Fabrication, Morphology Analysis, and Mechanical Properties of Ti Foams Manufactured Using the Space Holder Method for Bone Substitute Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Preparation
2.2. Morphology, Porosity, and Phase Analysis
2.3. Mechanical Testing
3. Results
3.1. Morphology and Phase Analysis
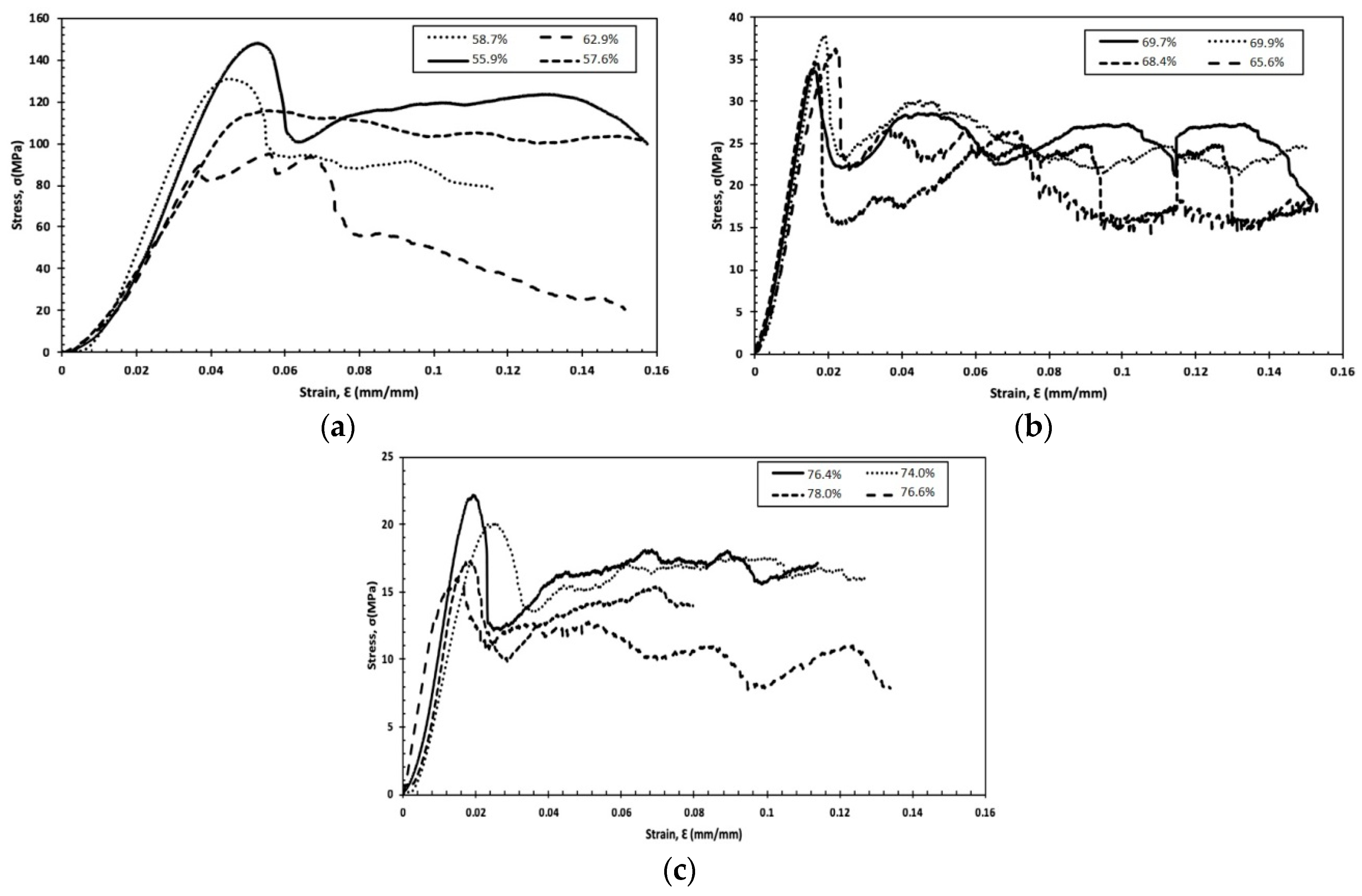
3.2. Mechanical Properties
3.2.1. Stress–Strain Curves
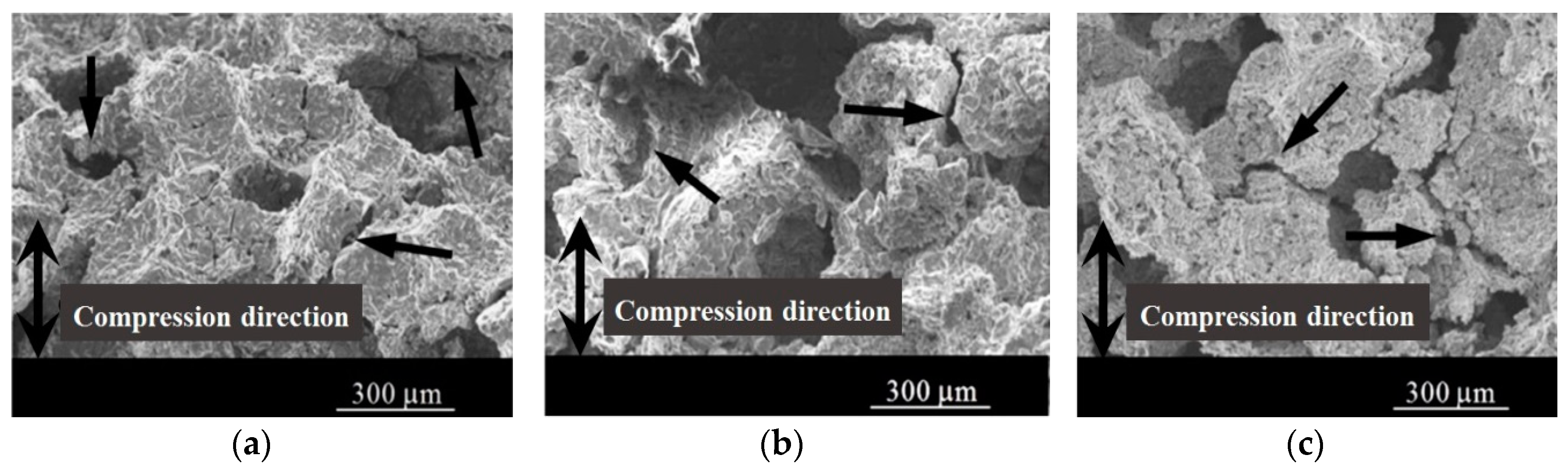
3.2.2. Macroscopic Deformation Band
3.2.3. Microscopic Deformation Mode of Cell Wall
4. Discussion
4.1. Structure Observation
4.2. Mechanical Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Lee, P.D.; Jones, J.R.; Poologasundarampillai, G.; Post, T.; Lindley, T.C.; Dashwood, R.J. Hierarchically structured titanium foams for tissue scaffold applications. Acta Biomater. 2010, 6, 4596–4604. [Google Scholar] [CrossRef] [PubMed]
- Esen, Z.; Bor, Ş. Characterization of Ti-6Al-4V alloy foams synthesized by space holder technique. Mater. Sci. Eng. A 2011, 528, 3200–3209. [Google Scholar] [CrossRef]
- Kashef, S.; Asgari, S.A.; Hodgson, P.; Yan, W.Y. Simulation of Three-Point Bending Test of Titanium Foam for Biomedical Applications. Adv. Mater. Res. 2008, 32, 237–240. [Google Scholar] [CrossRef]
- Imwinkelried, T. Mechanical properties of open-pore titanium foam. J. Biomed. Mater. Res. Part A 2007, 81, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Fujibayashi, S.; Kokubo, T. Titanium foam for bone tissue engineering. Met. Foam Bone Process. Modif. Charact. Prop. 2016, 17, 111–130. [Google Scholar]
- Barrabés, M.; Sevilla, P.; Planell, J.A.; Gil, F.J. Mechanical properties of nickel-titanium foams for reconstructive orthopaedics. Mater. Sci. Eng. C 2008, 28, 23–27. [Google Scholar] [CrossRef]
- Kato, K.; Ochiai, S.; Yamamoto, A.; Daigo, Y.; Honma, K.; Matano, S.; Omori, K. Novel multilayer Ti foam with cortical bone strength and cytocompatibility. Acta Biomater. 2013, 9, 5802–5809. [Google Scholar] [CrossRef]
- Tuncer, N.; Arslan, G. Designing compressive properties of titanium foams. J. Mater. Sci. 2009, 44, 1477–1484. [Google Scholar] [CrossRef]
- Xue, X.B.; Zhao, Y.Y.; Kearns, V.; Williams, R.L. Mechanical and Biological Properties of Titanium Syntactic Foams. Miner. Met. Mater. Soc. 2010, 2, 129–136. [Google Scholar]
- Mansourighasri, A.; Muhamad, N.; Sulong, A.B. Processing titanium foams using tapioca starch as a space holder. J. Mater. Process. Technol. 2012, 212, 83–89. [Google Scholar] [CrossRef]
