The Potential of Magnesium Based Materials in Mandibular Reconstruction
Abstract
1. Introduction
2. Biomechanics of Mandible
3. Modular Endoprosthesis
4. Magnesium/Magnesium Alloys
5. Magnesium/Magnesium Alloys in Mandibular Reconstruction
6. Challenges of Mg-Based Biomaterials in Clinical Applications
- 1.
- Biosafety and biocompatibility: Elements such as Al and Be have been identified as neurotoxic [68] and should preferably be avoided for alloying, as they are not suitable candidates for biomedical applications.
- 2.
- Requisite strength and ductility: As for orthopedic implants, materials possessing yield strength of >200 MPa, elongation > 10%, and a degradation rate < 0.5 mm/y in simulated fluids at 37 °C, ensures a productive lifetime of 90–180 days; whereas, for cardiovascular stents, higher ductility and moderate strength are desirable for cardiovascular stents, with elongation > 20% [80].
- 3.
- Uniform degradation: Most studied magnesium alloys are highly susceptible to localized corrosion [61]. Regardless, uniform and controllable degradation characteristics are essential for accurate predictions of an implant serving its lifetime. Also, Mg has a high negative standard electrode potential which leads to the rapid corrosion of Mg-based alloys in chloride physiological conditions producing Mg(OH)2 and H2 gas. Pure Mg dissolves in physiological solutions through the following electrochemical reactions [62]:
- 4.
- Cost effective eco-friendly production technique: Design and development of the biomaterial economically-conscious processing methodologies by the usage of eco-friendly protective gases like argon (Ar) over greenhouse cases like sulfur hexafluoride (SF6) to synthesize the material [58]. Disintegrated melt deposition and microwave sintering assisted powder metallurgy techniques can be identified as suitable processes for the primary synthesis of the biomaterial [58]. Incorporation of these processes will go a long way in reducing the cost of the technology and eventual cost borne by the end user.
7. Methods to Enhance the Corrosion Resistance of Magnesium and its Alloys
8. Current Research on Nanocomposites as Orthopaedic Materials and Future of Mg-Based Nanocomposites in the Field of Mandibular Reconstruction
9. Conclusions and Future Scope
Author Contributions
Funding
Conflicts of Interest
References
- Chim, H.; Salgado, C.J.; Mardini, S.; Chen, H.-C. Seminars in Plastic Surgery; Thieme Medical Publishers: New York, NY, USA, 2010; p. 188. [Google Scholar]
- Hadlock, T.A.; Vacanti, J.P.; Cheney, M.L. Tissue engineering in facial plastic and reconstructive surgery. Facial Plast. Surg. 1998, 14, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Goh, B.; Tideman, H.; Stoelinga, P. Modular endoprosthesis for mandibular reconstruction: A preliminary animal study. Int. J. Oral Maxillofac. Surg. 2008, 37, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Goh, B.; Tideman, H.; Stoelinga, P.; Jansen, J. Modular endoprosthesis for mandibular reconstruction: A clinical, microcomputed, tomographic and histologic evaluation in 8 Macaca fascicularis. Int. J. Oral Maxillofac. Surg. 2009, 38, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Lee, S.; Tideman, H.; Merkx, M.; Jansen, J.; Liao, K. Effect of replacement of mandibular defects with a modular endoprosthesis on bone mineral density in a monkey model. Int. J. Oral