Correlated Unique Variation of Electrical Resistivity to Crystallization Behavior of the Zr52.5Cu17.9Ni14.6Al10Ti5 Metallic Glass
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Variation of Electrical Resistivity during Crystallization
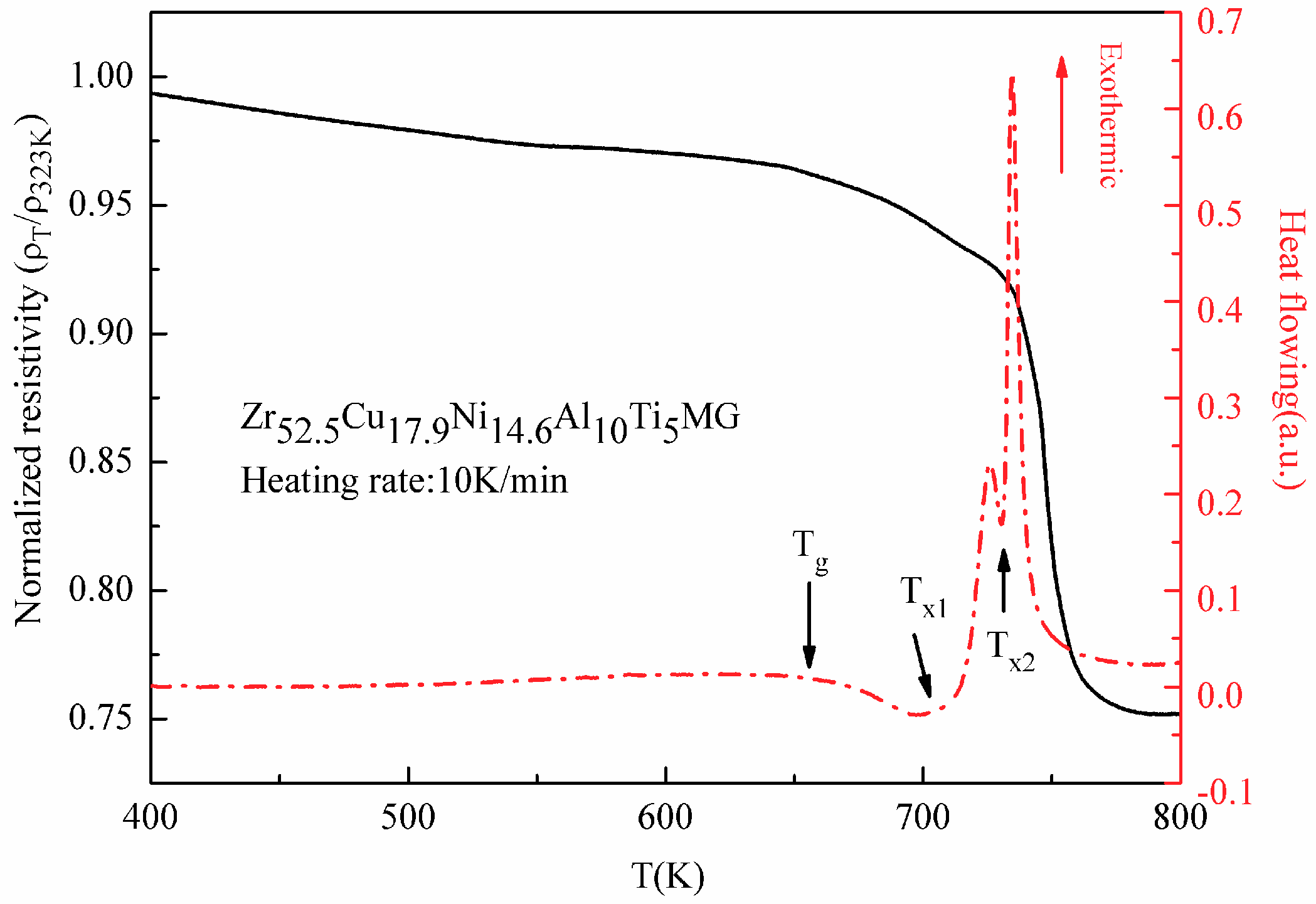
3.1.1. Variation of Electrical Resistivity under Isochronal Measurement
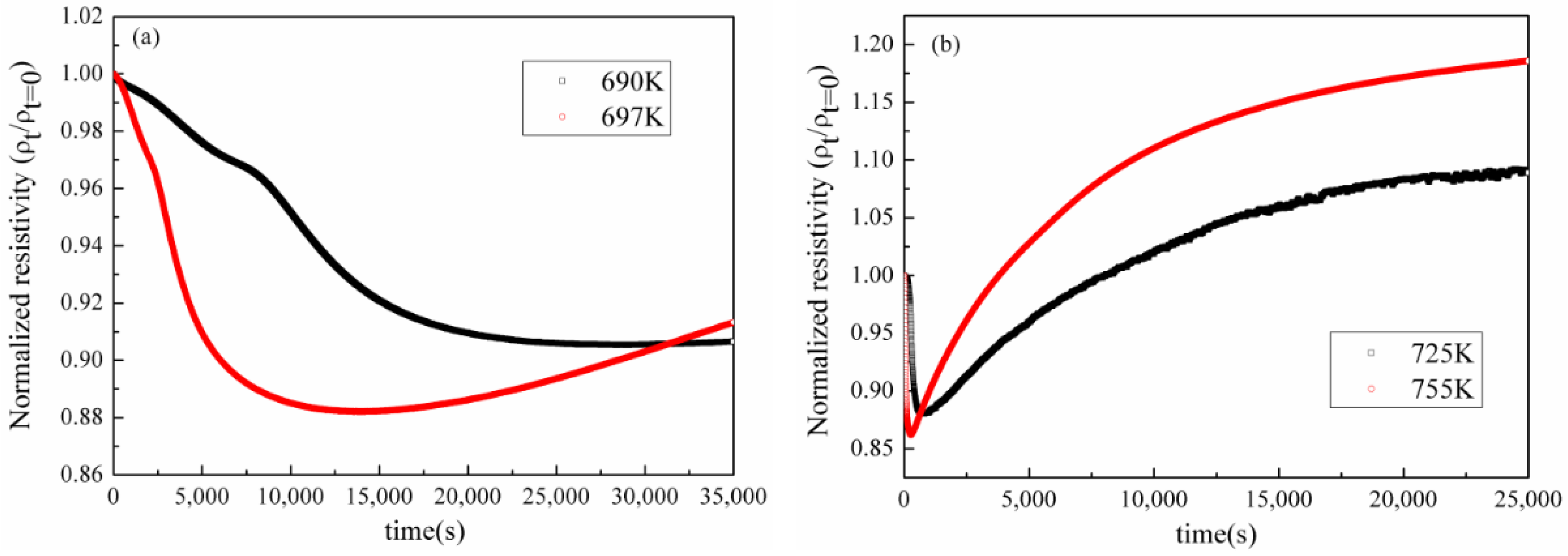
3.1.2. Variation of Electrical Resistivity under Isothermal Annealing
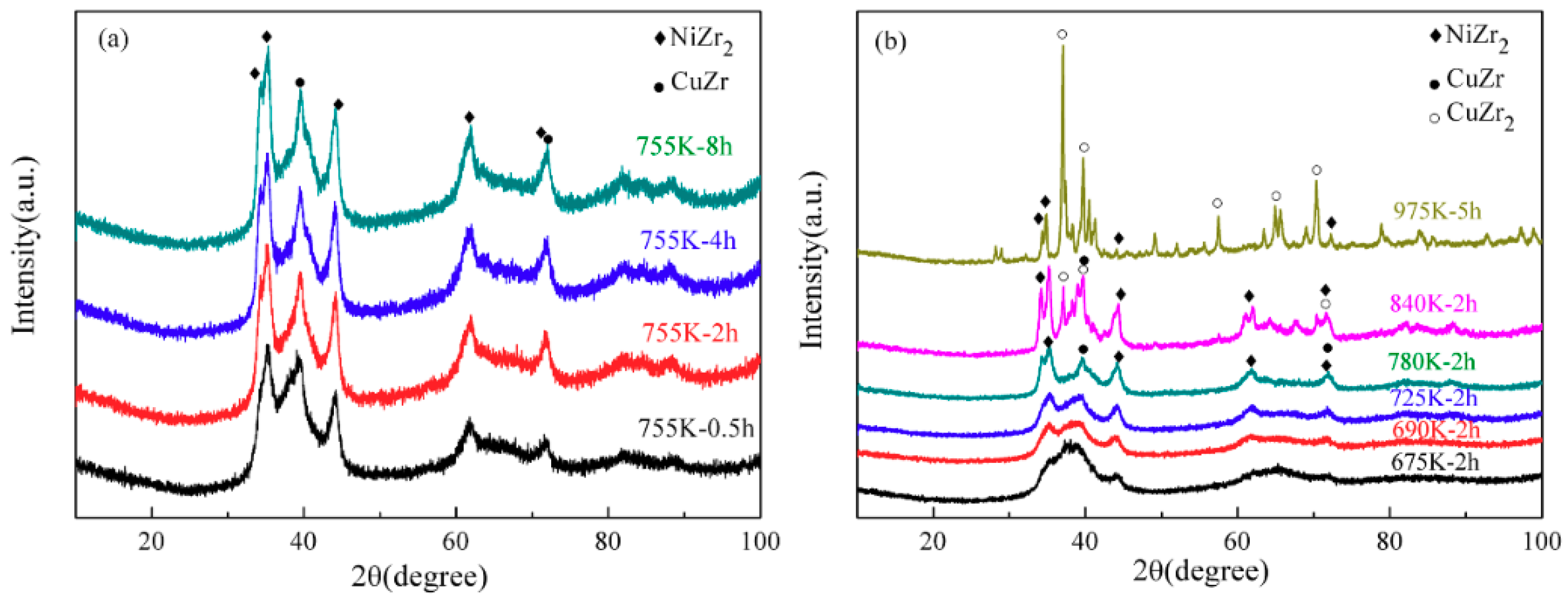
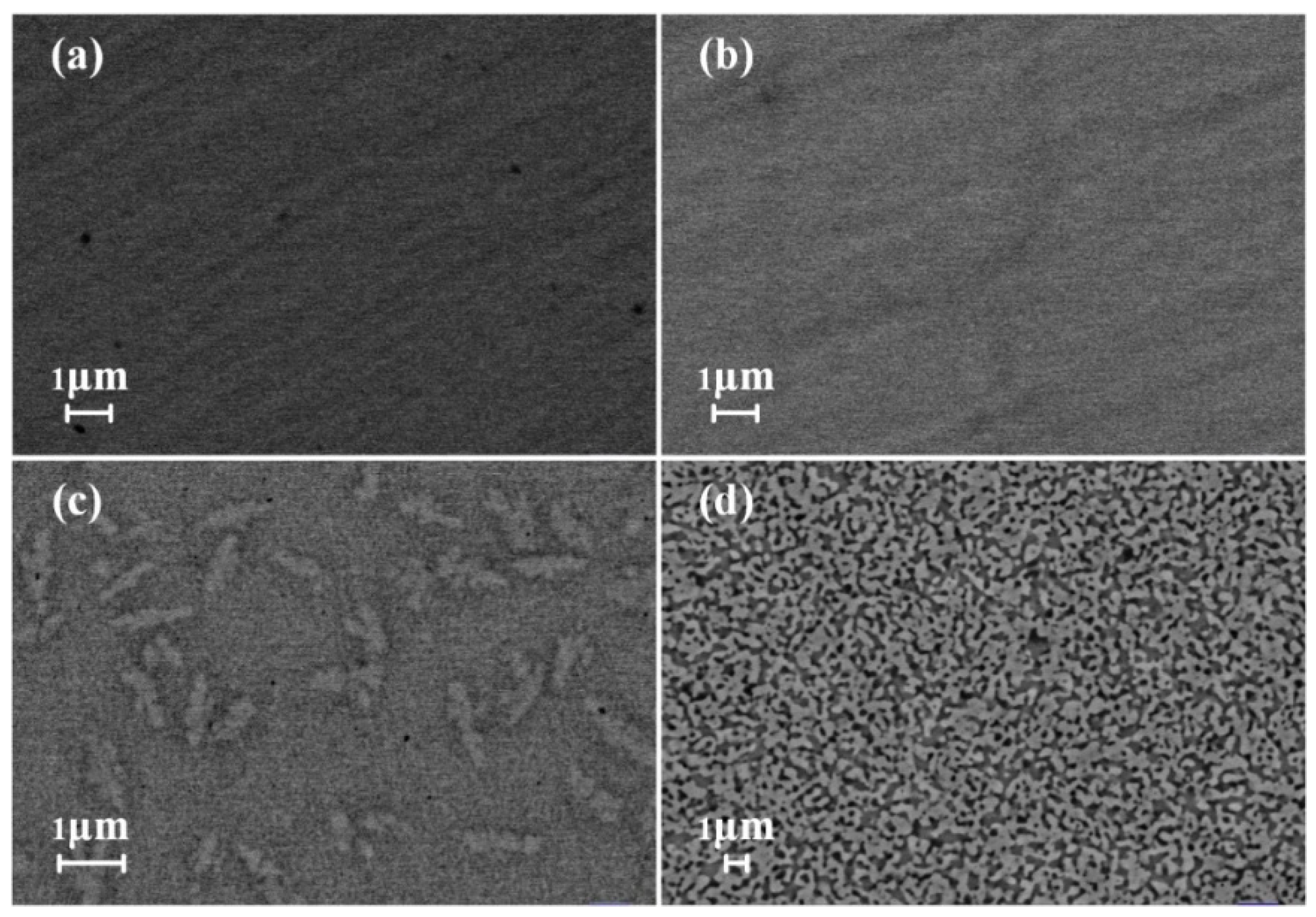
3.2. Phase Transformation of the Zr52.5Cu17.9Ni14.6Al10Ti5 MG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhuang, Y.X.; Duan, T.F.; Shi, H.Y. Calorimetric study of non-isothermal crystallization kinetics of Zr60Cu20Al10Ni10 bulk metallic glass. J. Alloys Compd. 2011, 509, 9019–9025. [Google Scholar] [CrossRef]
- Hu, L.; Ye, F. Crystallization kinetics of Ca65Mg15Zn20 bulk metallic glass. J. Alloys Compd. 2013, 557, 160–165. [Google Scholar] [CrossRef]
- Saida, J.; Matsushita, M.; Inoue, A. Transformation kenetics of nanoicosahedral phase from a supercooled liquid region in Zr70Pd30 binary glassy alloy. J. Appl. Phys. 2000, 88, 6081–6083. [Google Scholar] [CrossRef]
