Synthesis and Characterization of Hollow Glass Sphere Containing Aluminum Syntactic Foam by Spark Plasma Sintering and Hot Pressing
Abstract
:1. Introduction
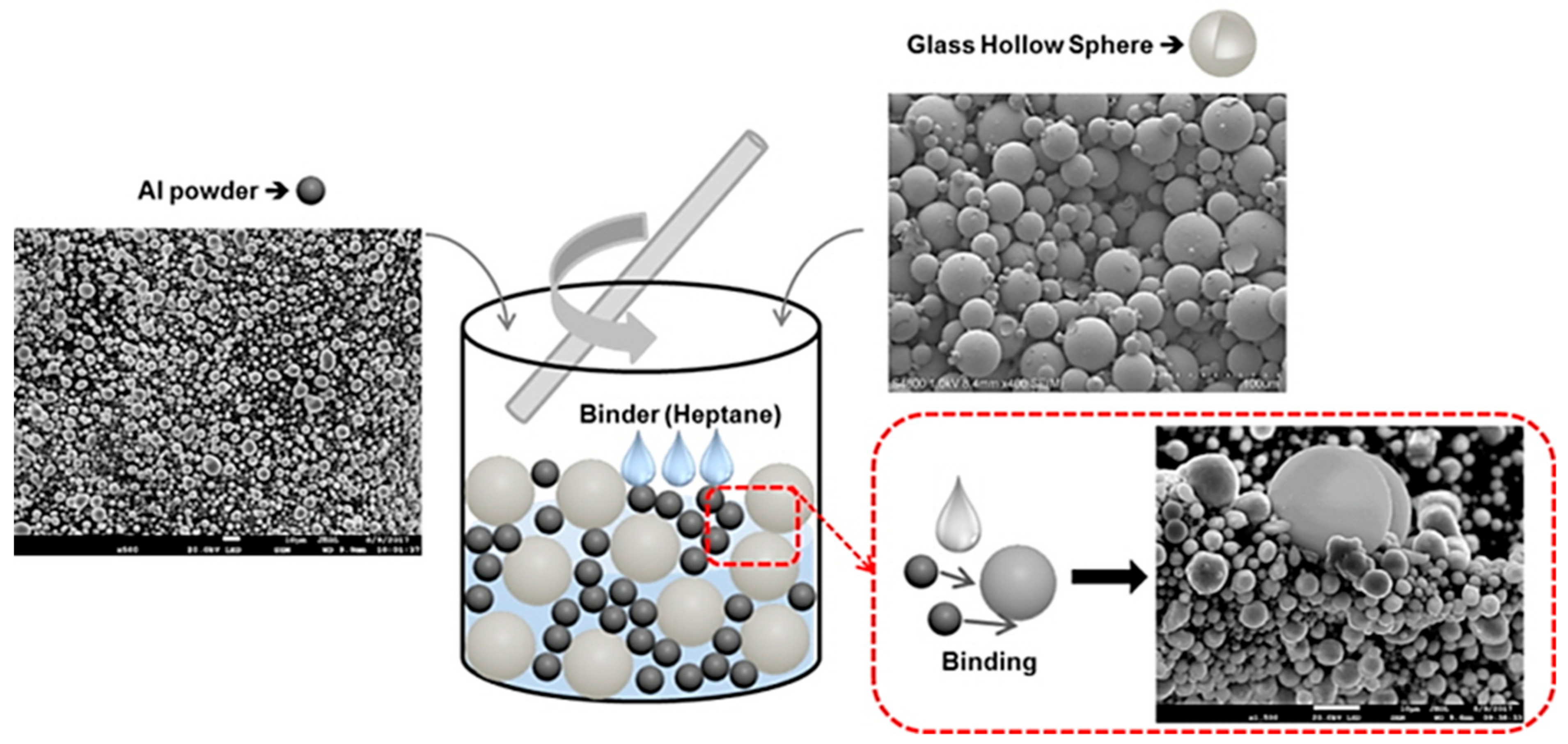
2. Experimental Procedure
- (1)
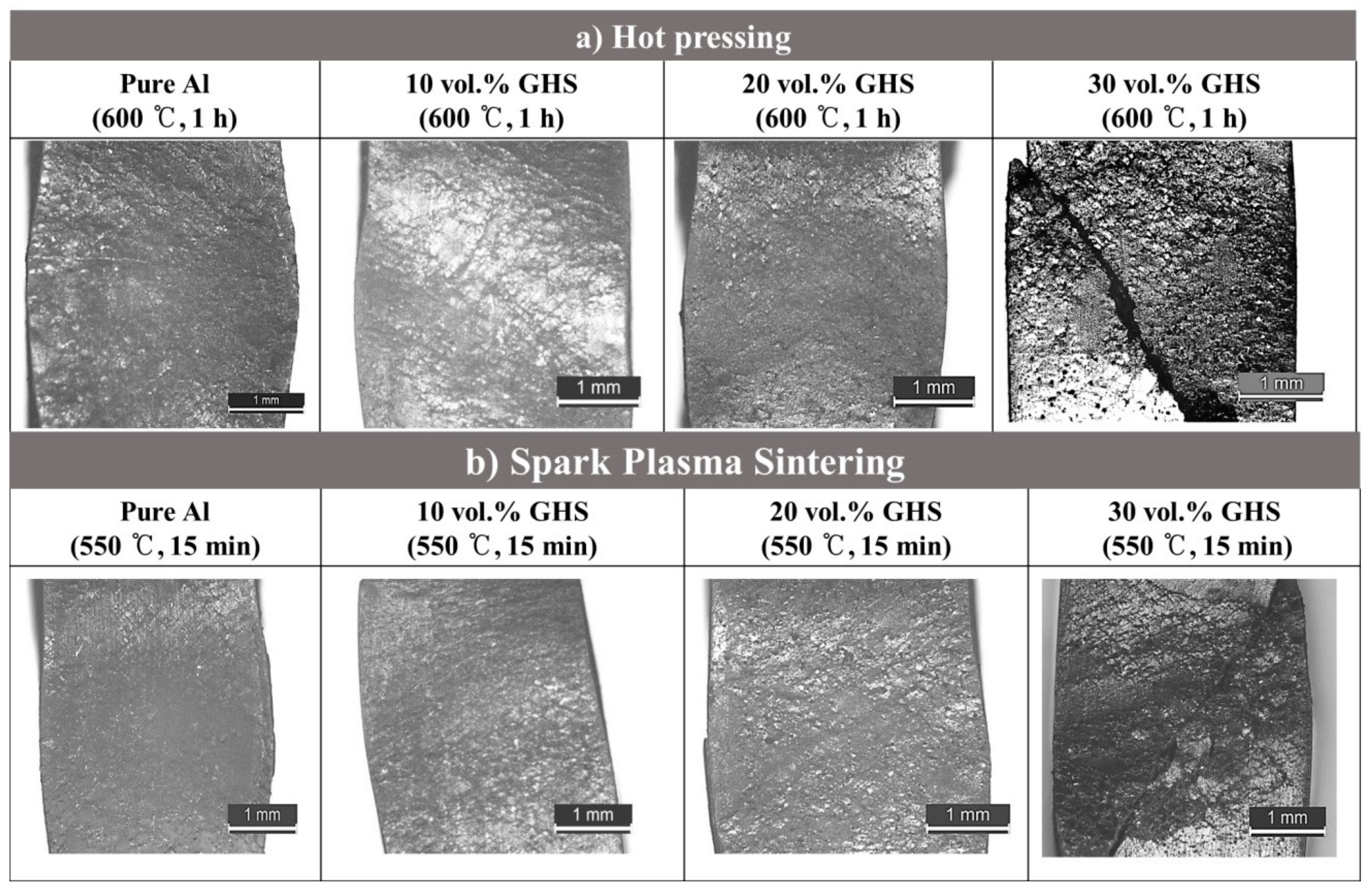
- Among the temperature range tested, the sintering temperature of 600 °C was the best for the HP process. When the sintering temperature is lowered to 550 °C, there was not enough bonding between the Al powders during the sintering process. Significant damage of the GHS occurred when higher sintering temperature of 650 °C was used.
- (2)
- In the case of the SPS process, both the sintering temperature and the holding time could be significantly reduced compared to the HP process. The sintering temperature of 550 °C with holding time of 15min was the best for the SPS process. Increasing the holding time to 30 min when keeping sintering temperature, the same significantly increased the final density of the foams, due to the softening of the GHS. Similar behavior was observed when the sintering temperature was raised to 600 °C.