- Kashef, S.; Asgari, A.; Hilditch, T.B.; Yan, W.; Goel, V.K.; Hodgson, P.D. Fatigue crack growth behavior of titanium foams for medical applications. Mater. Sci. Eng. A 2011, 528, 1602–1607. [Google Scholar] [CrossRef]
- Jian, X.; Hao, C.; Qiu, G.; Yang, Y.; Lyu, X. Investigation on relationship between porosity and spacer content of titanium foams. Mater. Des. 2015, 88, 132–137. [Google Scholar] [CrossRef]
- Ahmad, S.; Kassim, A. Fabrication of titanium foam by using compaction method. In Proceedings of the 26th Regional Conference on Solid State Science & Technology 2011, Negeri Sembilan, Malaysia, 22–24 November 2011. [Google Scholar]
- Spoerke, E.D.; Murray, N.G.; Li, H.; Brinson, L.C.; Dunand, D.C.; Stupp, S.I. A bioactive titanium foam scaffold for bone repair. Acta Biomater. 2005, 1, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Y.; Li, Y.C.; Wang, X.J.; Hodgson, P.D.; Wen, C.E. Titanium-nickel shape memory alloy foams for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2008, 1, 269–273. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, Y.; Qiu, G.B.; Liao, Y.L.; Cui, H.; Lü, X.W. Volume change of macropores of titanium foams during sintering. Trans. Nonferrous Met. Soc. Chin. (Engl. Ed.) 2015, 25, 3834–3839. [Google Scholar] [CrossRef]
- Li, J.P.; Li, S.H.; de Groot, K.; Layrolle, P. Preparation and Characterization of Porous Titanium. Key Eng. Mater. 2001, 218–220, 51–54. [Google Scholar] [CrossRef]
- Xie, B.; Fan, Y.Z.; Mu, T.Z.; Deng, B. Fabrication and energy absorption properties of titanium foam with CaCl2as a space holder. Mater. Sci. Eng. A 2017, 708, 419–423. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Dunand, D.C. Titanium and NiTi Foams for Bone Replacement; Woodhead Publishing Limited: Cambridge, UK, 2014; ISBN 9780857099037. [Google Scholar]
- Sargeant, T.D.; Guler, M.O.; Oppenheimer, S.M.; Mata, A.; Satcher, R.L.; Dunand, D.C.; Stupp, S.I. Hybrid bone implants: Self-assembly of peptide amphiphile nanofibers within porous titanium. Biomaterials 2008, 29, 161–171. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomater. 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Seong, W.-J.; Kim, U.-K.; Swift, J.Q.; Heo, Y.-C.; Hodges, J.S.; Ko, C.-C. Elastic properties and apparent density of human edentulous maxilla and mandible. Int. J. Oral Maxillofac. Surg. 2009, 38, 1088–1093. [Google Scholar] [CrossRef] [Green Version]
