Corrosion Behavior of AA6012 Aluminum Alloy Processed by ECAP and Cryogenic Treatment
Abstract
:1. Introduction
2. Materials and Methods
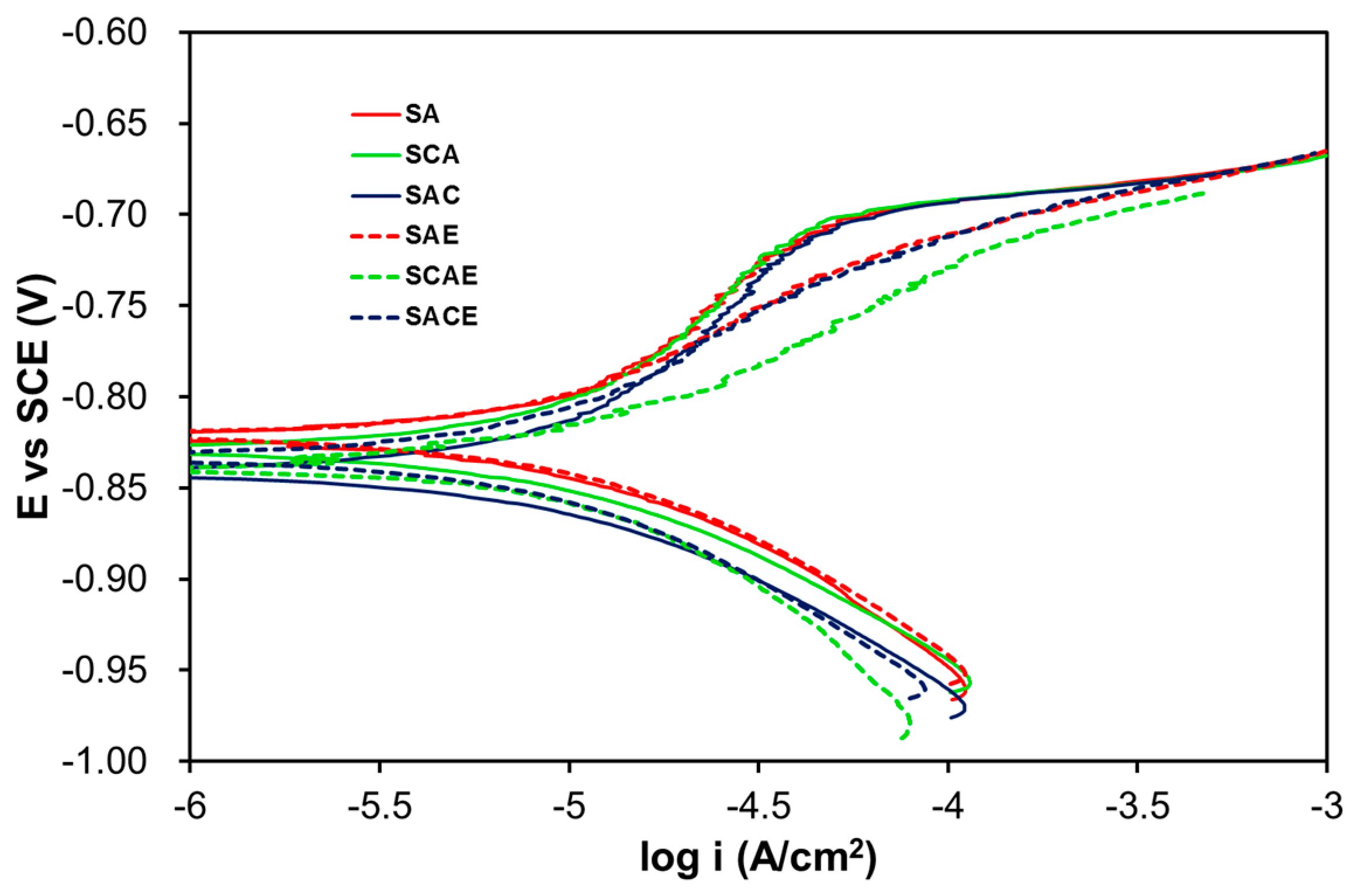
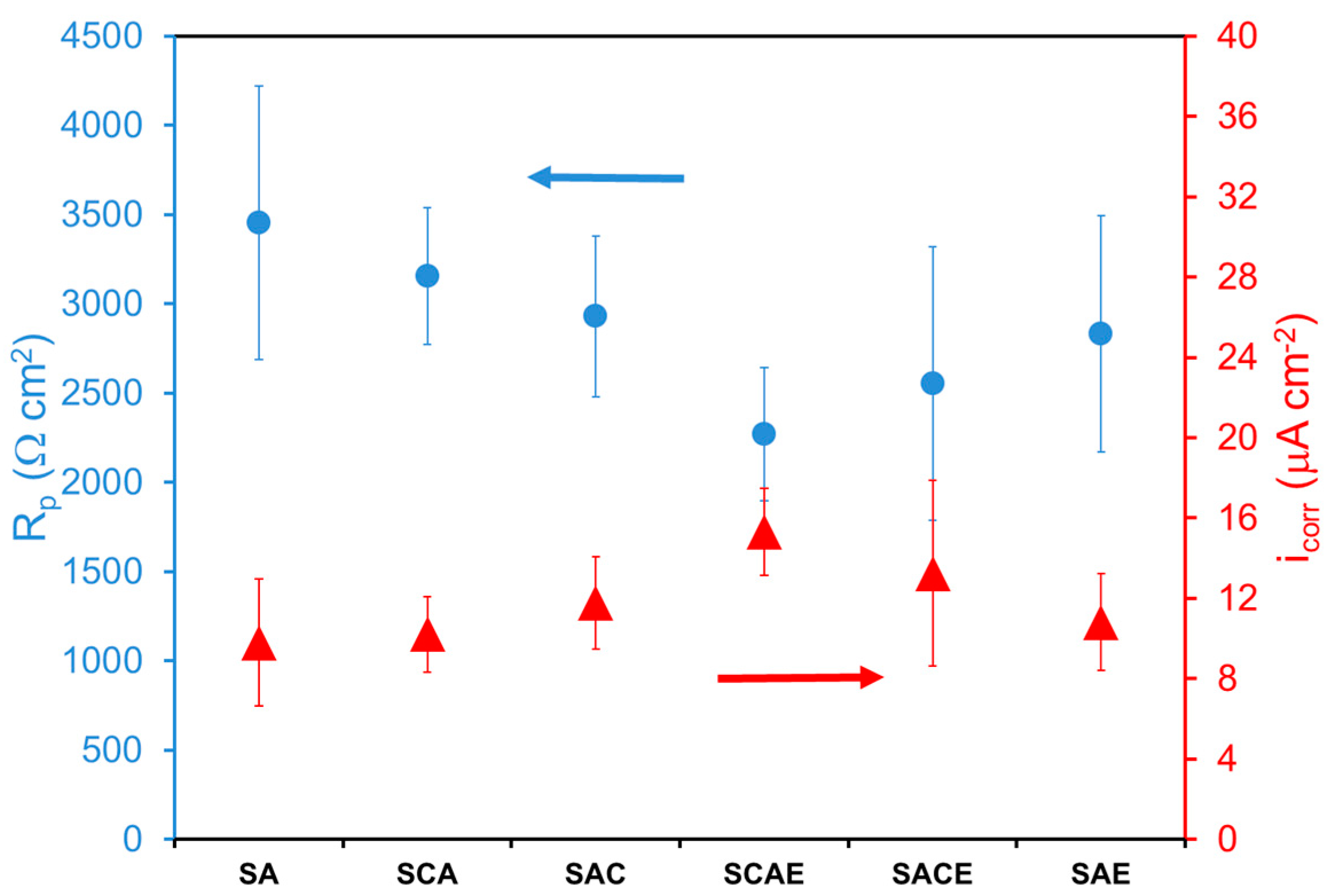
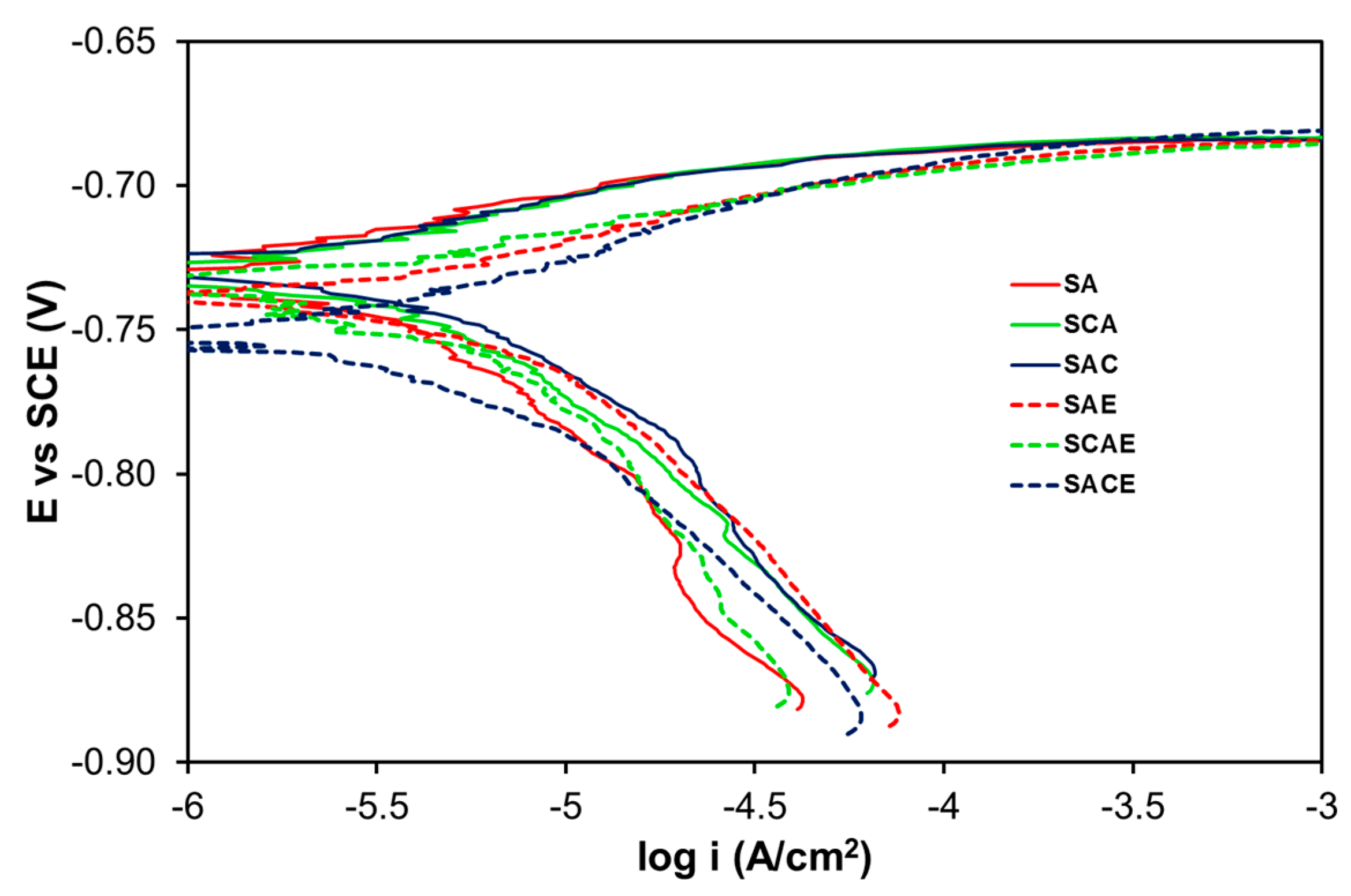
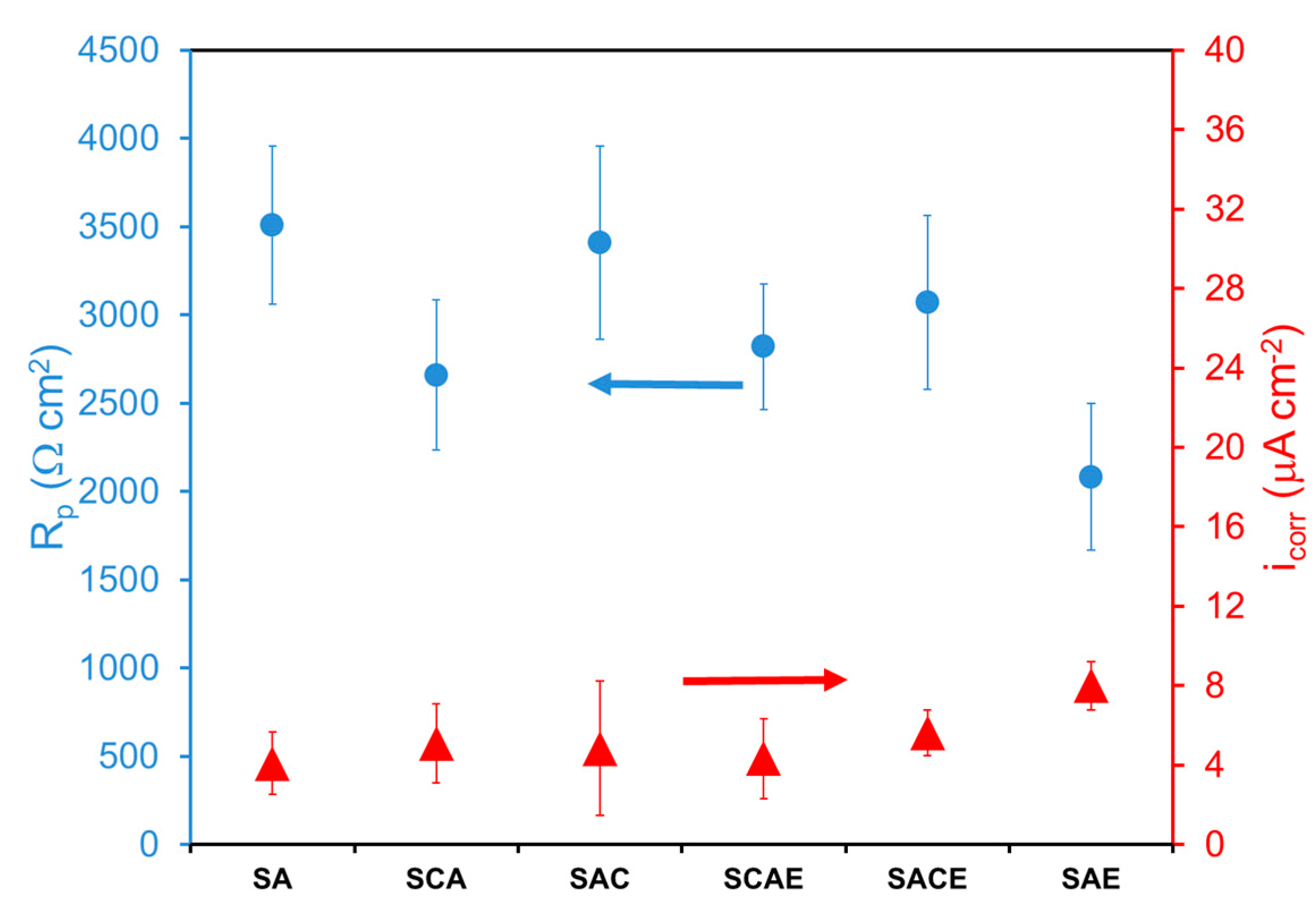
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langdon, T.G. Twenty-five years of ultrafine-grained materials: Achieving exceptional properties through grain refinement. Acta Mater. 2013, 61, 7035–7059. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Veveçka, A.; Cabibbo, M.; Langdon, T.G. A characterization of microstructure and microhardness on longitudinal planes of an Al–Mg–Si alloy processed by ECAP. Mater. Charact. 2013, 84, 126–133. [Google Scholar] [CrossRef]
- Furukawa, M.; Horita, Z.; Nemoto, M.; Langdon, T.G. Review: Processing of metals by equal-channel angular pressing. J. Mater. Sci. 2001, 36, 2835–2843. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayaganthan, R. Effect of ageing on microstructure and mechanical properties of bulk, cryorolled, and room temperature rolled Al 7075 alloy. J. Alloys Compd. 2011, 509, 9609–9616. [Google Scholar] [CrossRef]
