Effects of Mo Concentration on the Structural and Corrosion Properties of Cu–Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Sintered
2.3. Characterization
2.4. Corrosion
3. Results
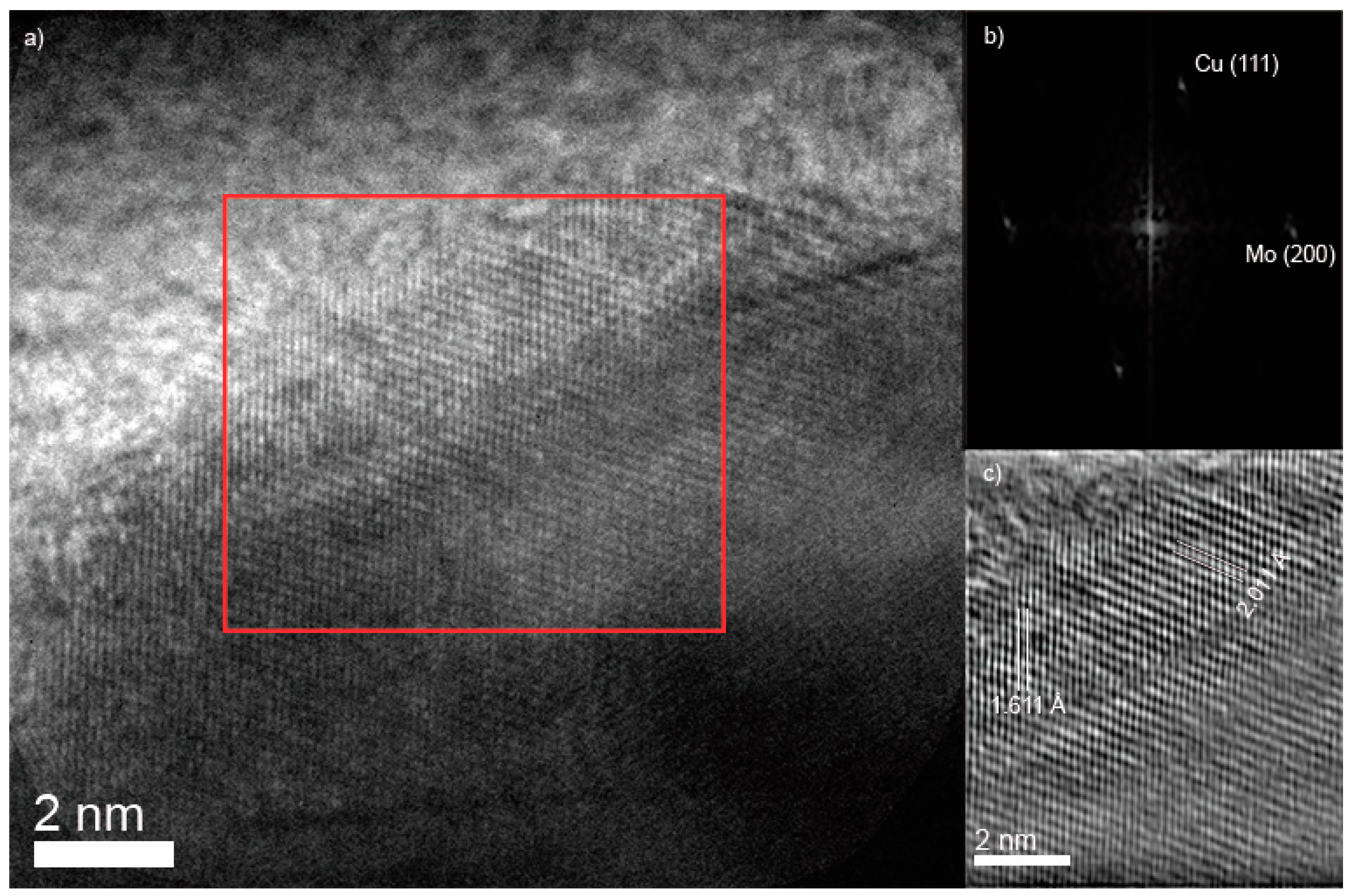
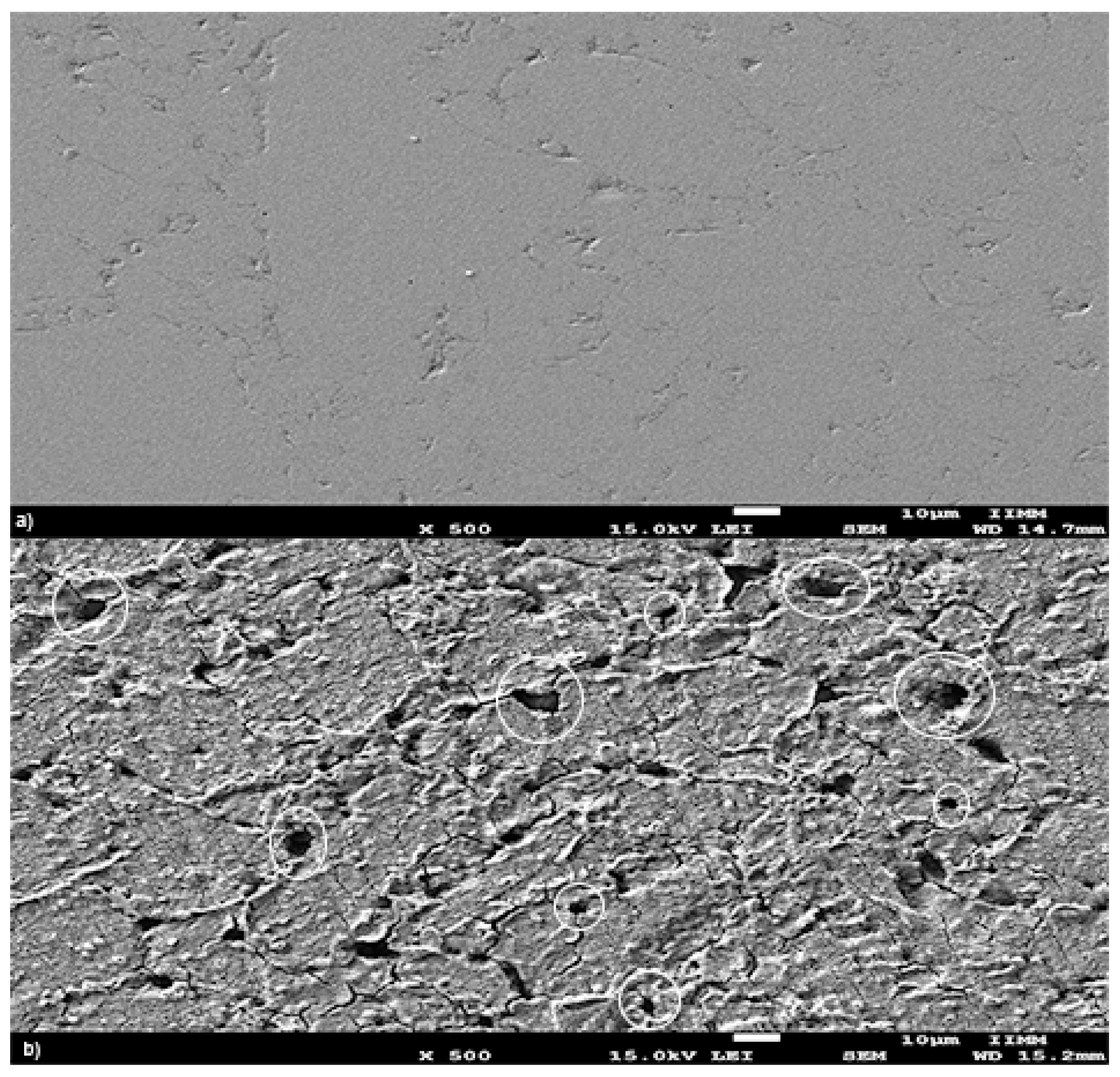
3.1. Characterization XRD, SEM, TEM
3.2. Density
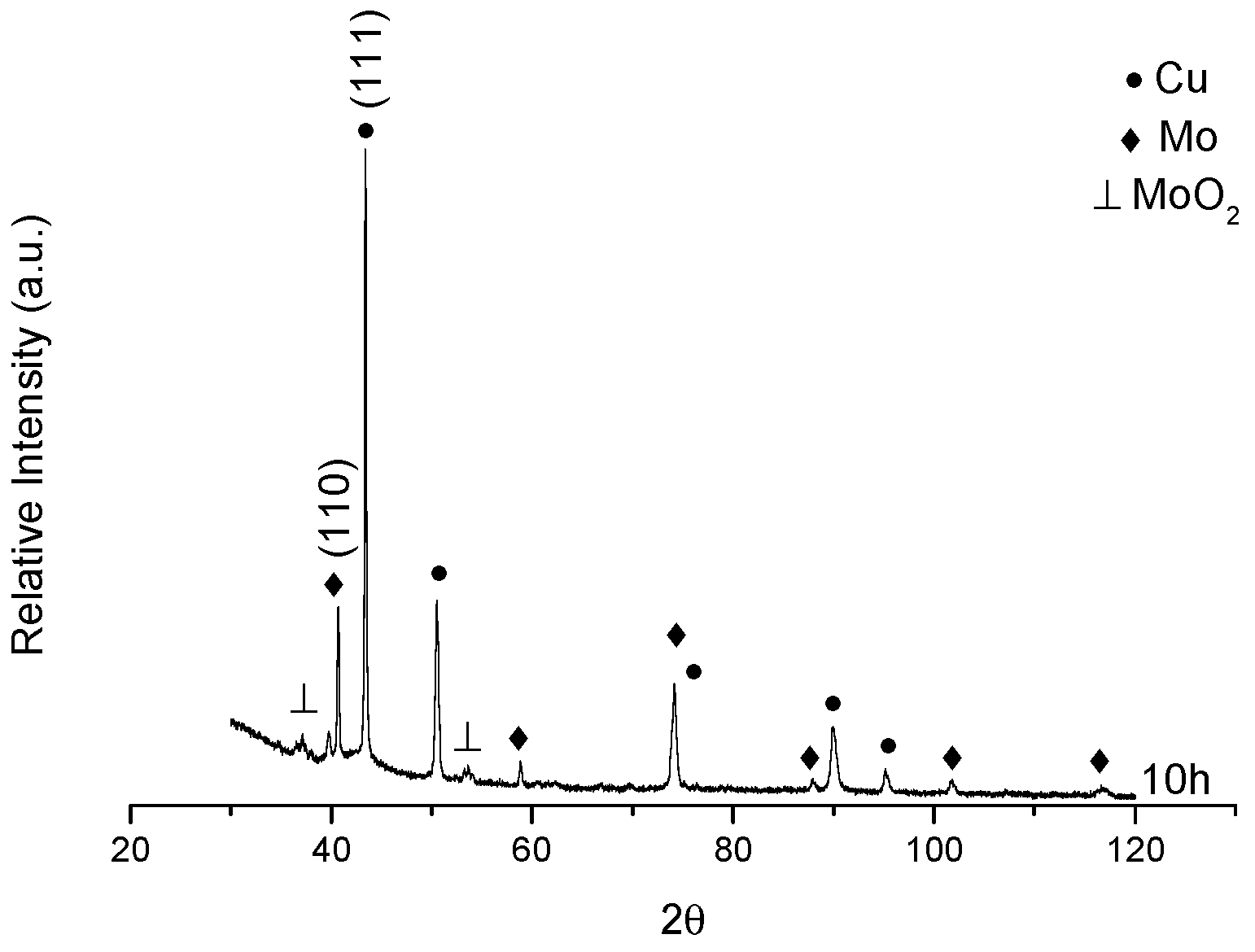
3.3. XRD after Sintering
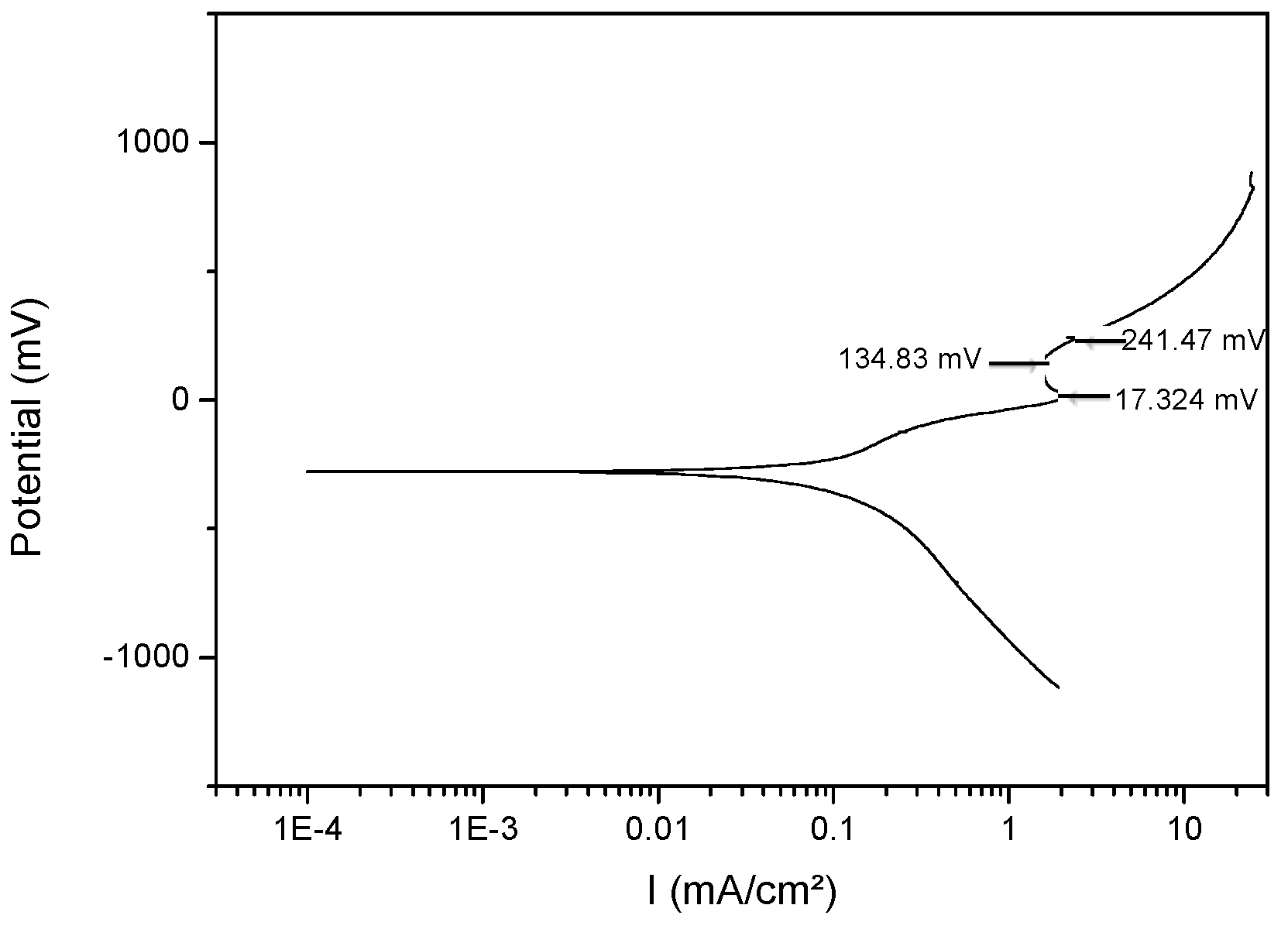
3.4. Corrosion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. (Ed.) Copper and Copper Alloys; ASM international: Materials Park, OH, USA, 2001. [Google Scholar]
- Ghorbani, A.; Sheibani, S.; Ataie, A. Microstructure and mechanical properties of consolidated Cu-Cr-CNT nanocomposite prepared via powder metallurgy. J. Alloys Compd. 2018, 732, 818–827. [Google Scholar] [CrossRef]
