Influence of Vanadium Microalloying on Deformation-Induced Pearlite Transformation of Eutectoid Steel
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Theoretical Calculation of Vanadium Solubility and Ar1 Temperatures
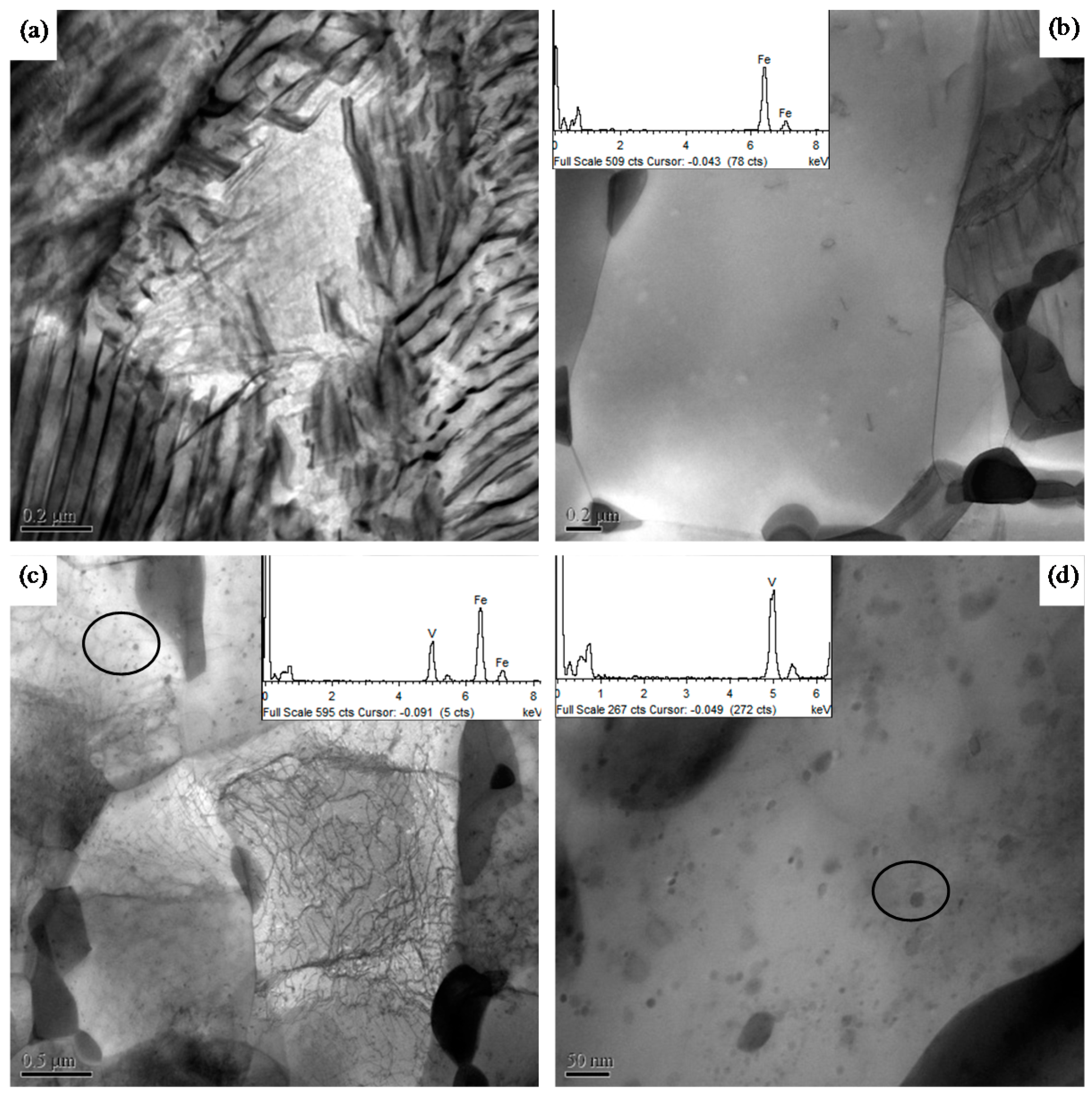
3.2. Precipitation of Vanadium Carbides, Nitrides, or Carbonitrides
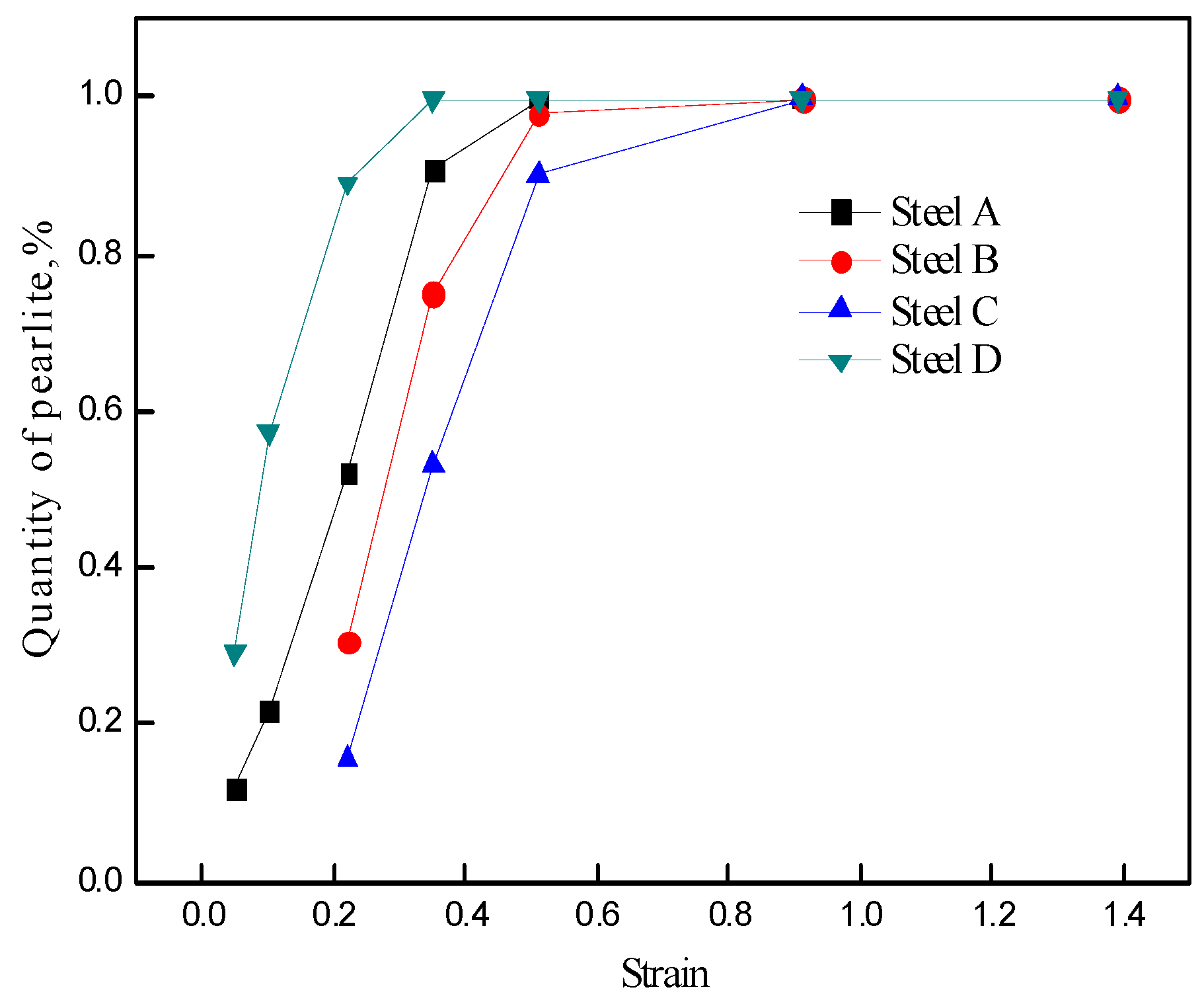
3.3. Fraction of Deformation-Induced Pearlite
3.4. Misorientation Angle and Grain Size of Ferrite
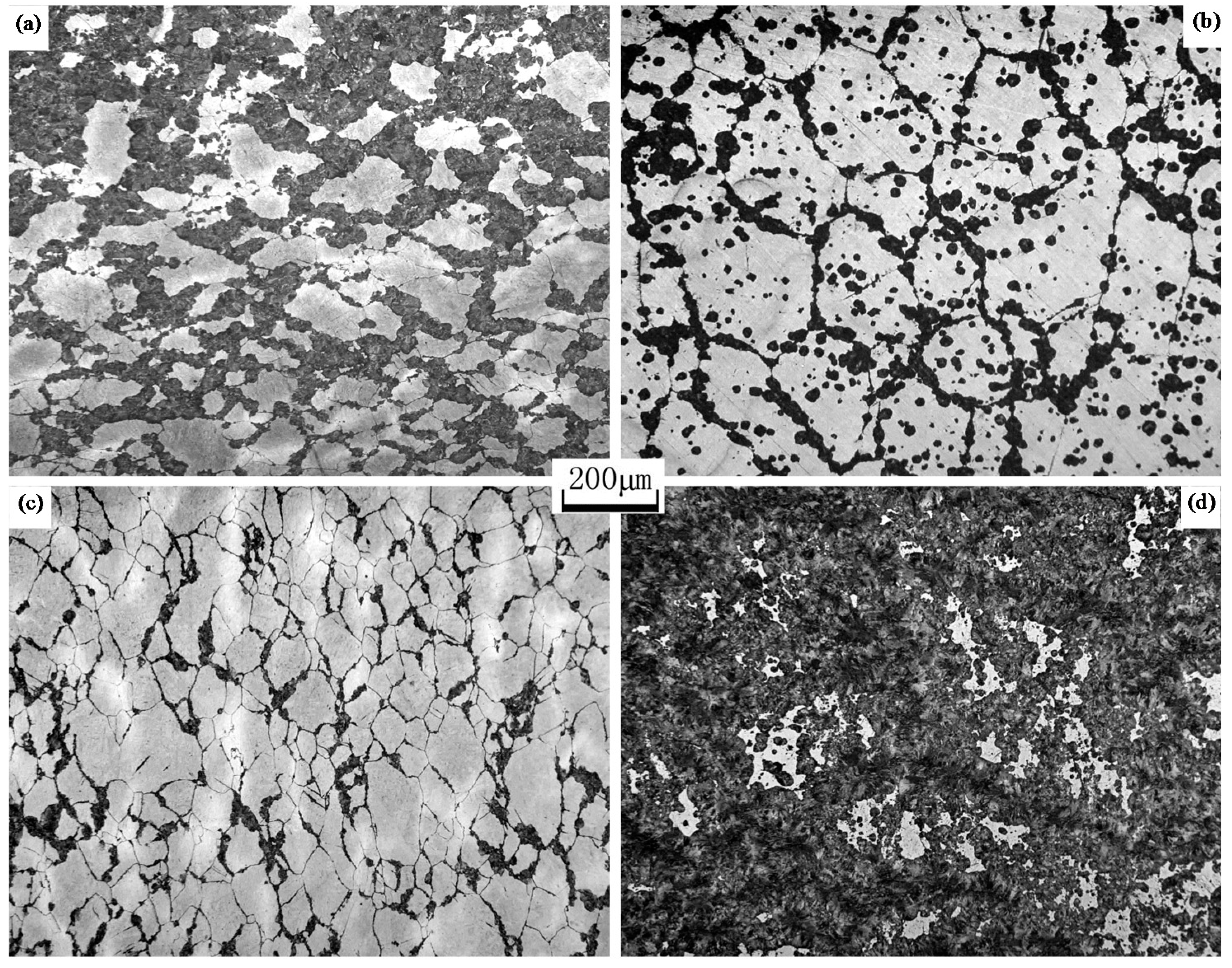
3.5. Spheroidization of Cementites
4. Conclusions
- (1)
- Vanadium in Steels B, C, and D was completely dissolved in austenite at an austenization temperature of 1150 °C. The pearlite transformation at a cooling rate of 20 °C/s was postponed and restrained at the dissolved vanadium content of 0.1 mass% in Steels B and D, especially at 0.27 wt% in Steel C. During the deformation, vanadium carbides in Steels B and C were precipitated in ferrite when the strain value was 0.91. However, vanadium nitrides or carbonitrides in Steel D were precipitated in austenite under a small deformation with a strain of 0.05 as vanadium has a higher affinity for nitrogen as compared to carbon, and the precipitation of vanadium can significantly be improved by the addition of N.
- (2)
- The fraction of deformation-induced pearlite increased with the increase of strain in all the steels, and the fractions in vanadium-microalloyed Steels B and C were lower as compared to that in vanadium-free Steel A at the same strain level before the contained vanadium began to precipitate because the dissolved vanadium postponed and restrained DIPT.
- (3)