Maxillofac. Surg. 2011, 40, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, A.; Hewicker-Trautwein, M.; Erdmann, N.; Angrisani, N.; Reifenrath, J.; Meyer-Lindenberg, A. Comparison of morphological changes in efferent lymph nodes after implantation of resorbable and non-resorbable implants in rabbits. Biomed. Eng. Online 2011, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Duygulu, O.; Kaya, R.A.; Oktay, G.; Kaya, A.A. Investigation on the potential of magnesium alloy AZ31 as a bone implant. Mater. Sci. Forum 2007, 546–549, 421–424. [Google Scholar] [CrossRef]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: A comparative in vivo study in rabbits. Acta Biomater. 2011, 7, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, N.; Bondarenko, A.; Hewicker-Trautwein, M.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Meyer-Lindenberg, A. Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: A comparative study in rabbits. Biomed. Eng. Online 2010, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Hampp, C.; Ullmann, B.; Reifenrath, J.; Angrisani, N.; Dziuba, D.; Bormann, D.; Seitz, J.M.; Meyer-Lindenberg, A. Research on the biocompatibility of the new magnesium alloy LANd442—An in vivo study in the rabbit tibia over 26 weeks. Adv. Eng. Mater. 2012, 14, B28–B37. [Google Scholar] [CrossRef]
- Hampp, C.; Angrisani, N.; Reifenrath, J.; Bormann, D.; Seitz, J.-M.; Meyer-Lindenberg, A. Evaluation of the biocompatibility of two magnesium alloys as degradable implant materials in comparison to titanium as non-resorbable material in the rabbit. Mater. Sci. Eng. C 2013, 33, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Huehnerschulte, T.A.; Reifenrath, J.; von Rechenberg, B.; Dziuba, D.; Seitz, J.M.; Bormann, D.; Windhagen, H.; Meyer-Lindenberg, A. In vivo assessment of the host reactions to the biodegradation of the two novel magnesium alloys ZEK100 and AX30 in an animal model. Biomed. Eng. Online 2012, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodeling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jo, J.H.; Lee, S.M.; Kang, M.H.; Kim, H.E.; Estrin, Y.; Lee, J.H.; Lee, J.W.; Koh, Y.H. Hydroxyapatite-coated magnesium implants with improved in vitro and in vivo biocorrosion, biocompatibility, and bone response. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2014, 102, 429–441. [Google Scholar]
- Krause, A.; von der Höh, N.; Bormann, D.; Krause, C.; Bach, F.-W.; Windhagen, H.; Meyer-Lindenberg, A. Degradation behaviour and mechanical properties of magnesium implants in rabbit tibiae. J. Mater. Sci. 2010, 45, 624. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Thomann, M.; Krause, A.; Bormann, D.; Rechenberg, B.V.; Windhagen, H. Untersuchungen zum Einsatz einer Magnesiumbasislegierung als neues resorbierbares Implantatmaterial für die Osteosynthese. Kleintierpraxis 2010, 55, 349–363. [Google Scholar]
- Thomann, M.; Krause, C.; Bormann, D.; von der Höh, N.; Windhagen, H.; Meyer-Lindenberg, A. Comparison of the resorbable magnesium. alloys LAE442 und MgCa0.8 concerning their mechanical properties, their progress of degradation and the bone-implant-contact after 12 months implantation duration in a rabbit model. Mater. Und Werkst. Entwickl. Fert. Prüfungeigenschaften Und Anwend. Tech. Werkst. 2009, 40, 82–87. [Google Scholar] [CrossRef]