- Hernández, O.; Aguilar, C.; Medina, A.; Béjar, L.; Parra, C.; Carreón, H.; Borjas-García, S.E. Structural characterization of Cu10Mo alloy aynthesized by mechanical alloying. Microsc. Microanal. 2018, 24 (Suppl. 1), 800–801. [Google Scholar]
- Hernández, O.; Aguilar, C.; Medina, A.; Borjas, S.; Béjar, L.; Bernal, J.; Vega, J. Characterization of Cu-30Mo alloys synthesized by mechanical alloying. Microsc. Microanal. 2017, 23 (Suppl. 1), 1944–1945. [Google Scholar]
- Suryanarayana, C. Mechanical Alloying and Milling; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Kazimierczak, H.; Morgiel, J.; Swiatek, Z.; Vega, J.M.; García-Lecina, E. Effect of Mo addition on corrosion of Zn coatings electrodeposited on steel. Corros. Sci. 2018, 135, 107–119. [Google Scholar] [CrossRef]
- Nikulina, A.V.; Konkov, V.F.; Peregud, M.M.; Vorobev, E.E. Effect of molybdenum on properties of zirconium components of nuclear reactor core. Nucl. Mater. Energy 2018, 14, 8–13. [Google Scholar] [CrossRef]
- Patil, V. A study on mechanical properties of aluminium-4.5% copper alloy metal matrix composites reinforced with alumina fibers using squeeze casting process. J. Polym. Compos. 2018, 6, 22–31. [Google Scholar]
- Thurber, C.R.; Ahmad, Y.H.; Calhoun, M.C.; Al-Shenawa, A.; D’Souza, N.; Mohamed, A.; Golden, T.D. Metal matrix composite coatings of cupronickel embedded with nanoplatelets for improved corrosion resistant properties. Int. J. Corros. 2018, 2018, 5250713. [Google Scholar] [CrossRef]
- Singh, M.K.; Gautam, R.K.; Prakash, R.; Ji, G. Mechanical and corrosion behaviors of developed copper-based metal matrix composites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Aponte, B.; Fernández de Romero, M.; Troconis de