- Prell, M.; Xu, C.; Langdon, T.G. The evolution of homogeneity on longitudinal sections during processing by ECAP. Mater. Sci. Eng. A 2008, 480, 449–455. [Google Scholar] [CrossRef]
- Cabibbo, M.; Santecchia, E.; Mengucci, P.; Bellezze, T.; Viceré, A. The role of cryogenic dipping prior to ECAP in the microstructure, secondary-phase precipitation, mechanical properties and corrosion resistance of AA6012 (Al-Mg-Si-Pb). Mater. Sci. Eng. A 2018. [Google Scholar] [CrossRef]
- Birbilis, N.; Muster, T.H.; Buchheit, R.G. Corrosion of Aluminum Alloys. In Corrosion Mechanism in Theory and Practice; Marcus, P., Ed.; CRC Press: New York, NY, USA, 2012; pp. 705–736. ISBN 9781420094626. [Google Scholar]
- Frankel, G.S. Pitting Corrosion of Metals. J. Electrochem. Soc. 1998, 145, 2186. [Google Scholar] [CrossRef]
- Chen, G.S.; Gao, M.; Wei, R.P. Microconstituent-Induced Pitting Corrosion in Aluminum Alloy 2024-T3. CORROSION 1996, 52, 8–15. [Google Scholar] [CrossRef]