3. Results and Discussions
4. Conclusions
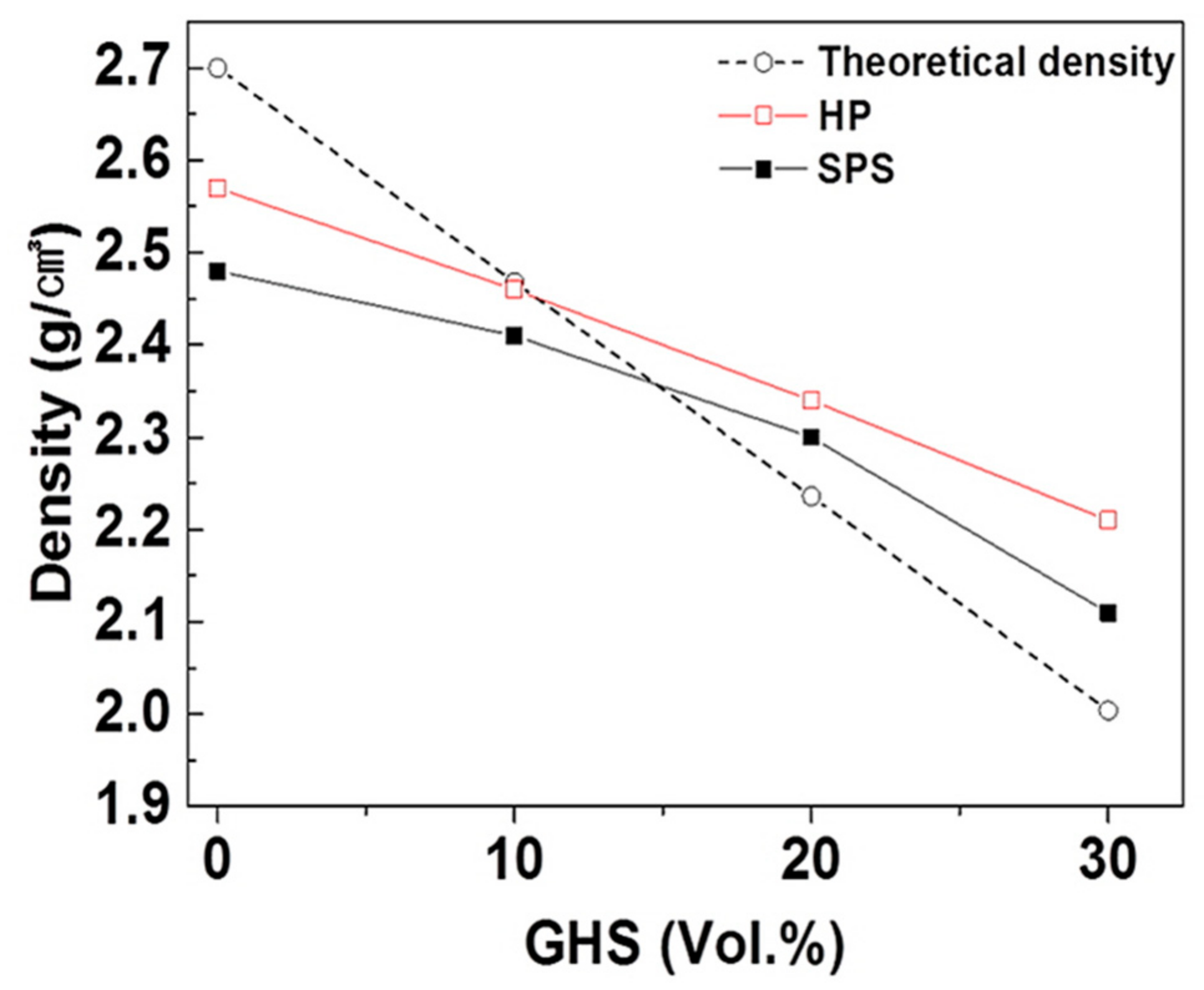
- By pressurized sintering processes of hot pressing and spark plasma sintering, aluminum syntactic foams with volume fractions of glass hollow microspheres up to 30% could be successfully fabricated. The pressurized sintering processes were characterized by short sintering time and low sintering temperatures. In the case of hot pressing, fully dense foams could be fabricated at a sintering temperature of 600 °C with a sintering time of 1 h. Spark plasma sintering process enabled fabricating near fully dense foams with even lower sintering temperature of 550 °C and very short sintering time of 15 min.