Rincón, O.; Arias, S.; Contreras, M.; Ramos, J.; Collantes, R. Costos por corrosión en el mantenimiento preventivo y correctivo de equipos e instalaciones en la industria. Rev. Téc. Ing. Univ. Zulia 2008, 31, 87–96. [Google Scholar]
- Schleich, W. Application of Copper-Nickel Alloy Uns C70600 for Seawater Service. In Proceedings of the CORROSION/2005 Annual Conference and Exhibition, Houston, TX, USA, 3–7 April 2005. [Google Scholar]
- Badawy, W.A.; Ismail, K.M.; Fathi, A.M. Effect of Ni content on the corrosion behavior of Cu–Ni alloys in neutral chloride solutions. Electrochim. Acta 2005, 50, 3603–3608. [Google Scholar] [CrossRef]
- Ma, A.; Jiang, S.; Zheng, Y.; Ke, W. Corrosion product film formed on the 90/10 copper–nickel tube in natural seawater: Composition/structure and formation mechanism. Corros. Sci. 2015, 91, 245–261. [Google Scholar] [CrossRef]
- Aguilar, C.; Medina, A.; Hernández, O.J.; Béjar, L.; Parra, C. Thermodynamic analysis of Fe contamination in Cu-Mo alloys processed by mechanical alloying. Philos. Mag. Lett. 2018, 98, 341–349. [Google Scholar] [CrossRef]
- ASTM D-1141-98, Standard Practice for the Preparation of Substitute Ocean Water; ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM G3-89, Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing; ASTM International: West Conshohocken, PA, USA, 2010.
- Gil, S.; Rodríguez, E. (Eds.) Física re-Creativa Experimentos de Física usando nuevas tecnologías; Prentice Hall: Buenos Aires, Argentina, 2001. [Google Scholar]
- ASTM G102-89, Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 1999.
- Niu, S.; Fang, Y.; Qiu, R.; Qiu, Z.; Xiao, Y.; Wang, P.; Chen, M. Superhydrophobic film based on Cu-dodecanethiol complex: Preparation and corrosion inhibition for Cu. Colloids Surf. A 2018, 550, 65–73. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, S.; Li, W.; Hou, B. Electrochemical and thermodynamic investigation of diniconazole and triadimefon as corrosion inhibitors for copper in synthetic seawater. Corros. Sci. 2010, 52, 2891–2896. [Google Scholar] [CrossRef]
- Otmačić, H.; Stupnišek-Lisac, E. Copper corrosion inhibitors in near neutral media. Electrochim. Acta 2003, 48, 985–991. [Google Scholar] [CrossRef]
- Khaled, K.F. Guanidine derivative as a new corrosion inhibitor for copper in 3% NaCl solution. Mater. Chem. Phys. 2008, 112, 104–111. [Google Scholar] [CrossRef]
- Al-Mobarak, N.A.; Khaled, K.F.; Hamed, M.N.; Abdel-Azim, K.M.; Abdelshafi, N.S. Corrosion inhibition of copper in chloride media by 2-mercapto-4-(p-methoxyphenyl)-6-oxo-1, 6-dihydropyrimidine-5-carbonitrile: Electrochemical and theoretical study. Arabian J. Chem. 2010, 3, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhang, D. Study of corrosion behavior of copper in 3.5 wt.%NaCl solution containing extracellular polymeric substances of an aerotolerantsulphate-reducing bacteria. Corros. Sci. 2018, 136, 275–284. [Google Scholar] [CrossRef]


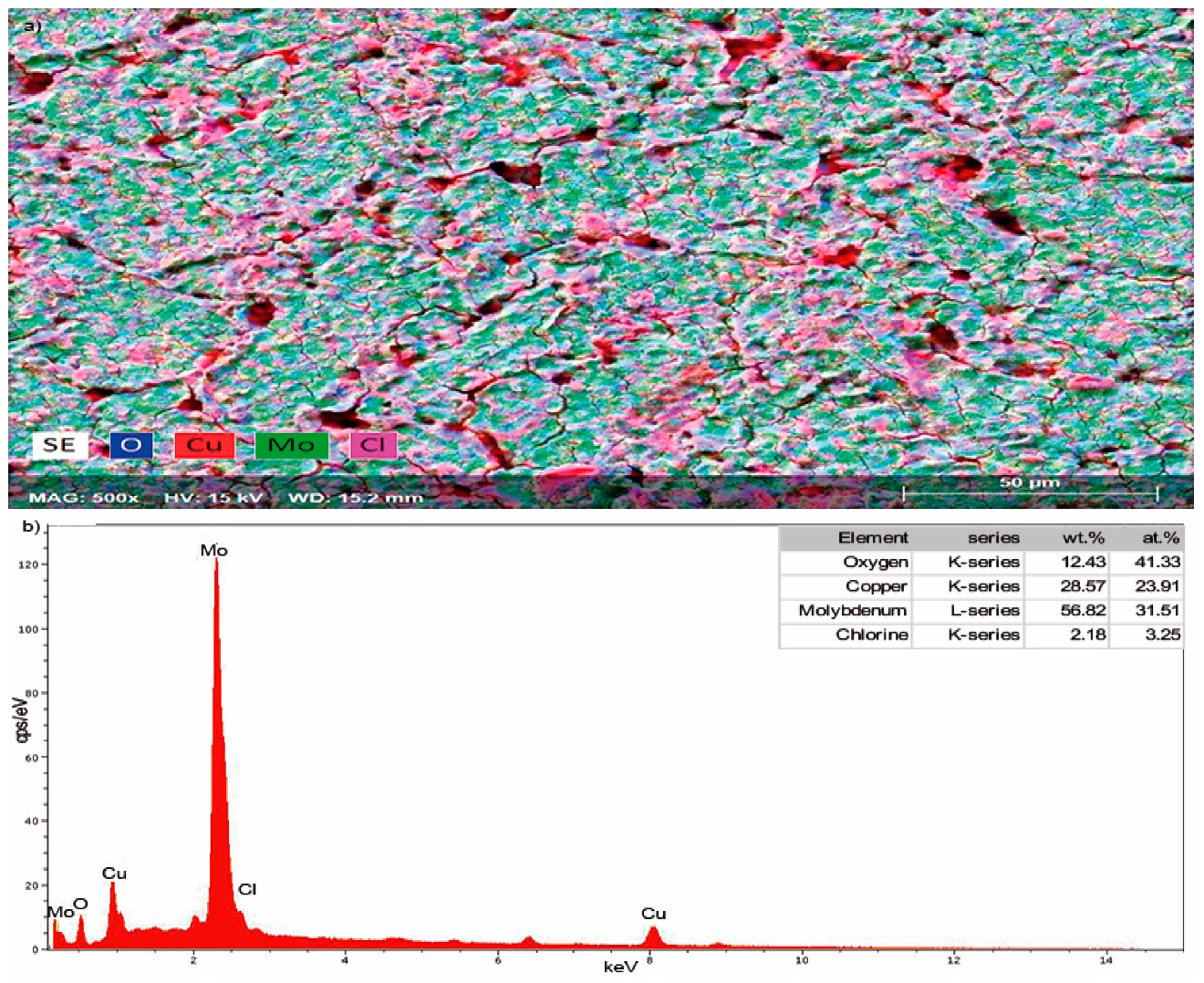
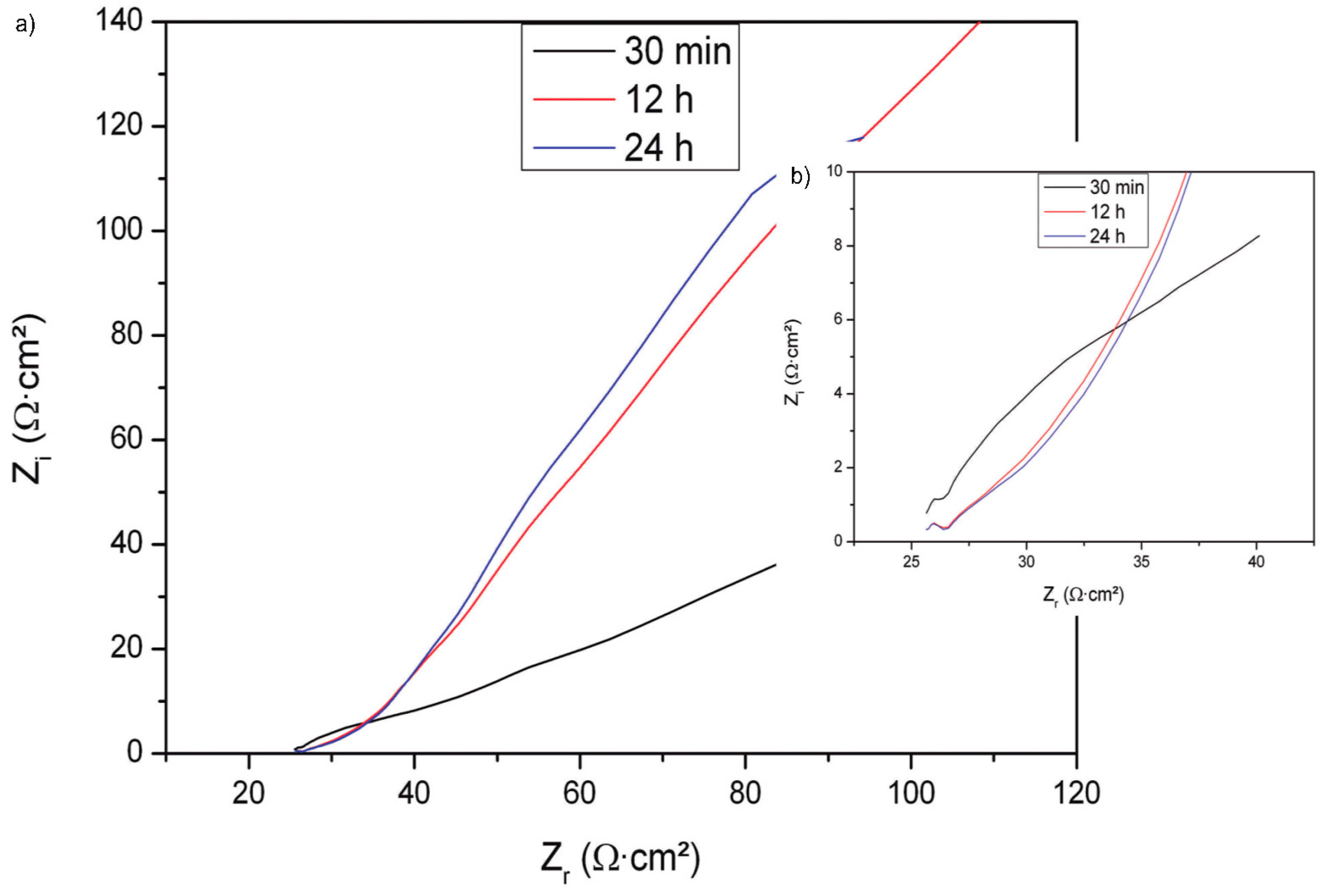

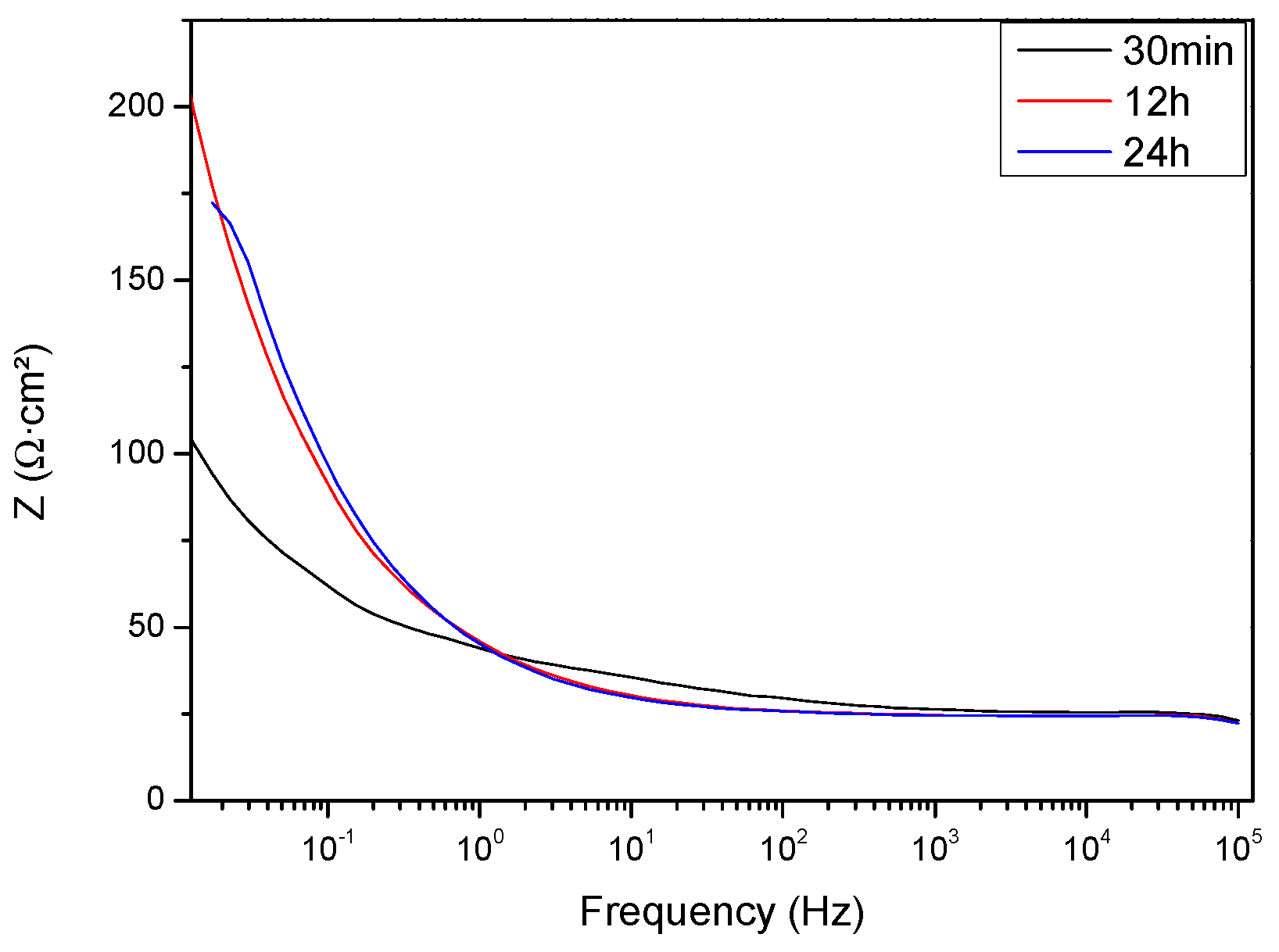
| Component | Concentration |
|---|---|
| NaCl | 19.62 g/L |
| MgCl2 | 4.16 g/L |
| Na2SO4 | 3.27 g/L |
| CaCl2 | 0.92 g/L |
| KCl | 0.55 g/L |
| NaHCO3 | 0.16 g/L |
| KBr | 0.08 g/L |
| H3BO3 | 0.02 g/L |
| SrCl2 | 0.02 g/L |
| NaF | 0.002 g/L |
| Sample | # pill | Density (g/cm3) |
|---|---|---|
| Cu-20 wt% Mo (10 h) | 1 | 7.76 ± 0.05 |
| 2 | 7.71 ± 0.05 | |
| 3 | 7.70 ± 0.05 |
| Sample | ba (mV/decade) | bc (mV/decade) | Ecorr (mV) | Icorr (mA/cm2) | Ep (mV) | Epitt (mV) | Erp (mV) |
|---|---|---|---|---|---|---|---|
| Cu-20 wt% Mo | 179.3 | 556.6 | −278.3 | 0.33 ± 1 × 10−4 | 17.3 | 134.8 | 241.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, O.; Aguilar, C.; Medina, A. Effects of Mo Concentration on the Structural and Corrosion Properties of Cu–Alloy. Metals 2019, 9, 1307. https://doi.org/10.3390/met9121307
Hernández O, Aguilar C, Medina A. Effects of Mo Concentration on the Structural and Corrosion Properties of Cu–Alloy. Metals. 2019; 9(12):1307. https://doi.org/10.3390/met9121307
Chicago/Turabian StyleHernández, Oscar, Claudio Aguilar, and Ariosto Medina. 2019. "Effects of Mo Concentration on the Structural and Corrosion Properties of Cu–Alloy" Metals 9, no. 12: 1307. https://doi.org/10.3390/met9121307