- Ly, R.; Hartwig, K.T.; Castaneda, H. Effects of strain localization on the corrosion behavior of ultra-fine grained aluminum alloy AA6061. Corros. Sci. 2018, 139, 47–57. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Walmsley, J.C.; Nordlien, J.H.; Nisancioglu, K. Effect of artificial aging on intergranular corrosion of extruded AlMgSi alloy with small Cu content. Corros. Sci. 2006, 48, 1528–1543. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Nordlien, J.H.; Nisancioglu, K. Effect of thermomechanical history on intergranular corrosion of extruded AlMgSi(Cu) model alloy. Corros. Sci. 2006, 48, 3969–3987. [Google Scholar] [CrossRef]
- Miyamoto, H. Corrosion of Ultrafine Grained Materials by Severe Plastic Deformation, an Overview. Mater. Trans. 2016, 57, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. CORROSION 2010, 66, 075005-075005-13. [Google Scholar] [CrossRef]
- Chung, M.-K.; Choi, Y.-S.; Kim, J.-G.; Kim, Y.-M.; Lee, J.-C. Effect of the number of ECAP pass time on the electrochemical properties of 1050 Al alloys. Mater. Sci. Eng. A 2004, 366, 282–291. [Google Scholar] [CrossRef]
- Sikora, E.; Wei, X.J.; Shaw, B.A. Corrosion Behavior of Nanocrystalline Bulk Al-Mg-Based Alloys. CORROSION 2004, 60, 387–398. [Google Scholar] [CrossRef]