- The fraction of deformation-induced pearlite in Steel D was higher as compared to that in Steel A because the precipitation of vanadium nitrides or carbonitrides facilitated the formation of proeutectoid ferrite along the boundary of austenite grain and pearlitic ferrite inside the grain by acting as a nucleus. Thus, the nucleation of pearlite along the boundary of austenite grain (AG pearlite) and intragranular pearlite (IG pearlite) was improved because of carbon gathering due to the formation of ferrite.
- (4)
- The spheroidization speed of cementites in Steels B, C, and D with vanadium microalloying was slower as compared to that in Steel A because vanadium carbides, nitrides, or carbonitrides and dissolved vanadium reduced the diffusion rate of carbon.
- (5)
- Steel D microalloyed with vanadium and with the addition of N showed the optimal microstructure with the maximum fraction of the recrystallized ferrite and the most uniform ferrite grain size and completely spheroidized cementites when the strain attained a value of 1.39, the reason is because the rate of pearlite transformation in Steel D was the fastest and the “pancake” ferrite took more time to recrystallize.
Author Contributions
Funding
Conflicts of Interest
References
- Lutsenko, V.A.; Matochkin, V.A.; Khudolei, Y.L.; Chernichenk, V.G.; Lutsenko, O.V. Influence of thermomechanical treatment and alloying on the properties of high carbon wire rod. Steel Transl. 2010, 40, 853–856. [Google Scholar] [CrossRef]
- Zhuchkov, S.M.; Matochkin, V.A.; Gorbanev, A.A. Production of high-quality wire rod. Steel Transl. 2007, 37, 448–452. [Google Scholar] [CrossRef]
- Verlinden, B.; Driver, J.; Samajdar, I.; Doherty, R.D. Thermo-mechanical processing of steel. In Thermo-Mechanical Processing of Metallic Materials; Elsevier: London, UK, 2007. [Google Scholar]
- Wu, S.; Li, X.C.; Zhang, J.; Shang, C.J. Effect of Nb on transformation and microstructure refinement in medium carbon steel. Acta Metall. Sin. 2014, 50, 400–408. [Google Scholar]
