Inclusion and Microstructure Characteristics in a Steel Sample with TiO2 Nanoparticle Addition and Mg Treatment
Abstract
:1. Introduction
2. Materials and Methods
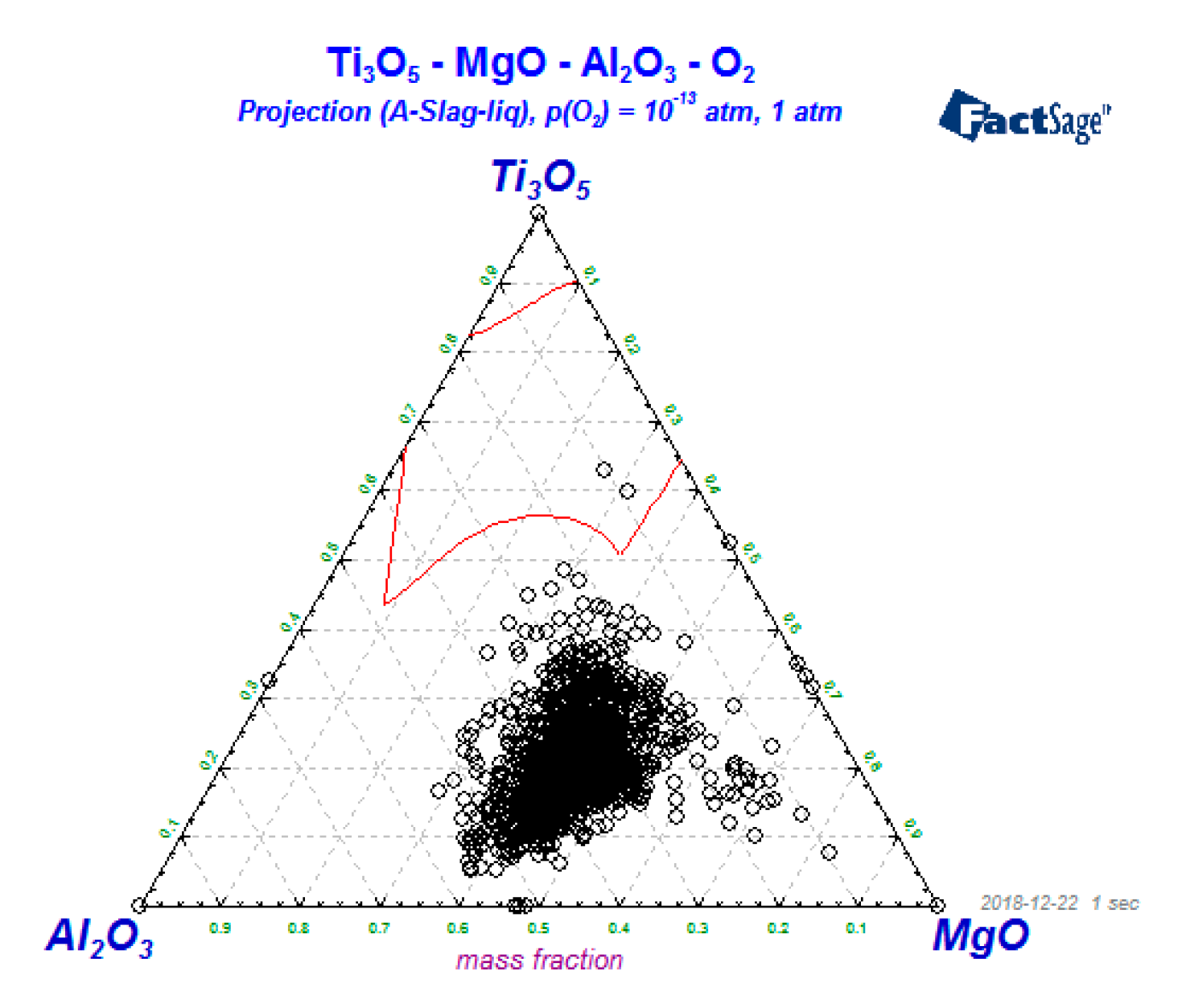
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Takamura, J.; Mizoguchi, S. Roles of oxides in steel performance. In Proceedings of the Sixth International Iron and Steel Congress, Nagoya, Japan, 21–26 October 1990; Iron and Steel Institute of Japan: Tokyo, Japan, 1990; Volume 1, pp. 591–597. [Google Scholar]
- Koseki, T.; Thewlis, G. Inclusion assisted microstructure control in C–Mn and low alloy steel welds. J. Mater. Sci. Technol. 2005, 21, 867–879. [Google Scholar] [CrossRef]
- Sarma, D.S.; Karasev, A.V.; Jönsson, P.G. On the role of non-metallic inclusions in the nucleation of acicular ferrite in steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Byun, J.S.; Shim, J.H.; Cho, Y.W. Influence of Mn on microstructural evolution in Ti-killed C–Mn steel. Scr. Mater. 2003, 48, 449–454. [Google Scholar] [CrossRef]
- Mu, W.Z.; Jönsson, P.G.; Nakajima, K.J. Recent Aspects on the effect of inclusion characteristics on the intragranular ferrite formation in low alloy steels: A review. High Temp. Mater. Proc. 2017, 36, 309–325. [Google Scholar] [CrossRef]
- Mu, W.Z.; Xuan, C.J.; Shibata, H.; Jönsson, P.G.; Nakajima, K.J. Wetting and Agglomeration Behavior of Inclusion and Intragranular Ferrite Formation Kinetics in Steel with TiO2 Addition According to Inclusion Engineering. In Proceedings of the AISTech 2017 Iron and Steel Technology Conference, Nashville, TN, USA, 8–11 May 2017; Association for Iron and Steel Technology, AISTECH: Warrendale, PA, USA, 2017; Volume 3, pp. 3027–3036. [Google Scholar]
- Kiviö, M.; Holappa, L.; Iung, T. Addition of Dispersoid Titanium Oxide Inclusions in Steel and Their Influence on Grain Refinement. Metall. Mater. Trans. B 2010, 41, 1194–1204. [Google Scholar] [CrossRef]
- Nedjad, S.H.; Farzaneh, A. Formation of fine intragranular ferrite in cast plain carbon steel inoculated by titanium oxide nanopowder. Scr. Mater. 2007, 57, 937–940. [Google Scholar] [CrossRef]
- Gregg, J.M.; Bhadeshia, H.K.D.H. Solid-state nucleation of acicular ferrite on minerals added to molten steel. Acta Mater. 1997, 45, 739–748. [Google Scholar] [CrossRef]