- Haruyama, O.; Miyazawa, T.; Saida, J.; Inoue, A. Change in electrical resistivity due to icosahedral phase precipitation in Zr70Pd20Ni10 and Zr65Al7.5Cu7.5Ni10Ag10 glasses. Appl. Phys. Lett. 2001, 79, 758–760. [Google Scholar] [CrossRef]
- Chung, S.J.; Hong, K.T.; Ok, M.R.; Yoon, J.K.; Kim, G.H.; Ji, Y.S.; Seong, B.S.; Lee, K.S. Analysis of the crystallization of Zr41Ti14Cu12.5Ni10Be22.5 bulk metallic glass using electrical resistivity measurement. Scrip. Mater. 2005, 53, 223–228. [Google Scholar]
- Young, S.J.; Chung, S.J.; Ok, M.R.; Hong, K.T.; Suh, J.Y.; Byeon, J.W.; Yoon, J.K.; Lee, K.H.; Lee, K.S. Analysis on the phase transition behavior of Cu base bulk metallic glass by electrical resistivity measurement. Mater. Sci. Eng. A 2007, 449, 521–525. [Google Scholar]
- Kim, Y.C.; Park, J.M.; Lee, J.K.; Bae, D.H.; Kim, W.T.; Kim, D.H. Amorphous and icosahedral phases in Ti–Zr–Cu–Ni–Be alloys. Mater. Sci. Eng. A 2004, 375, 749–753. [Google Scholar] [CrossRef]
- Kailath, A.J.; Dutta, K.; Alex, T.C.; Mitra, A. Crystallization study of Cu56Zr7Ti37 metallic glass by electrical resistivity measurement. J. Mater. Sci. Technol. 2011, 27, 275–279. [Google Scholar] [CrossRef]
- Kokanovice, I. Effect of disorder on the electrical resistivity in the partially crystalline Zr76Ni24 metallic glasses. J. Alloys Comp. 2006, 421, 12–18. [Google Scholar] [CrossRef]