- Thomann, M.; Krause, C.; Angrisani, N.; Bormann, D.; Hassel, T.; Windhagen, H.; Meyer-Lindenberg, A. Influence of a magnesium-fluoride coating of magnesium-based implants (MgCa0.8) on degradation in a rabbit model. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 93, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Höh, N.V.D.; Bormann, D.; Lucas, A.; Denkena, B.; Hackenbroich, C.; Meyer-Lindenberg, A. Influence of different surface machining treatments of magnesium-based resorbable implants on the degradation behavior in rabbits. Adv. Eng. Mater. 2009, 11, B47–B54. [Google Scholar] [CrossRef]
- Von der Höh, N.; Bormann, D.; Lucas, A.; Thorey, F.; Meyer-Lindenberg, A. Comparison of the in vivo degradation progress of solid magnesium alloy cylinders and screw-shaped magnesium alloy cylinders in a rabbit model. Mater. Sci. Forum 2010, 638, 742–747. [Google Scholar] [CrossRef]
- Von der Höh, N.; von Rechenberg, B.; Bormann, D.; Lucas, A.; Meyer-Lindenberg, A. Influence of different surface machining treatments of resorbable magnesium alloy implants on degradation–EDX-analysis and histology results. Mater. Und Werkst. Entwickl. Fert. Prüfungeigenschaften Und Anwend. Tech. Werkst. 2009, 40, 88–93. [Google Scholar] [CrossRef]
- Willbold, E.; Kalla, K.; Bartsch, I.; Bobe, K.; Brauneis, M.; Remennik, S.; Shechtman, D.; Nellesen, J.; Tillmann, W.; Vogt, C. Biocompatibility of rapidly solidified magnesium alloy RS66 as a temporary biodegradable metal. Acta Biomater. 2013, 9, 8509–8517. [Google Scholar] [CrossRef] [PubMed]
- Willbold, E.; Kaya, A.; Kaya, R.; Beckmann, F.; Witte, F. Corrosion of magnesium alloy AZ31 screws is dependent on the implantation site. Mater. Sci. Eng. B 2011, 176, 1835–1840. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Ulrich, H.; Palm, C.; Willbold, E. Biodegradable magnesium scaffolds: Part II: Peri-implant bone remodeling. J. Biomed. Mater. Res. Part A 2007, 81, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Ulrich, H.; Rudert, M.; Willbold, E. Biodegradable magnesium scaffolds: Part 1: Appropriate inflammatory response. J. Biomed. Mater. Res. Part A 2007, 81, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Vogt, C.; Vogt, J.; Donath, T.; Beckmann, F. In vivo corrosion and corrosion protection of magnesium alloy LAE442. Acta Biomater. 2010, 6, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, G.; Zhang, E.; Pan, F.; Yang, K. In vivo corrosion behavior of Mg-Mn-Zn alloy for bone implant application. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pan, F.; Yu, G.; Yang, L.; Zhang, E.; Yang, K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials 2009, 30, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cui, F.; Lee, I.; Zhang, Y.; Yin, Q.; Xia, H.; Yang, S. In vivo biocompatibility and degradation behavior of Mg alloy coated by calcium phosphate in a rabbit model. J. Biomater. Appl. 2012, 27, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. Part A: Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Turvey, T.; Proffit, W.; Phillips, C. Biodegradable fixation for craniomaxillofacial surgery: A 10-year experience involving 761 operations and 745 patients. Int. J. Oral Maxillofac. Surg. 2011, 40, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Meslemani, D.; Kellman, R.M. Recent advances in fixation of the craniomaxillofacial skeleton. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Suuronen, R.; Pohjonen, T.; Vasenius, J.; Vainionpää, S. Comparison of absorbable self-reinforced multilayer poly-1-lactide and metallic plates for the fixation of mandibular body osteotomies: An experimental study in sheep. J. Oral Maxillofac. Surg. 1992, 50, 255–262. [Google Scholar] [CrossRef]