- Wu, T.; Gao, Y.W.; Wang, M.Z. Influence of initial microstructure on warm deformation processability and microstructure of an ultrahigh carbon steel. J. Iron Steel Res. Int. 2014, 21, 52–59. [Google Scholar] [CrossRef]
- Holtzman, A.H.; Danko, J.C.; Stout, R.D. Spheroidization of cold-worked pearlite. Trans. Met. Soc. AIME 1958, 212, 475–478. [Google Scholar]
- Lu, Z.Q.; Zhang, H.F.; Meng, Q. Effect of cyclic annealing on microstructure and mechanical properties of medium carbon steel. J. Iron Steel Res. Int. 2016, 23, 145–150. [Google Scholar] [CrossRef]
- Ji, C.; Wang, L.; Zhu, M.Y. Effect of subcritical annealing temperature on microstructure and mechanical properties of SCM435 steel. J. Iron Steel Res. Int. 2015, 22, 1031–1036. [Google Scholar] [CrossRef]
- Tao, W.; Wang, M.Z.; Gao, Y.W.; Li, X.P.; Zhao, Y.C.; Zou, Q. Effects of plastic warm deformation on cementite spheroidization of a eutectoid steel. J. Iron Steel Res. Int. 2012, 19, 60–66. [Google Scholar]
- Li, L.; Yang, W.; Sun, Z. Effects of Particle Size on Mechanical Properties of a TiC Containing Tool Steel by Hot Isostatic Press. Met. Mat. Trans. 2008, 39, 624–629. [Google Scholar] [CrossRef]
- Hickson, M.R.; Gibbs, R.K.; Hodgson, P.D. The Effect of Chemistry on the Formation of Ultrafine Ferrite in Steel. ISIJ Int. 1999, 39, 1176–1182. [Google Scholar] [CrossRef]
- Zhang, S.L.; Sun, X.J.; Dong, H. Mechanism of Austenite Evolution during Deformation of Ultra-High Carbon Steel. J. Iron Steel Res Int. 2008, 15, 42–46. [Google Scholar] [CrossRef]
- Huang, Q.S.; Li, L.F.; Yang, W. Dynamic Transformation of Undercooling Austenite and Microstructure Refinement in a Eutectoid Steel. Acta Metall. Sin. 2007, 43, 724–730. [Google Scholar]