- Haruyama, O.; Tando, M.; Nishiyama, N.; Kimura, H.M.; Inoue, A.; Arai, J. Electrical resistivity and Mössbauer studies for the structural relaxation process in Pd–Cu–Ni–P glasses. Mater. Trans. 2002, 43, 1931–1936. [Google Scholar] [CrossRef]
- Liu, B.B.; Zuo, N.N.; Ye, F. Abnormal change of electrical resistivity in the Cu46Zr46Al8 bulk metallic glass during crystallization. Mater. Lett. 2016, 171, 285–288. [Google Scholar] [CrossRef]
- Busch, R.; Schneider, S.; Peker, A.; Johnson, W.L. Decomposition and primary crystallization in undercooled Zr41.2Ti13.8Cu12.5Ni10.0Be22.5 melts. Appl. Phys. Lett. 1995, 67, 1544–1546. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.L.; Porter, W.D.; Lu, Z.P.; Stoica, A.D.; Payzart, E.A.; Almer, J.; Shi, D.L. Consecutive nucleation events during divetrification of Zr52.5Cu17.9Ni14.6Al10Ti5 bulk metallic glass. Adv. Eng. Mater. 2008, 10, 1043–1047. [Google Scholar] [CrossRef]
- Xing, L.Q.; Eckert, J.; Löser, W.; Schultz, L. Effect of cooling rate on the precipitation of quasicrystals from the Zr-Cu-Al-Ni-Ti amorphous alloy. Appl. Phys. Lett. 1998, 73, 2110–2112. [Google Scholar] [CrossRef]
- Mizutani, U. Electronic structure of metallic glasses. Prog. Mater. Sci. 1983, 28, 97–228. [Google Scholar] [CrossRef]