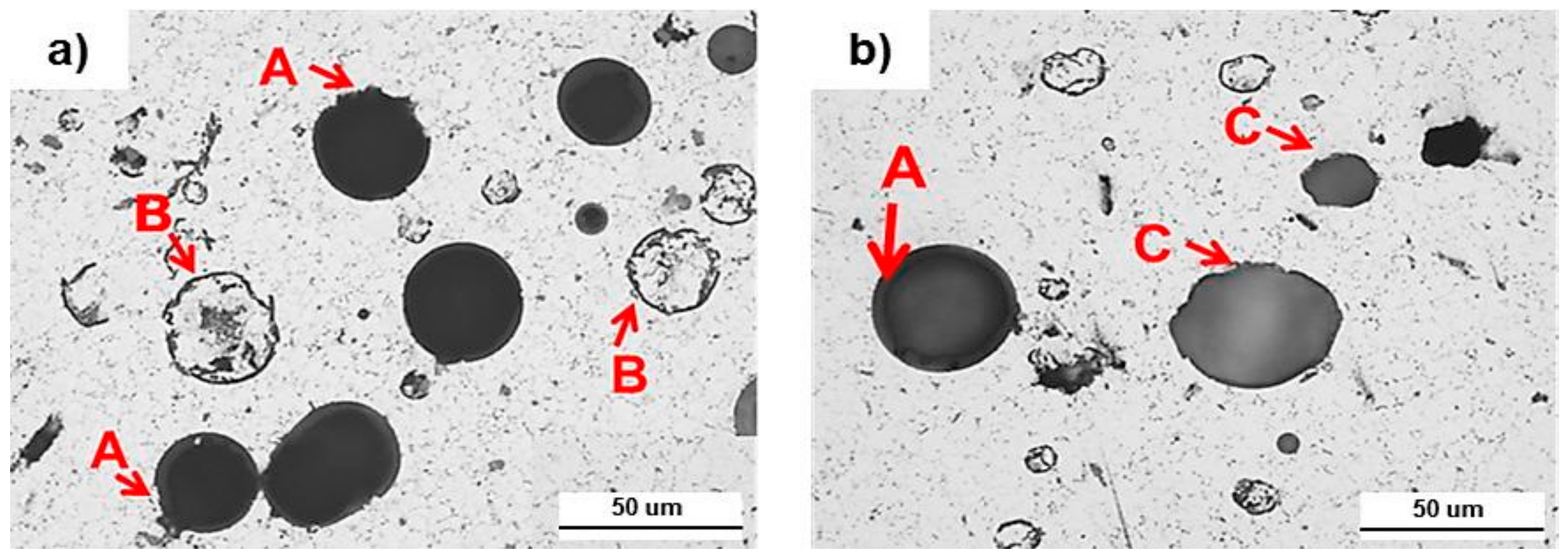
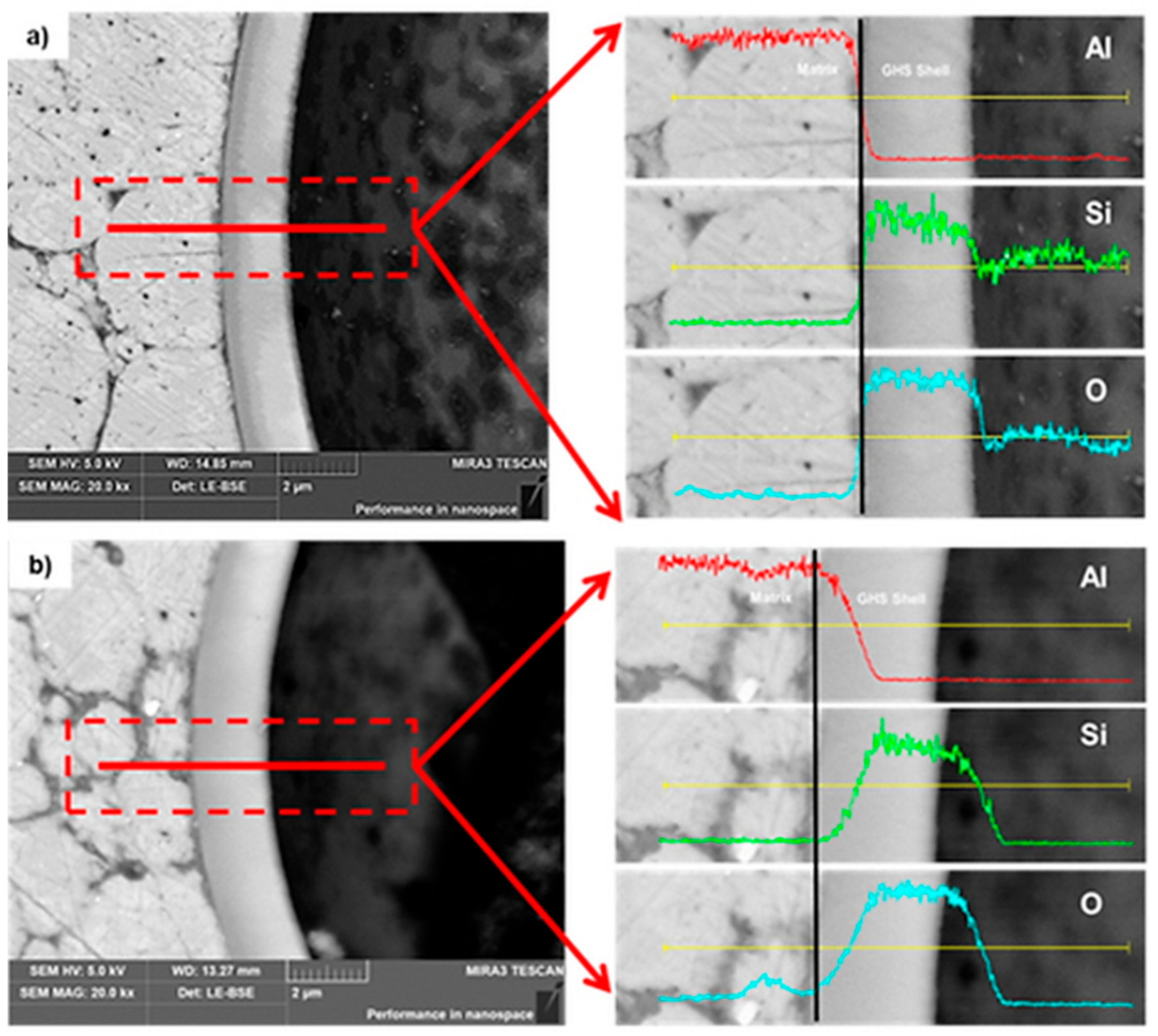
- In the microstructure, some fractured microspheres were observed during the process in both the hot pressing and spark plasma sintering. The fracture of the microspheres seems to be occurred by the pressure applied during the sintering processes. Unbroken microspheres in the hot pressed foam remained in spherical shape, while those in the spark plasma sintered foam became ellipsoidal, due to softening of the glass shell. The deformation of the microsphere in the spark plasma sintered foam is probably, due to local temperature increments around the glass hollow spheres during the sintering process by the plasma.
