Alloying Element Segregation and Grain Boundary Reconstruction, Atomistic Modeling
Abstract
1. Introduction
2. Methods of Modeling
2.1. Ab Initio Calculations of the Solute-GB Interaction
2.2. MD/MC Finite Temperature Simulations
3. Results
3.1. First Principles Modeling of Solute–GB Interactions and GB Reconstruction
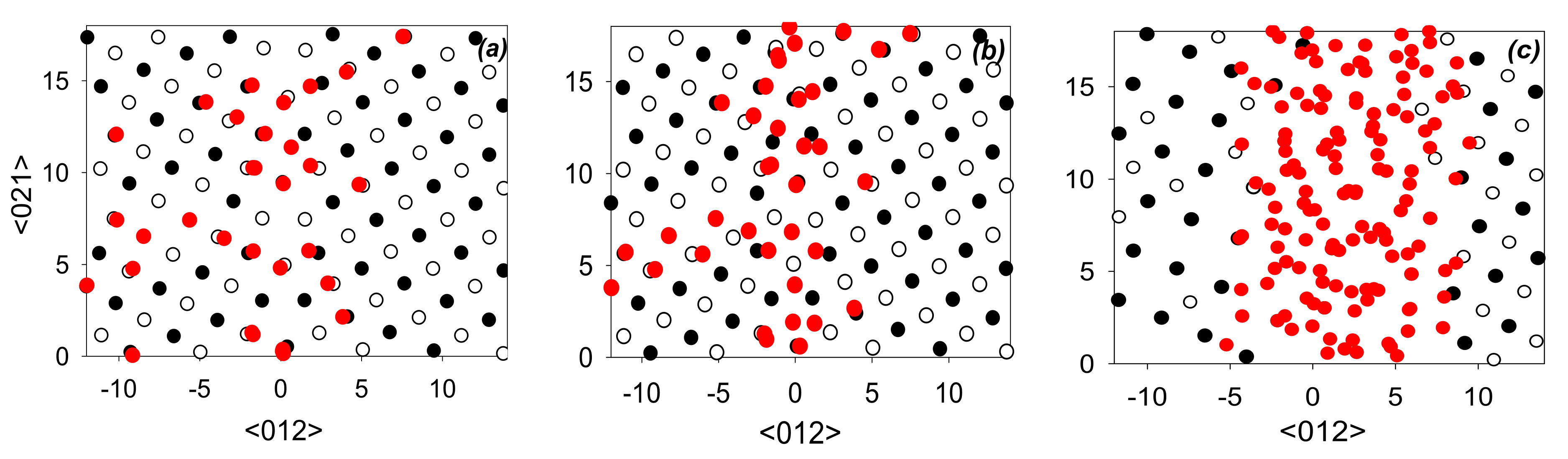
3.2. MD/MC Simulations of the Segregation Formation and GB Reconstruction
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lejček, P. Grain Boundary Segregation in Metals; Springer: Berlin, Germany, 2010. [Google Scholar]
- Valiev, R.Z. Nanostructuring of metals by severe plastic deformation for advanced properties. Nat. Mater. 2004, 3, 511–516. [Google Scholar] [CrossRef]
- Sauvage, X.; Wilde, G.; Divinski, S.V.; Horita, Z.; Valiev, R.Z. Grain boundaries in ultrafine in ultrafine grained materials processed by severe plastic deformation and related phenomena. Mater. Sci. Eng. A 2012, 540, 1–12. [Google Scholar] [CrossRef]
- Raabe, D.; Sandlobes, S.; Millan, J.; Ponge, D.; Assadi, H.; Herbig, M.; Choi, P.-P. Segregation engineering enables nanoscale martensite to austenite phase transformation at grain boundaries: A pathway to ductile martensite. Acta Mater. 2013, 61, 6132–6152. [Google Scholar] [CrossRef]
- Chatterjee, P.P.; Pabi, S.K.; Manna, I. An allotropic transformation induced by mechanical alloying. J. Appl. Phys. 1999, 86, 5912–5914. [Google Scholar] [CrossRef]
- Ben Makhlouf, M.; Bachaga, T.; Suñol, J.J.; Mohamed Dammak, M.; Khitouni, M. Synthesis and characterization of nanocrystalline Al-20 at% Cu powders produced by mechanical alloying. Metals 2016, 6, 145. [Google Scholar] [CrossRef]
- Weissmuller, J. Alloy effects in nanostructures. Nanostruct. Mater. 1993, 3, 261–272. [Google Scholar] [CrossRef]
- Kirchheim, R. Grain coarsening inhibited by solute segregation. Acta Mater. 2002, 50, 413–419. [Google Scholar] [CrossRef]
- Trelewicz, J.R.; Schuh, C.A. Grain boundary segregation and thermodynamically stable binary nanocrystalline alloys. Phys. Rev. B 2009, 79, 094112. [Google Scholar] [CrossRef]
- Chookajorn, T.; Murdoch, H.A.; Schuh, C.A. Design of stable nanocrystalline alloys. Science 2012, 337, 951–954. [Google Scholar] [CrossRef]
- Lozovoi, A.Y.; Paxton, A.T.; Finnis, M.W. Structural and chemical embrittlement of grain boundaries by impurities: A general theory and first-principles calculations for copper. Phys. Rev. B 2006, 74, 155416. [Google Scholar] [CrossRef]
- Wachowicz, E.; Ossowski, T.; Kiejna, A. Cohesive and magnetic properties of grain boundaries in bcc Fe with Cr additions. Phys. Rev. B 2010, 81, 094104. [Google Scholar] [CrossRef]
- Zhu, Q.; Samanta, A.; Li, B.; Rudd, R.E.; Frolov, T. Predicting phase behavior of grain boundaries with evolutionary search and machine learning. Nat. Commun. 2018, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Rupert, T.J. Amorphous intergranular films as toughening structural features. Acta Mater. 2015, 89, 205–214. [Google Scholar] [CrossRef]
- Krakauer, B.W.; Seidman, D.N. Subnanometer scale study of segregation at grain boundaries in an Fe(Si) alloy. Acta Mater. 1998, 46, 6145–6161. [Google Scholar] [CrossRef]
- Salem, A.A.; Langdon, T.G.; McNelley, T.R.; Kalidindi, S.R.; Semiatin, S.L. Strain-path effects on the evolution of microstructure and texture during the severe-plastic deformation of aluminum. Metall. Mater. Trans. A 2006, 37, 2879–2891. [Google Scholar] [CrossRef]