- Bell, R.B.; Kindsfater, C.S. The use of biodegradable plates and screws to stabilize facial fractures. J. Oral Maxillofac. Surg. 2006, 64, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Waizy, H.; Weizbauer, A.; Maibaum, M.; Witte, F.; Windhagen, H.; Lucas, A.; Denkena, B.; Meyer-Lindenberg, A.; Thorey, F. Biomechanical characterisation of a degradable magnesium-based (MgCa0.8) screw. J. Mater. Sci. Mater. Med. 2012, 23, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Denkena, B.; Podolsky, C.; Lucas, A.; Hassel, T.; Witte, F.; Palm, O. Processing of degradable surgical implants made of magnesium alloys. Biomaterialien 2005, 6, 171. [Google Scholar]
- Denkena, B.; Witte, F.; Podolsky, C.; Lucas, A. Degradable Implants Made of Magnesium Alloys. In Proceedings of the 5th Euspen International Conference, Montpellier, France, 8–11 May 2005; pp. 1–4. [Google Scholar]
- Witte, F.; Podolsky, C.; Hassel, T.; Lucas, A. Fertigung von magnesium-implantaten. Werkstattstechnik 2004, 94, 692–696. [Google Scholar]
- Reichert, J.C.; Hutmacher, D.W. Bone tissue engineering. In Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2011; pp. 431–456. [Google Scholar]
- Mravic, M.; Péault, B.; James, A.W. Current trends in bone tissue engineering. Biomed Res. Int. 2014, 2014, 865270. [Google Scholar] [CrossRef] [PubMed]
- Yusop, A.; Bakir, A.; Shaharom, N.; Kadir, M.A.; Hermawan, H. Porous biodegradable metals for hard tissue scaffolds: A review. Int. J. Biomater. 2012, 2012, 641430. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R: Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Ma, R.; Lai, Y.-X.; Li, L.; Tan, H.-L.; Wang, J.-L.; Li, Y.; Tang, T.-T.; Qin, L. Bacterial inhibition potential of 3D rapid-prototyped magnesium-based porous composite scaffolds–An in vitro efficacy study. Sci. Rep. 2015, 5, 13775. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Yi, W.-J.; Heo, M.-S.; Lee, S.-S.; Choi, S.-C.; Huh, K.-H.; Lee, S.-P. Direct measurement of trabecular bone anisotropy using directional fractal dimension and principal axes of inertia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 104, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Malawer, M.M.; Chou, L.B. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. JBJS 1995, 77, 1154–1166. [Google Scholar] [CrossRef]
- Riede, U.; Lüem, M.; Ilchmann, T.; Eucker, M.; Ochsner, P.E. The ME Müller straight stem prosthesis: 15 year follow-up. Survivorship and clinical results. Arch. Orthop. Trauma Surg. 2007, 127, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Tideman, H.; Lee, S. The TL endoprosthesis for mandibular reconstruction—A metallic yet biological approach. Asian J Oral Maxfac Surg 2006, 18, 5. [Google Scholar]
- Wong, R.C.; Tideman, H.; Merkx, M.A.; Jansen, J.; Goh, S.M. The modular endoprosthesis for mandibular body replacement. Part 2: Finite element analysis of endoprosthesis reconstruction of the mandible. J. Cranio-Maxillofac. Surg. 2012, 40, e487–e497. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.T.; Lee, S.; Tideman, H.; Stoelinga, P.J. Replacement of the condyle and ascending ramus by a modular endoprosthesis in Macaca fascicularis—Part 1: a clinical and radiographic study. J. Oral Maxillofac. Surg. 2009, 67, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Kujur, M.S.; Manakari, V.; Parande, G.; Tun, K.S.; Mallick, A.; Gupta, M. Enhancement of thermal, mechanical, ignition and damping response of magnesium using nano-ceria particles. Ceram. Int. 2018, 44, 15035–15043. [Google Scholar] [CrossRef]