- Chen, W.; Li, L.F.; Yang, W.Y. Microstructure Evolution of Hypereutectoid Steels During Warm Deformation I. Formation of Equiaxial Ferrite and Effects of Al. Acta Metall. Sin. 2009, 45, 151–155. [Google Scholar]
- Rastegari, H.; Kermanpur, A.; Najafizadeh, A.; Porter, D.; Somani, M. Warm Deformation Processing Maps for the Pain Eutectoid Steels. J. Alloy Compd. 2015, 626, 136–144. [Google Scholar] [CrossRef]
- Xiong, Y.X.; Fu, W.T.; Li, Y. Warm Deformation Behavior of High Carbon Steel. J. Iron Steel Res. Int. 2007, 19, 58–63. [Google Scholar]
- Zhang, S.L.; Sun, X.J.; Dong, H. Effect of Deformation on the Evolution of Spheroidization for the Ultra High Carbon Steel. Mater. Sci. Eng. A 2006, 432, 324–332. [Google Scholar] [CrossRef]
- Li, J.; Choi, P.; Borchers, C.; Westerkamp, S.; Goto, S.; Raabe, D.; Kirchheim, R. Atomic-scale Mechanisms of Deformation-induced Cementite Decomposition in Pearlite. Acta Mater. 2011, 59, 3965–3977. [Google Scholar] [CrossRef]
- Han, K.; Mottishaw, T.D.; Smith, G.D.; Edmonds, D.V.; Stacey, A.G. Effects of Vanadium Additions on Microstructure and Hardness of Hypereutectoid Pearlitic Steels. Mater. Sci. Eng. A 1995, 190, 207–213. [Google Scholar] [CrossRef]
- Jaiswal, S.; McIvor, I.D. Metallurgy of Vanadium-Microalloyed High-carbon Steel rod. Mater. Sci. Technol. 1985, 1, 276–283. [Google Scholar] [CrossRef]
- Izotov, B.I. Precipitation of Disperse Vanadium Carbides at the Interphase Boundary upon the Pearlitic Transformation of a Steel. PMM 2011, 111, 592–597. [Google Scholar] [CrossRef]
- Li, L.; Virta, J. Ultrahigh Strength Steel Wires Processed by Severe Plastic Deformation for Ultrafine Grained Microstructure. Mater. Sci. Tech. 2011, 27, 845–862. [Google Scholar] [CrossRef]