- In the case of hot pressing, there was almost no chemical interaction on interfaces between the aluminum matrix and the microspheres. Whereas, the pronounced diffusional process occurred at the interfaces in the foams fabricated by the spark plasma sintering even though lower sintering temperature than the hot pressing was used. This result indicates that the aluminum syntactic foams fabricated by spark plasma sintering has a stronger interfacial strength of the reaction layer than the aluminum syntactic foams fabricated by hot pressing process, due to diffusion bonding.
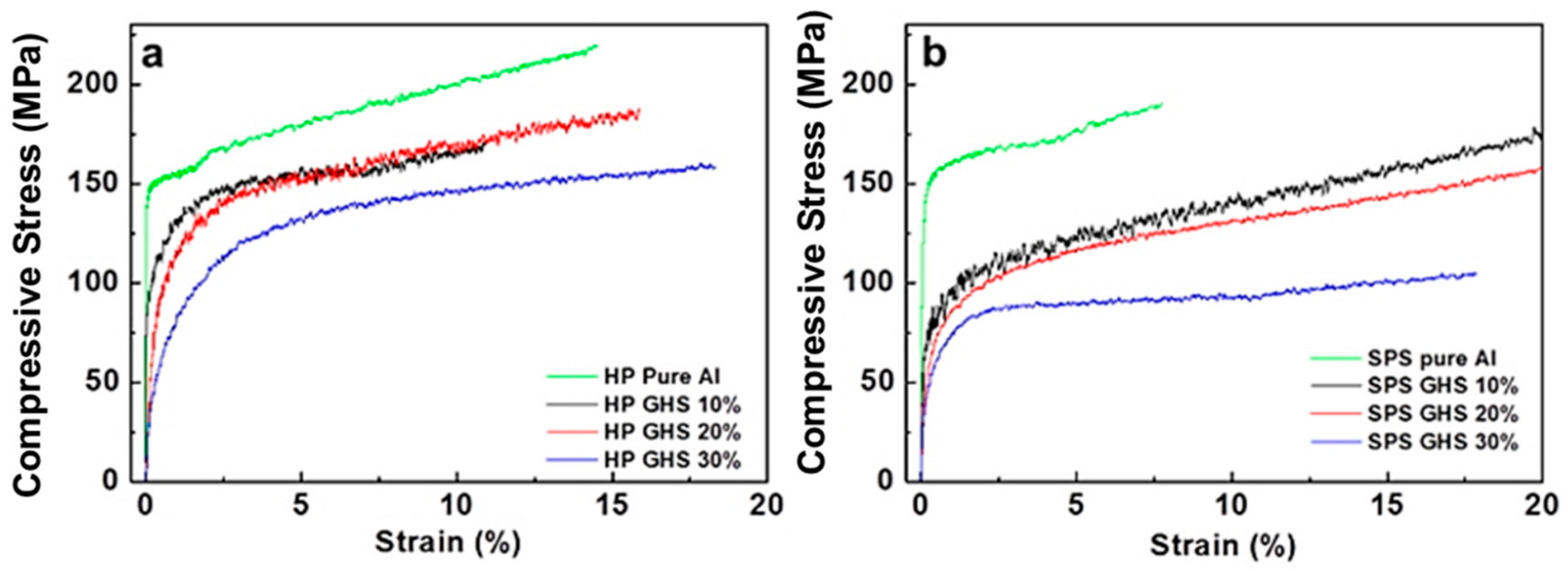
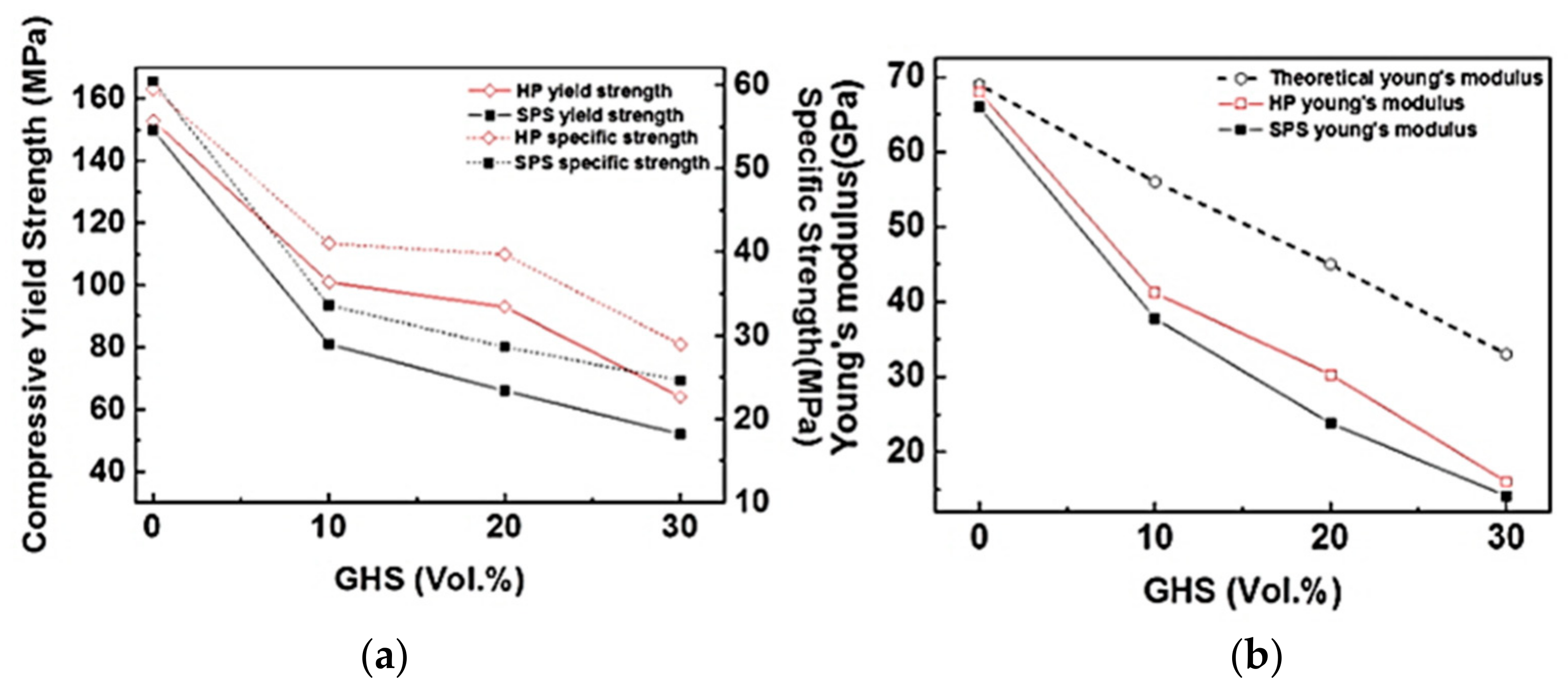
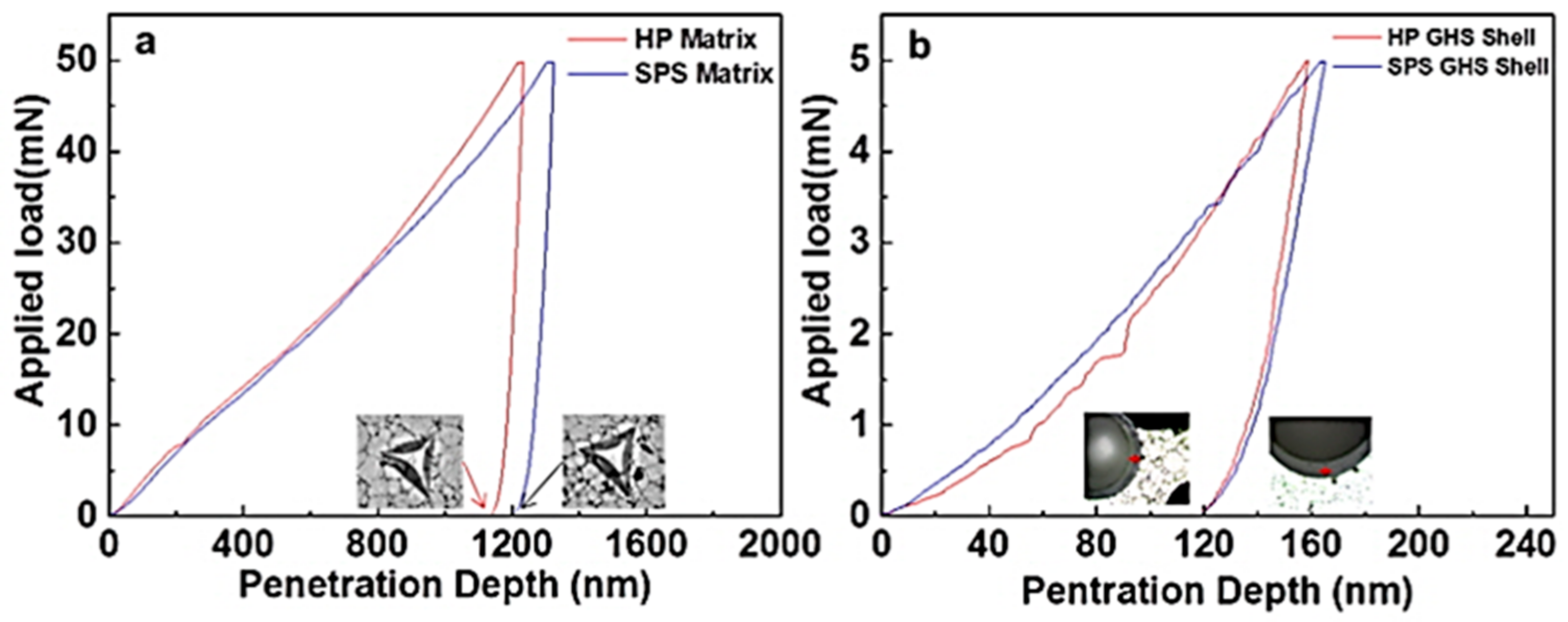
- The aluminum syntactic foams fabricated by spark plasma sintering process had slightly lower hardness and compressive strength than the foams fabricated by hot pressing. Nanoindentation results showed that the main reason for the lower strength of the spark plasma sintered foam is lower strength of aluminum matrix in the spark plasma sintered foam than in the hot pressed one. On the other hand, the maximum compressive strain was higher in the spark plasma sintered foam than those fabricated by hot pressing process, due to the strong chemical bonding at the interfaces between the microsphere and the aluminum matrix.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, N.; Rohatgi, P.K. Metal. Matrix Syntactic Foams: Processing, Microstructure, Properties and Applications; DEStech Publications, Inc.: Lancaster, PA, USA, 2015. [Google Scholar]
- Singh, N.P.; Purgert, R.M.; Sobczak, J. Aluminum Syntactic Foams Alfa for Automotive Applications. J. KONES Intern. Combust. Engines 2003, 10, 3–4. [Google Scholar]
- Tao, X.F.; Zhao, Y.Y. Compressive behavior of Al matrix syntactic foams toughened with Al particles. Scr. Mater. 2009, 61, 461–464. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Q.; Wu, G. Interfacial microstructure and compressive properties of Al-Mg syntactic foam reinforced with glass cenospheres. J. Alloy. Compd. 2016, 655, 301–308. [Google Scholar] [CrossRef]