- Grong, Ø.; Kolbeinsen, L.; Eijk, C.V.D.; Tranell, G. Microstructure Control of Steels through Dispersoid Metallurgy Using Novel Grain Refining Alloys. ISIJ. Int. 2006, 46, 824–831. [Google Scholar] [CrossRef]
- Gao, X.Z.; Yang, S.F.; Li, J.S.; Yang, Y.D.; Chattopadhyay, K.; Mclean, A. Effects of MgO Nanoparticle Additions on theStructure and Mechanical Properties of Continuously Cast Steel Billets. Metall. Mater. Trans. A 2016, 47, 461–470. [Google Scholar] [CrossRef]
- Guo, H.; Yang, S.F.; Li, J.S.; Zhao, M.J.; Chen, Z.Y.; Zhang, X.L.; Li, J.K. Effects of Surface-Modified MgO Nanoparticles on Inclusion Characteristics and Microstructure in Carbon Structural Steel. JOM 2018, 70, 1136–1142. [Google Scholar] [CrossRef]
- Chai, F.; Yang, C.F.; Su, H.; Zhang, Y.Q.; Xu, Z. Effect of Magnesium on Inclusion Formation in Ti-Killed Steels and Microstructural Evolution in Welding Induced Coarse-Grained Heat Affected Zone. J. Iron Steel Res. Int. 2009, 16, 69–74. [Google Scholar] [CrossRef]
- Wu, Z.H.; Zheng, W.; Li, G.Q.; Matsuura, H.; Tsukihashi, F. Effect of Inclusions’ Behavior on the Microstructure in Al-Ti Deoxidized and Magnesium-Treated Steel with Different Aluminum Contents. Metall. Mater. Trans. B 2015, 46, 1226–1241. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.L.; Li, G.Q.; Wang, Y.; Zheng, W.; Hou, Y.H. Grain refinement in coarse-grained heat-affected zone of Al–Ti–Mg complex deoxidised steel. Sci. Technol. Weld. Join. 2019, 24, 1–10. [Google Scholar] [CrossRef]
- Hou, Y.H.; Zheng, W.; Wu, Z.H.; Li, G.Q.; Moelans, N.; Guo, M.X.; Khan, B.S. Study of Mn absorption by complex oxide inclusions in Al-Ti-Mg killed steels. Acta Mater. 2016, 118, 8–16. [Google Scholar] [CrossRef]
- Kong, H.; Hao, L.; Qiang, Y.; Cai, Z.Y. Effects of Ti-Mg Complex Inclusions on Acicular Ferrite Nucleation. High Temp. Mater. Proc. 2017, 36, 459–465. [Google Scholar]
- Song, M.M.; Song, B.; Hu, C.L.; Xin, W.B.; Song, G.Y. Formation of Acicular Ferrite in Mg Treated Ti-bearing C-Mn Steel. ISIJ Int. 2015, 55, 1468–1473. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, M.Y.; Li, J.L.; Ran, S.L.; Shan, M.W.; Yue, Q.; Kong, H. The effect of MgTiO3 Adding on Inclusion Characteristics. High Temp. Mater. Proc. 2019. accepted. [Google Scholar]
