloys (ICAA13), TMS (The Minerals, Metals & Materials Society), Pittsburgh, PA, USA, 3–7 June 2012; pp. 195–200. [Google Scholar]
- Mukhachov, A.P.; Kharitonova, E.A.; Skipochka, D.G. Scandium and its alloys with aluminum. Probl. At. Sci. Technol. 2016, 101, 45–50. [Google Scholar]
- Sokolova, Y.V.; Pirozhenko, K.Y.; Makhov, S.V. Concentration of scandium during processing the sublimate of production of the aluminum-scandium master alloy. Russ. J. Non-Ferrous Met. 2015, 56, 10–14. [Google Scholar] [CrossRef]
- Harata, M.; Nakamura, T.; Yakushiji, H.; Okabe, T.H. Production of scandium and Al-Sc alloy by metallothermic reduction. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2008, 117, 95–99. [Google Scholar] [CrossRef]
- Shinmei, M.; Machida, T. Vaporization of AlF3 from the Slag CaF2-Al2O3. Metall. Trans. 1973, 4, 1996–1997. [Google Scholar] [CrossRef]
- Dyke, J.; Kirby, C.; Morris, A.; Gravenor, B. A Study of Aluminium Monofluoride and Aluminium Trifluoride by High-Temperature Photoelectron Spectroscopy. Chem. Phys. 1984, 88, 289–298. [Google Scholar] [CrossRef]










| Targeted Sc Concentrations in wt.-% | Sc in wt.-% | Al in wt.-% | Ca in wt.-% |
|---|---|---|---|
| 2 | 1.8 | 75.9 | 22.3 |
| 5 | 4.4 | 74.3 | 21.3 |
| 10 | 9.0 | 72.2 | 18.9 |
| 20 | 18.5 | 68.2 | 13.3 |
| 50 | 42.8 | 49.0 | 8.3 |
| Compound | ScF | AlO | CaF | Ca |
|---|---|---|---|---|
| Weight factor | 1.00 | 6.33 | 7.00 | 4.52 |
| ScF | NaO | AlO | SiO | PO | CaO | FeO | CuO | SO |
|---|---|---|---|---|---|---|---|---|
| 99.584% | 0.113% | 0.122% | 0.047% | 0.005% | 0.014% | 0.021% | 0.013% | 0.080% |
| Material | >90 m | <90 m | <63 m | <45 m | total |
| ScF | 37% | 7% | 14% | 37% | 95% |
| CaF | 11% | 6% | 2% | 75% | 94% |
| AlO | 48% | 37% | 7% | 1% | 93% |
| >2.5 mm | <2.5 mm | <2 mm | |||
| Ca | 54% | 31% | 14% |
| Temperature in C | Pressure in mbar | Retention Time in min | Ca Surplus in % |
|---|---|---|---|
| 1200–1450 | 200–800 | 15–45 | 100–200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinkmann, F.; Mazurek, C.; Friedrich, B. Metallothermic Al-Sc Co-Reduction by Vacuum Induction Melting Using Ca. Metals 2019, 9, 1223. https://doi.org/10.3390/met9111223
Brinkmann F, Mazurek C, Friedrich B. Metallothermic Al-Sc Co-Reduction by Vacuum Induction Melting Using Ca. Metals. 2019; 9(11):1223. https://doi.org/10.3390/met9111223
Chicago/Turabian StyleBrinkmann, Frederic, Carolin Mazurek, and Bernd Friedrich. 2019. "Metallothermic Al-Sc Co-Reduction by Vacuum Induction Melting Using Ca" Metals 9, no. 11: 1223. https://doi.org/10.3390/met9111223
APA StyleBrinkmann, F., Mazurek, C., & Friedrich, B. (2019). Metallothermic Al-Sc Co-Reduction by Vacuum Induction Melting Using Ca. Metals, 9(11), 1223. https://doi.org/10.3390/met9111223