- Rajan, T.P.D.; Pillai, R.M.; Pai, B.C.; Satyanarayana, K.G.; Rohatgi, P.K. Fabrication and characterisation of Al-7Si-0.35Mg/fly ash metal matrix composites processed by different stir casting routes. Compos. Sci. Technol. 2007, 67, 3369–3377. [Google Scholar] [CrossRef]
- Hashim, J.; Looney, L.; Hashmi, M.S.J. Metal matrix composites: Production by the stir casting method. J. Mater. Process. Technol. 1999, 92, 1–7. [Google Scholar] [CrossRef]
- Mondal, D.P.; Das, S.; Ramakrishnan, N.; Bhasker, K.U. Cenosphere filled aluminum syntactic foam made through stir-casting technique. Compos. Part. A Appl. Sci. Manuf. 2009, 40, 279–288. [Google Scholar] [CrossRef]
- Akbari, M.K.; Mirzaee, O.; Baharvandi, H.R. Fabrication and study on mechanical properties and fracture behavior of nanometric Al2O3 particle-reinforced A356 composites focusing on the parameters of vortex method. Mater. Des. 2013, 46, 199–205. [Google Scholar] [CrossRef]
- Vogiatzis, C.A.; Skolianos, S.M. On the sintering mechanisms and microstructure of aluminium-ceramic cenospheres syntactic foams produced by powder metallurgy route. Compos. Part. A Appl. Sci. Manuf. 2016, 82, 8–19. [Google Scholar] [CrossRef]
- Guo, R.Q.; Rohatgi, P.K.; Nath, D. Preparation of aluminium-fly ash particulate composite by powder metallurgy technique. J. Mater. Sci. 1997, 32, 3971–3974. [Google Scholar] [CrossRef]
- Spratt, M.; Newkirk, J.W.; Chandrashekhara, K. Aluminum Matrix Syntactic Foam Fabricated with Additive Manufacturing. Solid Free Fabr. Symp. 2017, 242–248. [Google Scholar]
- Vogiatzis, C.A.; Tsouknidas, A.; Kountouras, D.T.; Skolianos, S. Aluminum-ceramic cenospheres syntactic foams produced by powder metallurgy route. Mater. Des. 2015, 85, 444–454. [Google Scholar] [CrossRef]
- Madhan, M.; Prabhakaran, G. Microwave versus conventional sintering: Microstructure and mechanical properties of Al2O3-SiC ceramic composites. Boletín de la Sociedad Española de Cerámica y Vidrio 2019, 58, 14–22. [Google Scholar] [CrossRef]