- Zhuo, X.J.; Wang, Y.Q.; Wang, X.H.; Lee, H.G. Thermodynamic Calculation and MnS Solubility of Mn-Ti Oxide Formation in Si-Mn-Ti Deoxidized Steel. J. Iron Steel Res. Int. 2010, 17, 10–16. [Google Scholar] [CrossRef]
- Xuan, C.J.; Mu, W.Z.; Olano, Z.I.; Jönsson, P.G.; Nakajima, K.J. Effect of the Ti, Al Contents on the Inclusion Characteristics in Steels with TiO2 and TiN Particle Additions. Steel Res. Int. 2016, 87, 911–920. [Google Scholar] [CrossRef]
- Xuan, C.J. Wettability and Agglomeration Characteristics of Non-Metallic Inclusions. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2016. [Google Scholar]
- Mizoguchi, T.; Ueshima, Y.; Sugiyama, M.; Mizukami, K. Influence of Unstable Non-equilibrium Liquid Iron Oxide on Clustering of Alumina Particles in Steel. ISIJ Int. 2013, 53, 639–647. [Google Scholar] [CrossRef]
- Miia, K.; Lauri, H. Addition of Titanium Oxide Inclusions into Liquid Steel to Control Nonmetallic Inclusions. Metall. Mater. Trans. B 2012, 43, 233–240. [Google Scholar]
- Eisenhüttenleute, V.D. Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995; p. 414. [Google Scholar]
- Zhang, T.S.; Liu, C.J.; Jiang, M.F. Effect of Mg on Behavior and Particle Size of Inclusions in Al-Ti Deoxidized Molten Steels. Metall. Mater. Trans. B 2016, 47, 2253–2262. [Google Scholar] [CrossRef]
- You, D.L.; Michelic, S.K.; Bernhard, C. Formation of Multi-Type Inclusion during the Cooling and Solidification of Steel: A Trend Model. Metals 2018, 8, 452. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hasegawa, T.; Takamura, J.I. Effect of Boron on Intra-granular Ferrite Formation in Ti-oxide Bearing Steels. ISIJ Int. 1996, 36, 80–86. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Kang, Y.B.; Lee, H.G. Thermodynamics Analysis of Mn-depleted Zone near Ti Oxide Inclusions for Intragranular Nucleation of Ferrite in Steel. ISIJ Int. 2010, 50, 501–508. [Google Scholar] [CrossRef]
- Zhang, F.W.; Zhang, Q.R.; Liu, T.Y.; Sun, Y.Y.; Tao, K.; Julian, G. Computer simulation of intrinsic defects in MgAl2O4. Univ. Shanghai Sci. Technol. 2005, 27, 104–106. [Google Scholar]