- Lorna, J.G.; Ashby, M.F. Cellular Solids; Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Rice, R.W. Evaluation and extension of physical property-porosity models based on minimum solid area. J. Mater. Sci. 1996, 31, 102–118. [Google Scholar] [CrossRef]
- Kashef, S.; Asgari, A.; Hilditch, T.B.; Yan, W.; Goel, V.K.; Hodgson, P.D. Fracture toughness of titanium foams for biomedical applications. Mater. Sci. Eng. A 2010, 527, 7689–7693. [Google Scholar] [CrossRef]
- Ghidelli, M.; Sebastiani, M.; Johannas, K.E.; Pharr, G.M. Effects of indenter angle on micro-scale fracture toughness measurement by pillar splitting. J. Am. Ceram. Soc. 2017, 100, 5731–5738. [Google Scholar] [CrossRef]
- Ohman, C.; Baleani, M.; Perilli, E.; Dall’Ara, E.; Tassani, S.; Baruffaldi, F.; Viceconti, M. Mechanical testing of cancellous bone from the femoral head: Experimental errors due to off-axis measurements. J. Biomech. 2007, 40, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Norman, T.L.; Vashishth, D.; Burr, D.B. Fracture toughness of human bone under tension. J. Biomech. 1995, 28, 309–320. [Google Scholar] [CrossRef]








| Element | As-Received Ti powder | Ti Foams |
|---|---|---|
| Oxygen (wt.%) | 0.048 | 0.207 |
| Hydrogen (wt.%) | 0.0038 | 0.0626 |
| Nitrogen (wt.%) | 0.0013 | 0.0980 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetinel, O.; Esen, Z.; Yildirim, B. Fabrication, Morphology Analysis, and Mechanical Properties of Ti Foams Manufactured Using the Space Holder Method for Bone Substitute Materials. Metals 2019, 9, 340. https://doi.org/10.3390/met9030340
Cetinel O, Esen Z, Yildirim B. Fabrication, Morphology Analysis, and Mechanical Properties of Ti Foams Manufactured Using the Space Holder Method for Bone Substitute Materials. Metals. 2019; 9(3):340. https://doi.org/10.3390/met9030340
Chicago/Turabian StyleCetinel, Oktay, Ziya Esen, and Bora Yildirim. 2019. "Fabrication, Morphology Analysis, and Mechanical Properties of Ti Foams Manufactured Using the Space Holder Method for Bone Substitute Materials" Metals 9, no. 3: 340. https://doi.org/10.3390/met9030340
APA StyleCetinel, O., Esen, Z., & Yildirim, B. (2019). Fabrication, Morphology Analysis, and Mechanical Properties of Ti Foams Manufactured Using the Space Holder Method for Bone Substitute Materials. Metals, 9(3), 340. https://doi.org/10.3390/met9030340