- Son, I.J.; Nakano, H.; Oue, S.; Kobayashi, S.; Fukushima, H.; Horita, Z. Pitting corrosion resistance of anodized aluminum-copper alloy processed by severe plastic deformation. Nippon Kinzoku Gakkaishi/J. Jpn. Inst. Met. 2008, 72, 353–359. [Google Scholar] [CrossRef]
- Hockauf, M.; Meyer, L.W.; Nickel, D.; Alisch, G.; Lampke, T.; Wielage, B.; Krüger, L. Mechanical properties and corrosion behavior of ultrafine-grained AA6082 produced by equal-channel angular pressing. J. Mater. Sci. 2008, 43, 7409–7417. [Google Scholar] [CrossRef]
- Son, I.-J.; Nakano, H.; Oue, S.; Kobayashi, S.; Fukushima, H.; Horita, Z. Pitting Corrosion Resistance of Anodized Aluminum Alloy Processed by Severe Plastic Deformation. Mater. Trans. 2007, 48, 21–28. [Google Scholar] [CrossRef]
- Viceré, A.; Cabibbo, M.; Paoletti, C.; Roventi, G.; Bellezze, T. Analisi del comportamento a corrosione di campioni di alluminio AA6012 sottoposti a ECAP e trattamento criogenico. La Metall. Ital. 2018, 2, 25–33. [Google Scholar]