- Braithwaite, J.S.; Rez, P. Grain boundary impurities in iron. Acta Mater. 2005, 53, 2715–2726. [Google Scholar] [CrossRef]
- Zhang, S.; Kontsevoi, O.Y.; Freeman, A.J.; Olson, G.B. Sodium-induced embrittlement of an aluminum grain boundary. Phys. Rev. B 2010, 82, 224107. [Google Scholar] [CrossRef]
- Scheiber, D.; Razumovskiy, V.I.; Puschnig, P.; Pippanc, R.; Romaner, L. Ab initio description of segregation and cohesion of grain boundaries in W–25 at% Re alloys. Acta Mater. 2015, 88, 180–189. [Google Scholar] [CrossRef]
- Miller, M.K.; Cerezo, A.; Hetherlington, M.G.; Smith, G.D.W. Atom Probe Field Ion Microscopy; Oxford Science: Oxford, UK, 1996. [Google Scholar]
- Cerezo, A.; Clifton, P.H.; Lozano-Perez, S.; Panayi, P.; Sha, G.; Smith, G.D.W. Overview: Recent Progress in Three-Dimensional Atom Probe Instruments and Applications. Microsc. Microanal. 2007, 13, 408–417. [Google Scholar] [CrossRef]
- Blavette, D.; Cadel, E.; Pereige, C.; Deconihout, B.; Caron, P. Phase Transformation and Segregation to Lattice Defects in Ni-Base Superalloys. Microsc. Microanal. 2007, 13, 464–483. [Google Scholar] [CrossRef]
- Sha, G.; Marceau, R.K.W.; Gao, X.; Muddle, B.C.; Ringer, S.P. Nanostructure of aluminum alloy 2024: Segregation, clustering and precipitation processes. Acta Mater. 2011, 59, 1659–1670. [Google Scholar] [CrossRef]
- Sha, G.; Yao, L.; Liao, X.; Ringer, S.P.; Duan, Z.C.; Langdon, T.G. Segregation of solute elements at grain boundaries in an ultrafine grained Al-Zn-Mg-Cu alloy. Ultramicroscopy 2011, 111, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Savage, X.; Ganeev, A.; Ivanisenko, Y.; Enikeev, N.; Murashkin, M.; Valiev, R. Grain Boundary Segregation in UFG Alloys Processed by Severe Plastic Deformation. Adv. Eng. Mater. 2012, 14, 968–974. [Google Scholar] [CrossRef]
- Udler, D.; Seidman, D.N. Congruent Phase Transition at a Twist Boundary Induced by Solute Segregation. Phys. Rev. Lett. 1996, 77, 3379. [Google Scholar] [CrossRef]
- Creuze, J.; Berthier, F.; Tétot, R.; Legrand, B. Wetting and Structural Transition Induced by Segregation at Grain Boundaries: A Monte Carlo Study. Phys. Rev. Lett. 2001, 86, 5735. [Google Scholar] [CrossRef] [PubMed]
- Kogtenkova, O.; Straumal, B.; Korneva, A.; Czeppe, T.; Wierzbicka-Miernik, A.; Faryna, M.; Zieba, P. Grain Boundary Complexions and Phase Transformations in Al- and Cu-Based Alloys. Metals 2019, 9, 10. [Google Scholar] [CrossRef]
- Pan, Z.; Rupert, T.J. Effect of grain boundary character on segregation-induced structural transitions. Phys. Rev. B 2016, 93, 134113. [Google Scholar] [CrossRef]
- Pan, Z.; Rupert, T.J. Atomistic modeling of interfacial segregation and structural transitions in ternary alloys. J. Mater. Sci. 2019, 54, 3975–3993. [Google Scholar]
- Khalajhedayati, A.; Pan, Z.L.; Rupert, T.J. Manipulating the interfacial structure of nanomaterials to achieve a unique combination of strength and ductility. Nat. Commun. 2016, 7, 10802. [Google Scholar] [CrossRef]
- Sauvage, X.; Enikeev, N.; Valiev, R.; Nasedkina, Y.; Murashkin, M. Atomic-scale analysis of the segregation and precipitation mechanisms in a severely deformed Al–Mg alloy. Acta Mater. 2014, 72, 125–136. [Google Scholar] [CrossRef]