- Haruyama, O.; Tando, M.; Kimura, H.M.; Nishiyama, N.; Inoue, A. Structural relaxation in Pd–Cu–Ni–P metallic glasses. Mater. Sci. Eng. A 2004, 375–377, 292–296. [Google Scholar] [CrossRef]
- Bai, H.Y.; Tong, C.Z.; Zheng, P. Electrical resistivity in Zr48Nb8Cu12Fe8Be24 glassy and crystallized alloys. J. Appl. Phys. 2004, 95, 1269–1273. [Google Scholar] [CrossRef]
- Pekarskaya, E.; Löffler, J.F.; Johnson, W.L. Microstructural studies of crystallization of a Zr-based bulk metallic glass. Acta Mater. 2003, 51, 4045–4057. [Google Scholar] [CrossRef]
- Xing, L.Q.; Eckert, J.; Löser, W.; Schultz, L. High-strength materials produced by precipitation of icosahedral quasicrystals in bulk Zr-Ti-Cu-Ni-Al amorphous alloys. Appl. Phys. Lett. 1999, 74, 664–666. [Google Scholar] [CrossRef]
- Liu, B.B.; Ye, F. Low temperature heat capacity and electrical resistivity of the Ti40Zr25Cu12Ni3Be20 glass forming alloy. Intermetallics 2016, 75, 31–35. [Google Scholar] [CrossRef]
- Kelton, K.F.; Kim, W.J.; Stroud, R.M. A stable Ti-based quasicrystal. Appl. Phys. Lett. 1997, 70, 3230–3232. [Google Scholar] [CrossRef]
- Sachdev, S.; Nelson, D.R. Theory of the Structure Factor of Metallic Glasses. Phys. Rev. Lett. 1984, 53, 1947–1950. [Google Scholar] [CrossRef]