- Kwon, H.; Park, D.H.; Park, Y.; Silvain, J.F.; Kawasaki, A.; Park, Y. Spark plasma sintering behavior of pure aluminum depending on various sintering temperatures. Met. Mater. Int. 2010, 16, 71–75. [Google Scholar] [CrossRef]
- Borrell, A.; Salvador, M.D. Advanced Ceramic Materials Sintered by Microwave Technology. Sinter. Technol.-Method Appl. 2018, 3–24. [Google Scholar]
- Cho, Y.J.; Lee, T.S.; Lee, W.; Lee, Y.C.; Park, Y.H. Preparation and Characterization of Iron Matrix Syntactic Foams with Glass Microspheres via Powder Metallurgy. Met. Mater. Int. 2019, 25, 794–804. [Google Scholar] [CrossRef]
- Movahedi, N.; Taherishargh, M.; Belova, I.V.; Murch, G.E.; Fiedler, T. Mechanical and microstructural characterization of an AZ91-activated carbon syntactic foam. Materials (Basel) 2018, 12, 3. [Google Scholar] [CrossRef]
- Song, S.X.; Wang, Z.; Shi, G.P. Heating mechanism of spark plasma sintering. Ceram. Int. 2013, 39, 1393–1396. [Google Scholar] [CrossRef]
- Lemus-Ruiz, J.; Ceja-Cárdenas, L.; Verduzco, J.A.; Flores, O. Joining of tungsten carbide to nickel by direct diffusion bonding and using a Cu-Zn alloy. J. Mater. Sci. 2008, 43, 6296–6300. [Google Scholar] [CrossRef]
- Ratnaparkhit, P.L.; Howe, J.M. Structure and mechanism of bonding at a diffusion-bonded A1/SiC interface. Acta Metall. meterialia. 1994, 42, 811–823. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Tao, X.F. Behaviour of Metal Matrix Syntactic Foams in Compression. Mater. Sci. Technol. 2009, 4, 1785–1794. [Google Scholar]
- Porfiri, M.; Gupta, N. Effect of volume fraction and wall thickness on the elastic properties of hollow particle filled composites. Compos. Part. B Eng. 2009, 40, 166–173. [Google Scholar] [CrossRef]
- Luong, D.D.; Strbik, O.M.; Hammond, V.H.; Gupta, N.; Cho, K. Development of high performance lightweight aluminum alloy/SiC hollow sphere syntactic foams and compressive characterization at quasi-static and high strain rates. J. Alloy. Compd. 2013, 550, 412–422. [Google Scholar] [CrossRef]