- Bobruk, E.V.; Sauvage, X.; Enikeev, N.A.; Straumal, B.B.; Valiev, R.Z. Ultrafine Grained Structures Resulting from SPD-Induced Phase Transformation in Al-Zn Alloys. Rev. Adv. Mater. Sci. 2015, 43, 45–51. [Google Scholar]
- Karkina, L.E.; Karkin, I.N.; Kuznetsov, A.R.; Razumov, I.K.; Korzhavyi, P.A.; Gornostyrev, Y.N. Solute-grain boundary interaction and segregation formation in Al: First principles calculations and molecular dynamics modeling. Comput. Mater. Sci. 2016, 112, 18–26. [Google Scholar] [CrossRef]
- Petrik, M.V.; Kuznetsov, A.R.; Enikeev, N.; Gornostyrev, Y.N.; Valiev, R.Z. Peculiarities of Interactions of Alloying Elements with Grain Boundaries and the Formation of Segregations in Al–Mg and Al–Zn Alloys. Phys. Met. Metallogr. 2018, 119, 607–612. [Google Scholar] [CrossRef]
- Schweinfest, R.; Paxton, A.T.; Finnis, M.W. Bismuth embrittlement of copper is an atomic size effect. Nature 2004, 432, 1008–1011. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Shiga, M.; Kaburaki, H. Grain Boundary Decohesion by Impurity Segregation in a Nickel-Sulfur System. Science 2005, 21, 393–397. [Google Scholar] [CrossRef]
- Zhang, L.; Shu, X.; Jin, S.; Zhang, Y.; Lu, G.H. First-principles study of He effects in a bcc Fe grain boundary: Site preference, segregation and theoretical tensile strength. J. Phys. Condens. Matter. 2010, 22, 375401. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Wang, J.; Zhang, H.J. First-principles investigation of Mg segregation at Σ = 11(113) grain boundaries in Al. J. Phys. Condens. Matter. 2005, 17, 4301–4308. [Google Scholar] [CrossRef]
- Christensen, M.; Angeliu, T.M.; Ballard, J.D.; Vollmer, J.; Najafabadi, R.; Wimmer, E. Effect of impurity and alloying elements on Zr grain boundary strength from first-principles computations. J. Nucl. Mater. 2010, 404, 121–127. [Google Scholar] [CrossRef]
- Yuasa, M.; Mabuchi, M.J. Effects of segregated Cu on an Fe grain boundary by first-principles tensile tests. J. Phys. Condens. Matter. 2010, 22, 505705. [Google Scholar] [CrossRef]
- Wachowicz, E.; Kiejna, A. Effect of impurities on grain boundary cohesion in bcc iron. Comput. Mater. Sci. 2008, 43, 736–743. [Google Scholar] [CrossRef]
- Pun, G.P.; Yamakov, V.; Mishin, Y. Interatomic potential for the ternary Ni–Al–Co system and application to atomistic modeling of the B2–L10 martensitic transformation. Model. Simul. Mater. Sci. Eng. 2015, 23, 065006. [Google Scholar]
- Koju, R.K.; Darling, K.A.; Kecskes, L.J.; Mishin, Y. Zener pinning of grain boundaries and structural stability of immiscible alloys. JOM 2016, 68, 1596–1604. [Google Scholar] [CrossRef]
- Li, A.; Szlufarska, I. Morphology and mechanical properties of nanocrystalline Cu/Ag alloy. J. Mater. Sci. 2017, 52, 4555–4567. [Google Scholar] [CrossRef]
- Dillon, S.J.; Tang, M.; Carter, W.C.; Harmer, M.P. Complexion: A new concept for kinetic engineering in materials science. Acta Mater. 2007, 55, 6208–6218. [Google Scholar] [CrossRef]
- Harmer, M.P. The phase behavior of interfaces. Science 2011, 332, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, P.R.; Tang, M.; Dillon, S.J.; Luo, J.; Rohrer, G.S.; Harmer, M.P. Grain boundary complexions. Acta Mater. 2014, 62, 1–48. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 1994, 6, 8245–8258. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- LAMMPS Molecular Dynamics Simulator. Available online: http://lammps.sandia.gov/index.html (accessed on 18 January 2019).