- Matlock, D.K.; Speer, J.G. Microalloying Concepts and Application in Long Products. Mater. Sci. Technol. 2009, 25, 1118–1125. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.M. The Effects of V on Phase Transformation of High Carbon Steel during Continuous Cooling. Acta Metall. Sin. 2010, 46, 1502–1510. [Google Scholar]
- Hu, X.J.; Zhao, Y.F.; Wang, L. Effect of Vanadium on the Microstructure and Properties of High Carbon Steel Wires. Iron & Steel 2014, 49, 71–75. [Google Scholar]
- Wang, K.; Yu, H.Y.; He, J.C. Influence of Cooling Rate on Microstructure Evolution Due to Deformation Induced Ferrite Transformation in Vanadium Microalloyed Steel. J. Northeastern Univ. 2009, 30, 1740–1742. [Google Scholar]
- Wang, K.; Wang, L.J.; Cui, W.F. Effect of Vanadium and Vanadium-N Microalloying on Deformation –Induced Ferrite Transformation in Low Carbon Steels. J. Mater. Sci. Technol. 2006, 22, 159–163. [Google Scholar] [CrossRef]
- Sharma, R.C.; Lakshmanan, K.; Kirkaldy, J.S. Solubility of Niobium Carbide and Niobium Carbonitride in Alloyed Austenite and Ferrite. Metall. Mater. Trans. A 1984, 15, 545–553. [Google Scholar] [CrossRef]
- Karmakar, A.; Mukherjee, S.; Kund, S.; Srivastava, D.; Mitra, R.; Chakrabarti, D. Effect of Composition and Isothermal Holding Temperature on the Precipitation Hardening in Vanadium-microalloyed steels. Mater. Charact. 2017, 132, 31–40. [Google Scholar] [CrossRef]
- Rune, L.; Bevis, H.; Tadeusz, S.; Stanislaw, Z. The Role of Vanadium in Microalloyed Steels. Scand. J. Metall. 1999, 28, 186–241. [Google Scholar]
- Liu, Q.C.; Yong, Q.L.; Zheng, Z.W. Effect of Nitrogen on the Vanadium Precipitation Behavior of Higher Yield Strength Weathering Steels. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Han, K.; Mottishaw, T.D.; Smith, G.D.W.; Edmonds, D.V.; Stacey, A.G. Effects of Vanadium Addition on Nucleation and Growth of Pearlite in High Carbon Steel. Mater. Sci. Tech. 1994, 10, 955–963. [Google Scholar] [CrossRef]