- Gupta, M.; Parande, G.; Manakari, V. An Insight into High Performance Magnesium Alloy/Nano-Metastable-Syntactic Composites. In Proceedings of the 17th Australian International Aerospace Congress: AIAC 2017, Engineers Australia, Royal Aeronautical Society, Melbourne, Australia, 26–28 February 2017; p. 270. [Google Scholar]
- Parande, G.; Manakari, V.; Meenashisundaram, G.K.; Gupta, M. Enhancing the tensile and ignition response of monolithic magnesium by reinforcing with silica nanoparticulates. J. Mater. Res. 2017, 32, 2169–2178. [Google Scholar] [CrossRef]
- Manakari, V.; Parande, G.; Gupta, M. Effects of hollow fly-ash particles on the properties of magnesium matrix syntactic foams: A review. Mater. Perform. Charact. 2016, 5, 116–131. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.; Fajardo, S.; Birbilis, N.; Frankel, G.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Manakari, V.; Parande, G.; Gupta, M. Selective laser melting of magnesium and magnesium alloy powders: A review. Metals 2016, 7, 2. [Google Scholar] [CrossRef]
- Manakari, V.; Parande, G.; Doddamani, M.; Gupta, M. Enhancing the ignition, hardness and compressive response of magnesium by reinforcing with hollow glass microballoons. Materials 2017, 10, 997. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Shi, Z.; Atrens, A. The influence of pH on the corrosion rate of high-purity Mg, AZ91 and ZE41 in bicarbonate buffered Hanks’ solution. Corros. Sci. 2015, 101, 182–192. [Google Scholar] [CrossRef]
- Toda-Caraballo, I.; Galindo-Nava, E.I.; Rivera-Díaz-del-Castillo, P.E. Understanding the factors influencing yield strength on Mg alloys. Acta Mater. 2014, 75, 287–296. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Meenashisundaram, G.K.; Gupta, M. Enhancing the hardness/compression/damping response of magnesium by reinforcing with biocompatible silica nanoparticulates. Int. J. Mater. Res. 2016, 107, 1091–1099. [Google Scholar] [CrossRef]
- Kujur, M.S.; Mallick, A.; Manakari, V.; Parande, G.; Tun, K.S.; Gupta, M. Significantly enhancing the ignition/compression/damping response of monolithic magnesium by addition of Sm2O3 nanoparticles. Metals 2017, 7, 357. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Kopparthy, S.D.S.; Gupta, M. Utilizing low-cost eggshell particles to enhance the mechanical response of Mg–2.5Zn magnesium alloy matrix. Adv. Eng. Mater. 2018, 20, 1700919. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef] [PubMed]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xiong, M.; Zeng, F.; Xu, B.; Yang, L.; Guo, H.; Niu, J.; Zhang, J.; Chen, C.; Pei, J. Enhancement of osteogenesis and biodegradation control by brushite coating on Mg–Nd–Zn–Zr alloy for mandibular bone repair. ACS Appl. Mater. Interfaces 2014, 6, 21525–21533. [Google Scholar] [CrossRef] [PubMed]