- Mendelev, M.I.; Asta, M.; Rahman, M.J.; Hoyt, J.J. Development of interatomic potentials appropriate for simulation of solid–liquid interface properties in Al–Mg alloys. Philos. Mag. 2009, 89, 3269–3285. [Google Scholar] [CrossRef]
- Purja Pun, G.P.; Mishin, Y. Development of an interatomic potential for the Ni-Al system. Phil. Mag. 2009, 89, 3245–3267. [Google Scholar] [CrossRef]
- Apostol, F.; Mishin, Y. Interatomic potential for the Al-Cu system. Phys. Rev. B 2011, 83, 54116. [Google Scholar] [CrossRef]
- Landa, A.; Wynblatt, P.; Siegel, D.J.; Adams, J.B.; Mryasov, O.N.; Liu, X.-Y. Development of glue-type potentials for the Al-Pb system: Phase diagram calculation. Acta Mater. 2000, 48, 1753–1761. [Google Scholar] [CrossRef]
- Interatomic Potentials (Force Fields). Available online: http://www.ctcms.nist.gov/potentials/ (accessed on 20 January 2019).
- Guyot, P.; Simon, J.-P. Theoretical aspects of the interaction between grain-boundaries and impurities. J. Phys. Colloq. 1975, 36, 141–149. [Google Scholar] [CrossRef]
- Trautt, Z.T.; Mishin, Y. Grain Boundary Migration and Grain Rotation Studied by Molecular Dynamics. Acta Mater. 2012, 60, 2407–2424. [Google Scholar] [CrossRef]
- Guinier, A. Heterogeneities in Solid Solutions. Solid State Phys. 1959, 9, 293–398. [Google Scholar]
- Joshi, K.; Chaudhuri, S. Empirical force field-based kinetic Monte Carlo simulation of precipitate evolution and growth in Al–Cu alloys. Model. Simul. Mater. Sci. Eng. 2016, 24, 075012. [Google Scholar] [CrossRef]
- Gorbatov, O.I.; Gornostyrev, Y.N.; Korzhavyi, P.A. Many-body mechanism of Guinier-Preston zones stabilization in Al–Cu alloys. Scr. Mater. 2017, 138, 130–133. [Google Scholar] [CrossRef]
- Karkina, L.E.; Karkin, I.N.; Kuznetsov, A.R.; Gornostyrev, Y.N. Grain boundary shear-migration coupling in Al bicrystalls. Atomistic modeling. Phys. Solid State 2018, 60, 1916–1923. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkina, L.; Karkin, I.; Kuznetsov, A.; Gornostyrev, Y. Alloying Element Segregation and Grain Boundary Reconstruction, Atomistic Modeling. Metals 2019, 9, 1319. https://doi.org/10.3390/met9121319
Karkina L, Karkin I, Kuznetsov A, Gornostyrev Y. Alloying Element Segregation and Grain Boundary Reconstruction, Atomistic Modeling. Metals. 2019; 9(12):1319. https://doi.org/10.3390/met9121319
Chicago/Turabian StyleKarkina, Lidia, Iliya Karkin, Andrey Kuznetsov, and Yuri Gornostyrev. 2019. "Alloying Element Segregation and Grain Boundary Reconstruction, Atomistic Modeling" Metals 9, no. 12: 1319. https://doi.org/10.3390/met9121319
APA StyleKarkina, L., Karkin, I., Kuznetsov, A., & Gornostyrev, Y. (2019). Alloying Element Segregation and Grain Boundary Reconstruction, Atomistic Modeling. Metals, 9(12), 1319. https://doi.org/10.3390/met9121319