- Bellezze, T.; Giuliani, G.; Roventi, G. Study of stainless steels corrosion in a strong acid mixture. Part 1: Cyclic potentiodynamic polarization curves examined by means of an analytical method. Corros. Sci. 2018, 130, 113–125. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S. Role of intermetallics in corrosion of aluminum alloys. Smart corrosion protection. Intermet. Matrix Compos. 2018, 425–462. [Google Scholar] [CrossRef]
- Bellezze, T.; Giuliani, G.; Viceré, A.; Roventi, G. Study of stainless steels corrosion in a strong acid mixture. Part 2: Anodic selective dissolution, weight loss and electrochemical impedance spectroscopy tests. Corros. Sci. 2018. [Google Scholar] [CrossRef]
- Orazem, M.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bai, L.; Conway, B.E. AC Impedance of Faradaic Reactions Involving Electrosorbed Intermediates: Examination of Conditions Leading to Pseudoinductive Behavior Represented in Three-Dimensional Impedance Spectroscopy Diagrams. J. Electrochem. Soc. 1991, 138, 2897. [Google Scholar] [CrossRef]
- Cao, C. On the impedance plane displays for irreversible electrode reactions based on the stability conditions of the steady-state—I. One state variable besides electrode potential. Electrochim. Acta 1990, 35, 831–836. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.H.; Zhao, Y.H.; Ma, A.B.; Wen, D.J.; Zhu, Y.T. Effect of equal-channel angular pressing and aging on corrosion behavior of ZK60 Mg alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3909–3920. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Carboneras, M.; Viejo, F.; Arrabal, R.; Muñoz, J. Influence of Cu and Sn content in the corrosion of AISI 304 and 316 stainless steels in H2SO4. Corros. Sci. 2006, 48, 1075–1092. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Matykina, E. Effect of Mo and Mn additions on the corrosion behavior of AISI 304 and 316 stainless steels in H2SO4. Corros. Sci. 2008, 50, 780–794. [Google Scholar] [CrossRef]
- Epelboin, I.; Gabrielli, C.; Keddam, M.; Takenouti, H. Alternating-Current Impedance Measurements Applied to Corrosion Studies and Corrosion-Rate Determination. In Electrochemical Corrosion Testing; ASTM International: West Conshohocken, PA, USA; pp. 150–166.