- Van den Bos, T.; Speijer, D.; Bank, R.; Brömme, D.; Everts, V. Differences in matrix composition between calvaria and long bone in mice suggest differences in biomechanical properties and resorption: Special emphasis on collagen. Bone 2008, 43, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S.; Yao, Y.; Cui, F. Study on vertical mandibular distraction osteogenesis using magnesium alloy on canine. Prog. Nat. Sci. Mater. Int. 2014, 24, 446–451. [Google Scholar] [CrossRef]
- Guo, C.-W.; Yu, Q.; Sun, B.-Z.; Wang, C.-Y.; Yang, J.-X. Evaluation of alveolar bone repair with mineralized collagen block reinforced with Mg–Ca alloy rods. J. Biomater. Tissue Eng. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Lee, J.-H.; Han, H.-S.; Kim, Y.-C.; Lee, J.-Y.; Lee, B.-K. Stability of biodegradable metal (Mg–Ca–Zn alloy) screws compared with absorbable polymer and titanium screws for sagittal split ramus osteotomy of the mandible using the finite element analysis model. J. Cranio-Maxillofac. Surg. 2017, 45, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Kang, C.-Y.; Zhang, S.-N.; Yang, J.-X.; Cheng, G. Study on repairing canine mandibular defect with Mg–Sr alloy. J. Biomater. Tissue Eng. 2018, 8, 11–19. [Google Scholar] [CrossRef]
- Ding, W. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.; Kannan, S.; Vincent, S.; Eddine, N.; Muhammed, A.; Gupta, M.; Karthikeyan, R.; Badari, V. Influence of Silica Nanospheres on Corrosion Behavior of Magnesium Matrix Syntactic Foam. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Dubai, UAE, 28–29 November 2017; IOP Publishing: Dubai, UAE, 2018; p. 012012. [Google Scholar]
- Gupta, M.; Meenashisundaram, G.K. Insight into Designing Biocompatible Magnesium Alloys and Composites: Processing, Mechanical and Corrosion Characteristics; Springer: Singapore, 2015. [Google Scholar]
- Erinc, M.; Sillekens, W.; Mannens, R.; Werkhoven, R. Applicability of Existing Magnesium Alloys as Biomedical Implant Materials. In Proceedings of the Magnesium Technology 2009, San Francisco, CA, USA, 15–19 February 2009. [Google Scholar]
- Albayrak, O.; El-Atwani, O.; Altintas, S. Hydroxyapatite coating on titanium substrate by electrophoretic deposition method: effects of titanium dioxide inner layer on adhesion strength and hydroxyapatite decomposition. Surf. Coat. Technol. 2008, 202, 2482–2487. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, J.; Xie, X.; Zhang, P.; Lai, Y.; Li, Y.; Qin, L. Surface coating reduces degradation rate of magnesium alloy developed for orthopaedic applications. J. Orthop. Transl. 2013, 1, 41–48. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Zhang, P.; Li, Y.; Wang, J.; Lai, Y.; Qin, L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: A general review. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2012, 100, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.L.; Chan, D.C. Hydroxyapatite and their use as coatings in dental implants: A review. Crit. Rev. ™ Biomed. Eng. 2000, 28, 1–41. [Google Scholar] [CrossRef]
- Chai, H.; Guo, L.; Wang, X.; Gao, X.; Liu, K.; Fu, Y.; Guan, J.; Tan, L.; Yang, K. In vitro and in vivo evaluations on osteogenesis and biodegradability of a β-tricalcium phosphate coated magnesium alloy. J. Biomed. Mater. Res. Part A 2012, 100, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vivo assessments of bioabsorbable AZ91 magnesium implants coated with nanostructured fluoridated hydroxyapatite by MAO/EPD technique for biomedical applications. Mater. Sci. Eng. C 2015, 48, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yu, D.; Luo, Y.; Wang, F. Preparation and characterization of HA microflowers coating on AZ31 magnesium alloy by micro-arc oxidation and a solution treatment. Appl. Surf. Sci. 2013, 264, 816–822. [Google Scholar] [CrossRef]