- Pierce, F.S.; Poon, S.J.; Guo, Q. Electron localization in metallic quasicrystals. Science 1993, 261, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, Q.D.; Li, X.Y.; Zu, F.Q. On crystallization behavior and thermal stability of Cu64Zr36 metallic glass by controlling the melt temperature. J. Non Cryst. Solids 2016, 452, 336–341. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Jiang, X.; Huo, G.; Zhang, Y.; Qiao, Y.; Ye, F.; Liu, B. Correlated Unique Variation of Electrical Resistivity to Crystallization Behavior of the Zr52.5Cu17.9Ni14.6Al10Ti5 Metallic Glass. Metals 2019, 9, 1298. https://doi.org/10.3390/met9121298
Zhang X, Jiang X, Huo G, Zhang Y, Qiao Y, Ye F, Liu B. Correlated Unique Variation of Electrical Resistivity to Crystallization Behavior of the Zr52.5Cu17.9Ni14.6Al10Ti5 Metallic Glass. Metals. 2019; 9(12):1298. https://doi.org/10.3390/met9121298
Chicago/Turabian StyleZhang, Xiao, Xin Jiang, Guangrui Huo, Yuxiang Zhang, Yi Qiao, Feng Ye, and Binbin Liu. 2019. "Correlated Unique Variation of Electrical Resistivity to Crystallization Behavior of the Zr52.5Cu17.9Ni14.6Al10Ti5 Metallic Glass" Metals 9, no. 12: 1298. https://doi.org/10.3390/met9121298
APA StyleZhang, X., Jiang, X., Huo, G., Zhang, Y., Qiao, Y., Ye, F., & Liu, B. (2019). Correlated Unique Variation of Electrical Resistivity to Crystallization Behavior of the Zr52.5Cu17.9Ni14.6Al10Ti5 Metallic Glass. Metals, 9(12), 1298. https://doi.org/10.3390/met9121298