- Khalid, F.A.; Edmonds, D.V. Effect of Vanadium on the Grain Boundary Carbide Nucleation of Pearlite in High-carbon Steels. Scr. Metal. Mater. 1994, 30, 1251–1255. [Google Scholar] [CrossRef]
- Hsu, T.Y. Additivity Hypothesis and Effects of Stress on Phase Transformations in Steel. Curr. Opin. Sol. Stat. Mater. Sci. 2005, 9, 256–268. [Google Scholar]
- Chen, W.; Li, L.F.; Yang, W.Y. Microstructure Evolution of Hypereutectoid Steels during Warm Deformation II. Cementite Spheroidization and Effects of Al. Acta Metall. Sin. 2009, 45, 156–160. [Google Scholar]
- Hales, S.; McNelley, T.; McQueen, H. Recrystallization and Superplasticity at 300 °C in an Aluminum-magnesium Alloy. Metall. Mater. Trans. A 1991, 22, 1037–1043. [Google Scholar] [CrossRef]
- Haessner, F. Recrystallization of Metallic Materials; Dr. Riederer Verlag: Stuttgart, Germany, 1978; p. 159. [Google Scholar]
- Hornbogen, E. Combined reactions. Metall. Mater. Trans. A 1979, 10, 947. [Google Scholar] [CrossRef]
- Baudelet, B.; Surey, M. Superplasticity; Centre National de la Recherche Scientifique: Paris, France, 1985; Volume 7, pp. 1–14. [Google Scholar]
- Storojeva, L.; Ponge, D.; Kaspar, R.; Raabe, D. Development of Microstructure and Texture of Medium Carbon Steel during Heavy Warm Deformation. Acta. Materialia. 2004, 52, 2209–2220. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Tan, Y.B.; Ji, X.M.; Xiang, Z.J.; He, Y.; Xiang, S. In Situ Study of Cementite Deformation and its Fracture Mechanism in Pearlitic Steels. Mater. Sci. Eng. A 2018, 731, 93–101. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sellars, S. Kinetics of Pearlite Spheroidisation during Static Annealing and during Hot Dformation. Acta Met. 1982, 30, 157–170. [Google Scholar] [CrossRef]