- Sun, J.E.; Wang, J.; Jiang, H.; Chen, M.; Bi, Y.; Liu, D. In vivo comparative property study of the bioactivity of coated Mg–3Zn–0.8 Zr alloy. Mater. Sci. Eng. C 2013, 33, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R: Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Wu, G.; Ibrahim, J.M.; Chu, P.K. Surface design of biodegradable magnesium alloys—A review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Xu, L.; Yamamoto, A. Characteristics and cytocompatibility of biodegradable polymer film on magnesium by spin coating. Colloids Surf. B: Biointerfaces 2012, 93, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Hayes, W.; Keaveny, T.; Boskey, A.; Einhorn, T.; Iannotti, J. Form and function of bone. Orthop. Basic Sci. 1994, 127–185. [Google Scholar]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Nanoceramic surface roughness enhances osteoblast and osteoclast functions for improved orthopaedic/dental implant efficacy. Scr. Mater. 2001, 44, 1639–1642. [Google Scholar] [CrossRef]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Increased osteoblast functions on nanophase titania dispersed in poly-lactic-co-glycolic acid composites. Nanotechnology 2005, 16, S601. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomaterials 1999, 20, 1221–1227. [Google Scholar] [CrossRef]
- Liu, H.; Webster, T.J. Bioinspired Nanocomposites for Orthopedic Applications. In Nanotechnology for the Regeneration of Hard and Soft Tissues; World Scientific: Cranston, RI, USA, 2007; pp. 1–51. [Google Scholar]
- Hickey, D.J.; Ercan, B.; Chung, S.; Webster, T.J.; Sun, L.; Geilich, B. MgO Nanocomposites as New Antibacterial Materials for Orthopedic Tissue Engineering Applications. In Proceedings of the Bioengineering Conference (NEBEC), 2014 40th Annual Northeast, IEEE, 2014, Boston, MA, USA, 25–27 April 2014; pp. 1–2. [Google Scholar]
- Kumar, A.; Meenashisundaram, G.K.; Manakari, V.; Parande, G.; Gupta, M. Lanthanum effect on improving CTE, damping, hardness and tensile response of Mg-3Al alloy. J. Alloy. Compd. 2017, 695, 3612–3620. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Ong, T.H.D.; Parande, G.; Manakari, V.; Xiang, S.; Gupta, M. Using lanthanum to enhance the overall ignition, hardness, tensile and compressive strengths of Mg-0.5 Zr alloy. J. Rare Earths 2017, 35, 723–732. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Wakeel, S.; Kujur, M.; Gupta, M. Enhancing Mechanical Response of Monolithic Magnesium Using Nano-NiTi (Nitinol) Particles. Metals 2018, 8, 1014. [Google Scholar] [CrossRef]
- Wakeel, S.; Manakari, V.; Parande, G.; Kujur, M.S.; Gupta, M. Synthesis and mechanical response of NiTi SMA nanoparticle reinforced Mg composites synthesized through microwave sintering process. Mater. Today: Proc. 2018, 5, 28203–28210. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Gupta, H.; Gupta, M. Magnesium-β-tricalcium phosphate composites as a potential orthopedic implant: A mechanical/damping/immersion perspective. Metals 2018, 8, 343. [Google Scholar] [CrossRef]
- Meenashisundaram, G.; Gupta, M. Emerging environment friendly, magnesium-based composite technology for present and future generations. JOM 2016, 68, 1890–1901. [Google Scholar] [CrossRef]