- Li, M.L. Theoretical Study on Properties of Magnesium Aluminates Spinel Materials. Ph.D. Thesis, Northeastern University, Shenyang, China, 2009. [Google Scholar]
- Kong, H.; Zhou, Y.H.; Lin, H.; Xia, Y.J.; Li, J.; Yue, A.; Cai, Z.Y. The Mechanism of Intragranular Acicular Ferrite Nucleation Induced by Mg-Al-O Inclusions. Adv. Mater. Sci. Eng. 2015, 2015, 378678. [Google Scholar]
- Lin, C.K.; Pan, Y.C.; Su, Y.H.F.; Lin, G.R.; Hwang, W.S.; Kuo, J.C. Effects of Mg-Al-O-Mn-S inclusion on the nucleation of acicular ferrite in magnesium-containing low-carbon steel. Mater. Charact. 2018, 141, 318–327. [Google Scholar] [CrossRef]






| Figure | Position | Ti | Mg | Al | Mn | S |
|---|---|---|---|---|---|---|
| 1a | Point 1 | 41.98 | 33.50 | 17.89 | 6.63 | - |
| Point 2 | 35.04 | 37.39 | 21.31 | 6.26 | - | |
| 1b | Point 1 | 39.94 | 34.78 | 18.02 | 7.26 | - |
| Point 2 | 22.00 | 28.45 | 13.68 | 23.23 | 12.64 |
| Figure | Position | Al | Mn | S |
|---|---|---|---|---|
| 2a | Point 1 | 100 | - | - |
| Point 2 | 100 | - | - | |
| 2b | Point 1 | 94.69 | 3.45 | 1.86 |
| Point 2 | 24.12 | 47.23 | 28.65 |
| Figure | Position | Ti | Mg | Al | Mn | S | Si |
|---|---|---|---|---|---|---|---|
| 6a | Point 1 | 26.93 | 41.44 | 25.91 | 5.72 | - | - |
| Point 2 | 19.29 | 31.87 | 18.45 | 18.75 | 9.44 | 2.20 | |
| 6b | Point 1 | 23.36 | 43.02 | 28.65 | 4.97 | - | - |
| Point 2 | 19.13 | 46.40 | 29.29 | 5.18 | - | - |
| Figure | Position | Al | Mn | S |
|---|---|---|---|---|
| 7a | Point 1 | 100 | - | - |
| Point 2 | 100 | - | - | |
| 7b | Point 1 | 91.15 | 5.31 | 3.54 |
| Point 2 | 24.56 | 45.89 | 29.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Kong, H. Inclusion and Microstructure Characteristics in a Steel Sample with TiO2 Nanoparticle Addition and Mg Treatment. Metals 2019, 9, 171. https://doi.org/10.3390/met9020171
Cai Z, Kong H. Inclusion and Microstructure Characteristics in a Steel Sample with TiO2 Nanoparticle Addition and Mg Treatment. Metals. 2019; 9(2):171. https://doi.org/10.3390/met9020171
Chicago/Turabian StyleCai, Zhengyu, and Hui Kong. 2019. "Inclusion and Microstructure Characteristics in a Steel Sample with TiO2 Nanoparticle Addition and Mg Treatment" Metals 9, no. 2: 171. https://doi.org/10.3390/met9020171