- Zhang, L.P.; Zhao, Y.Y. Mechanical response of Al matrix syntactic foams produced by pressure infiltration casting. J. Compos. Mater. 2007, 41, 2105–2117. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Q.; Ma, X.; Wu, G. Mechanical behavior of pure Al and Al-Mg syntactic foam composites containing glass cenospheres. Compos. Part. A Appl. Sci. Manuf. 2016, 87, 194–202. [Google Scholar] [CrossRef]


| Sample Type (Vol.%) | Sintering Method | Sintering Temp (°C) | Holding Time | Sintering Behavior |
|---|---|---|---|---|
| GHS 30% | HP | 550 | 1 h | Poor bonding |
| 600 | Good | |||
| 650 | GHS Softening or Broken | |||
| SPS | 550 | 15 min | Good | |
| 550 | 30 min | GHS Softening or Broken | ||
| 600 | 15 min | GHS Softening or Broken |
| Powder Type | True Density (g/cm3) | Vol.% Glass | Vol.% Gas |
|---|---|---|---|
| GHS | 0.38 | 15 | 85 |
| Al | 2.7 | - | - |
| Material | Elastic Modulus (GPa) | Poisson’s Ratio | η | Shear Modulus (GPa) | Bulk Modulus (k) (GPa) |
|---|---|---|---|---|---|
| Matrix | 68 | 0.36 | - | 25 | 62 |
| GHS | 37.1 | 0.17 | 0.952 | 31.2 | 36.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.G.; Lee, Y.C.; Jung, S.S.; Kwon, H.S.; Lee, W.; Park, Y. Synthesis and Characterization of Hollow Glass Sphere Containing Aluminum Syntactic Foam by Spark Plasma Sintering and Hot Pressing. Metals 2019, 9, 1266. https://doi.org/10.3390/met9121266
Son YG, Lee YC, Jung SS, Kwon HS, Lee W, Park Y. Synthesis and Characterization of Hollow Glass Sphere Containing Aluminum Syntactic Foam by Spark Plasma Sintering and Hot Pressing. Metals. 2019; 9(12):1266. https://doi.org/10.3390/met9121266
Chicago/Turabian StyleSon, Yong Guk, Young Cheol Lee, Sung Su Jung, Han Sang Kwon, Wookjin Lee, and Yongho Park. 2019. "Synthesis and Characterization of Hollow Glass Sphere Containing Aluminum Syntactic Foam by Spark Plasma Sintering and Hot Pressing" Metals 9, no. 12: 1266. https://doi.org/10.3390/met9121266
APA StyleSon, Y. G., Lee, Y. C., Jung, S. S., Kwon, H. S., Lee, W., & Park, Y. (2019). Synthesis and Characterization of Hollow Glass Sphere Containing Aluminum Syntactic Foam by Spark Plasma Sintering and Hot Pressing. Metals, 9(12), 1266. https://doi.org/10.3390/met9121266