- Ong, T.H.D.; Yu, N.; Meenashisundaram, G.K.; Schaller, B.; Gupta, M. Insight into cytotoxicity of Mg nanocomposites using MTT assay technique. Mater. Sci. Eng. C 2017, 78, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Khalajabadi, S.Z.; Abu, A.H.; Ahmad, N.; Kadir, M.; Ismail, A.; Nasiri, R.; Haider, W.; Redzuan, N. Biodegradable Mg/HA/TiO2 nanocomposites coated with MgO and Si/MgO for orthopedic applications: A study on the corrosion, surface characterization, and biocompatibility. Coatings 2017, 7, 154. [Google Scholar] [CrossRef]
- Haghshenas, M. Mechanical characteristics of biodegradable magnesium matrix composites: A review. J. Magnes. Alloy. 2017, 5, 189–201. [Google Scholar] [CrossRef]
- Prakash, C.; Singh, S.; Gupta, M.; Mia, M.; Królczyk, G.; Khanna, N. Synthesis, characterization, corrosion resistance and in-vitro bioactivity behavior of biodegradable Mg–Zn–Mn–(Si–HA) composite for orthopaedic applications. Materials 2018, 11, 1602. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Molecular hydrogen: A therapeutic antioxidant and beyond. Med Gas Res. 2016, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Glocker, D.; Ranade, S. Medical Coatings and Deposition Technologies; John Wiley & Sons: Beverly, MA, USA, 2016. [Google Scholar]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, W.; Zheng, Y.; Cheng, Y.; Wei, S.; Zhong, S.; Xi, T.; Chen, L. Corrosion fatigue behaviors of two biomedical Mg alloys–AZ91D and WE43–in simulated body fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef] [PubMed]





| Properties | Human Bone | Magnesium | Titanium Alloy | Co-Cr Alloy | Stainless Steel | Hydroxyapatite |
|---|---|---|---|---|---|---|
| Density (g/cm3) | 1.8 to 2.1 | 1.74 to 2.0 | 4.4 to 4.5 | 8.3 to 9.2 | 8.9 to 8.1 | 3.1 |
| Modulus of Elasticity (GPa) | 3–20 | 41–45 | 110–117 | 230 | 185–205 | 73–117 |
| Compressive Strength (MPa) | 130–180 | 65–100 | 758–1117 | 450–1000 | 170–310 | 600 |
| Tensile Strength (MPa·m2) | 3–6 | 15–40 | 55–115 | indeterminate | 50–200 | 0.7 |
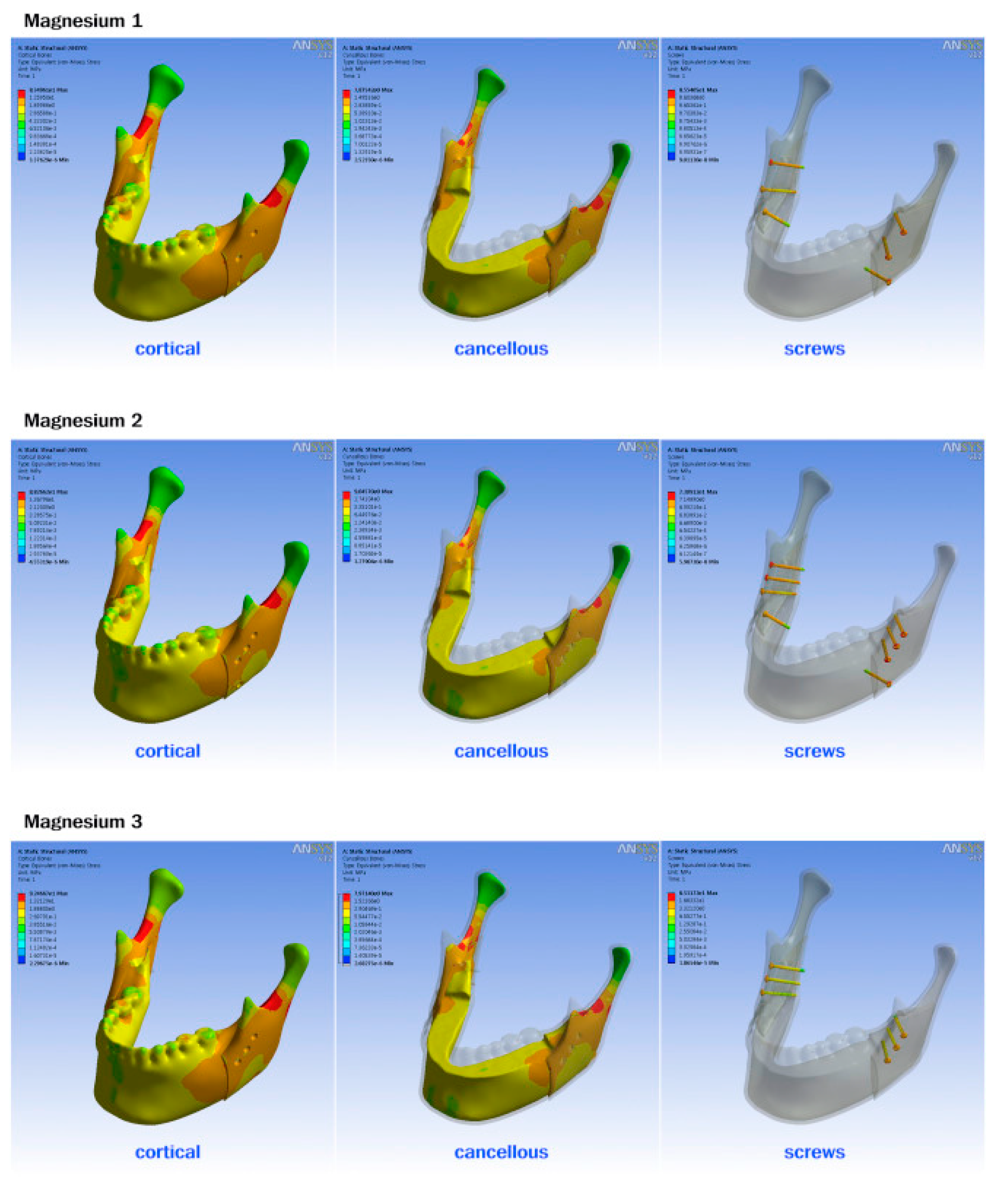
| Component | Titanium T | Polymer 1 T | Polymer 2 RT | Polymer 3 L | Mg 1 T | Mg 2 RT | Mg 3 L |
|---|---|---|---|---|---|---|---|
| Cortical | 83.49 | 83.53 | 88.38 | 92.55 | 83.49 | 88.26 | 92.46 |
| Cancellous | 7.87 | 7.88 | 9.06 | 7.98 | 7.87 | 9.04 | 7.97 |
| Screw | 129.95 | 7.19 | 8.77 | 9.65 | 85.54 | 73.09 | 85.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasadh, S.; Ratheesh, V.; Manakari, V.; Parande, G.; Gupta, M.; Wong, R. The Potential of Magnesium Based Materials in Mandibular Reconstruction. Metals 2019, 9, 302. https://doi.org/10.3390/met9030302
Prasadh S, Ratheesh V, Manakari V, Parande G, Gupta M, Wong R. The Potential of Magnesium Based Materials in Mandibular Reconstruction. Metals. 2019; 9(3):302. https://doi.org/10.3390/met9030302
Chicago/Turabian StylePrasadh, Somasundaram, Vaishnavi Ratheesh, Vyasaraj Manakari, Gururaj Parande, Manoj Gupta, and Raymond Wong. 2019. "The Potential of Magnesium Based Materials in Mandibular Reconstruction" Metals 9, no. 3: 302. https://doi.org/10.3390/met9030302
APA StylePrasadh, S., Ratheesh, V., Manakari, V., Parande, G., Gupta, M., & Wong, R. (2019). The Potential of Magnesium Based Materials in Mandibular Reconstruction. Metals, 9(3), 302. https://doi.org/10.3390/met9030302