- Tian, Y.L.; Kraft, R.W. Kinetics of Pearlite Spheroidization. Metall. Trans. A. 1987, 18, 1359–1369. [Google Scholar] [CrossRef]
- Cree, A.M.; Faulkner, R.G.; Lyne, A.T. Cementite Particle Coarsening During Spheroidisation of Bearing Steel SAE 52100. Mater. Sci. Technol. 1995, 11, 566–571. [Google Scholar] [CrossRef]
- Jorge-badiola, D.; Iza-mendia, A.; López, B.; Rodriguez-ibabe, J.M. Role of Vanadium Microalloying in Austenite Conditioning and Pearlite Microstructure in Thermomechanically Processed Eutectoid Steels. ISIJ Int. 2009, 49, 1615–1623. [Google Scholar] [CrossRef] [Green Version]













| Steel | C | Si | Mn | P | S | V | N |
|---|---|---|---|---|---|---|---|
| A | 0.798 | 0.21 | 0.33 | <0.015 | <0.01 | - | - |
| B | 0.80 | 0.21 | 0.32 | <0.015 | <0.01 | 0.094 | - |
| C | 0.78 | 0.22 | 0.33 | <0.015 | <0.01 | 0.27 | - |
| D | 0.79 | 0.22 | 0.35 | <0.015 | <0.01 | 0.098 | 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Mao, X.; Bao, S.; Zhao, G.; Xu, Y. Influence of Vanadium Microalloying on Deformation-Induced Pearlite Transformation of Eutectoid Steel. Metals 2019, 9, 268. https://doi.org/10.3390/met9020268
Cai Z, Mao X, Bao S, Zhao G, Xu Y. Influence of Vanadium Microalloying on Deformation-Induced Pearlite Transformation of Eutectoid Steel. Metals. 2019; 9(2):268. https://doi.org/10.3390/met9020268
Chicago/Turabian StyleCai, Zhen, Xinping Mao, Siqian Bao, Gang Zhao, and Yaowen Xu. 2019. "Influence of Vanadium Microalloying on Deformation-Induced Pearlite Transformation of Eutectoid Steel" Metals 9, no. 2: 268. https://doi.org/10.3390/met9020268
APA StyleCai, Z., Mao, X., Bao, S., Zhao, G., & Xu, Y. (2019). Influence of Vanadium Microalloying on Deformation-Induced Pearlite Transformation of Eutectoid Steel. Metals, 9(2), 268. https://doi.org/10.3390/met9020268