- Hsu, C.H.; Mansfeld, F. Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance. CORROSION 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Czechowski, M. Low-cycle fatigue of friction stir welded Al–Mg alloys. J. Mater. Process. Technol. 2005, 164–165, 1001–1006. [Google Scholar] [CrossRef]
- Mizuno, K.; Nylund, A.; Olefjord, I. Surface reactions during pickling of an aluminium–magnesium–silicon alloy in phosphoric acid. Corros. Sci. 2001, 43, 381–396. [Google Scholar] [CrossRef]
- Leblanc, P.; Frankel, G.S. A Study of Corrosion and Pitting Initiation of AA2024-T3 Using Atomic Force Microscopy. J. Electrochem. Soc. 2002, 149, B239. [Google Scholar] [CrossRef]
- Perovic, A.; Perovic, D.; Weatherly, G.; Lloyd, D. Precipitation in aluminum alloys AA6111 and AA6016. Scr. Mater. 1999, 41, 703–708. [Google Scholar] [CrossRef]
- Qian, M.; Taylor, J.; Yao, J.; Couper, M.; StJohn, D. A practical method for identifying intermetallic phase particles in aluminium alloys by electron probe microanalysis. J. Light Met. 2001, 1, 187–193. [Google Scholar] [CrossRef]
- Cabibbo, M.; Evangelista, E.; Vedani, M. Influence of severe plastic deformations on secondary phase precipitation in a 6082 Al-Mg-Si alloy. Metall. Mater. Trans. A 2005, 36, 1353–1364. [Google Scholar] [CrossRef]
- Tan, L.; Allen, T.R. Effect of thermomechanical treatment on the corrosion of AA5083. Corros. Sci. 2010, 52, 548–554. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, Y.-H.; Li, N.; Huang, S.-K. Remarkable improvement of recovery stress of Fe–Mn–Si shape memory alloy fabricated by equal channel angular pressing. Mater. Sci. Eng. A 2007, 454–455, 19–23. [Google Scholar] [CrossRef]
- Murayama, M.; Horita, Z.; Hono, K. Microstructure of two-phase Al–1.7 at% Cu alloy deformed by equal-channel angular pressing. Acta Mater. 2001, 49, 21–29. [Google Scholar] [CrossRef]
- Cabibbo, M. Microstructure strengthening mechanisms in different equal channel angular pressed aluminum alloys. Mater. Sci. Eng. A 2013, 560, 413–432. [Google Scholar] [CrossRef]
- Cabibbo, M. Microstructure strengthening mechanisms in an Al–Mg–Si–Sc–Zr equal channel angular pressed aluminium alloy. Appl. Surf. Sci. 2013, 281, 38–43. [Google Scholar] [CrossRef]
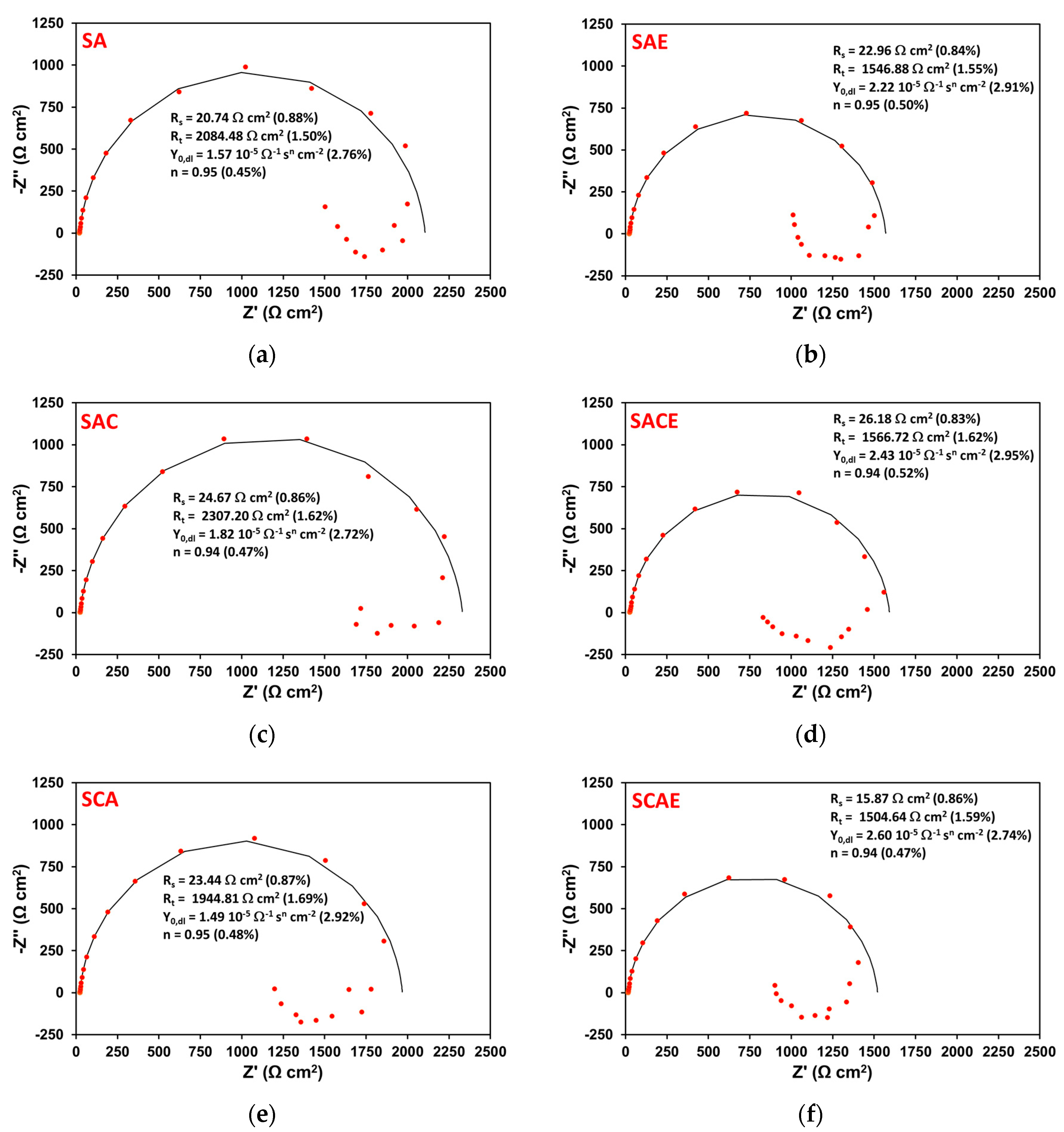
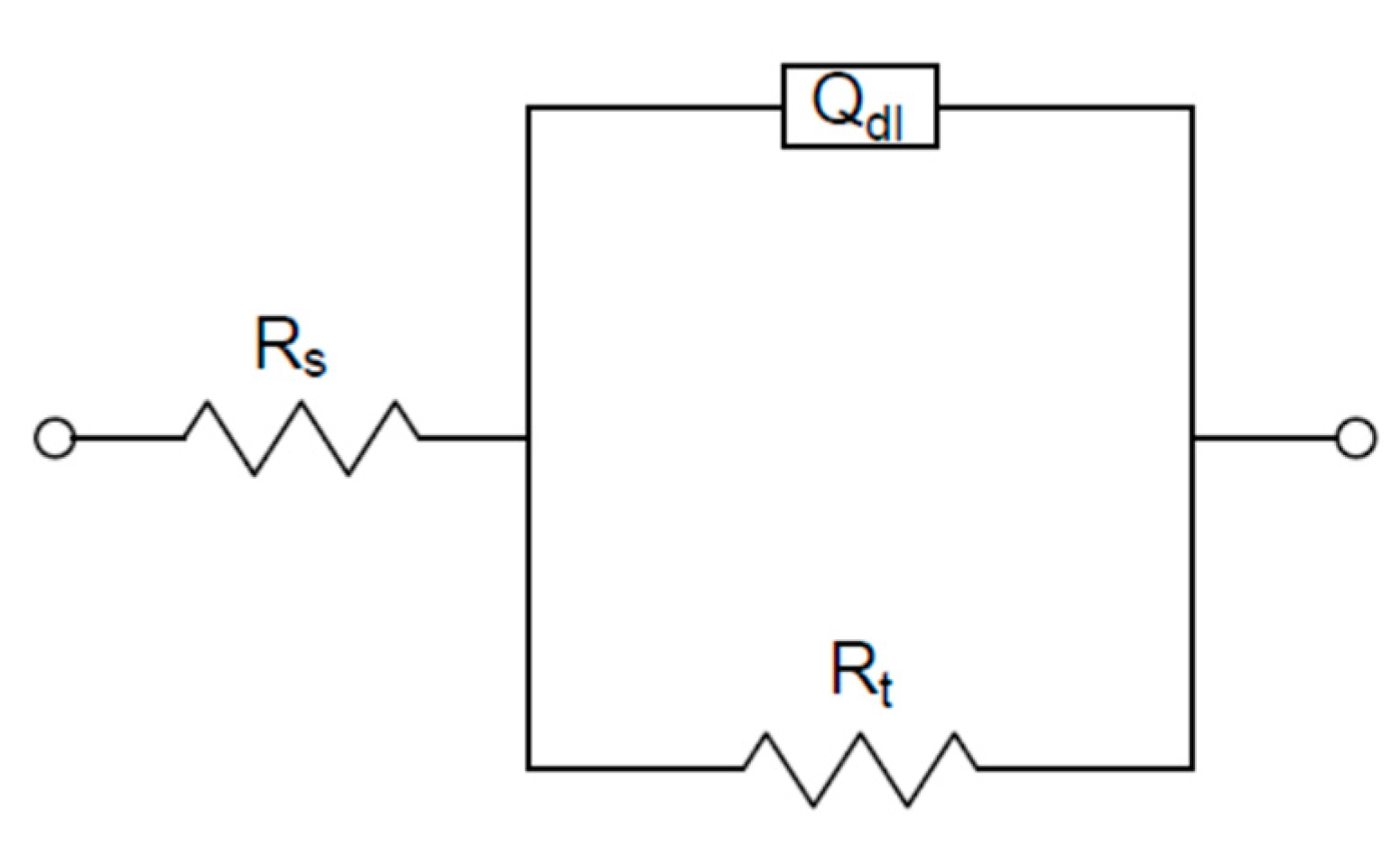
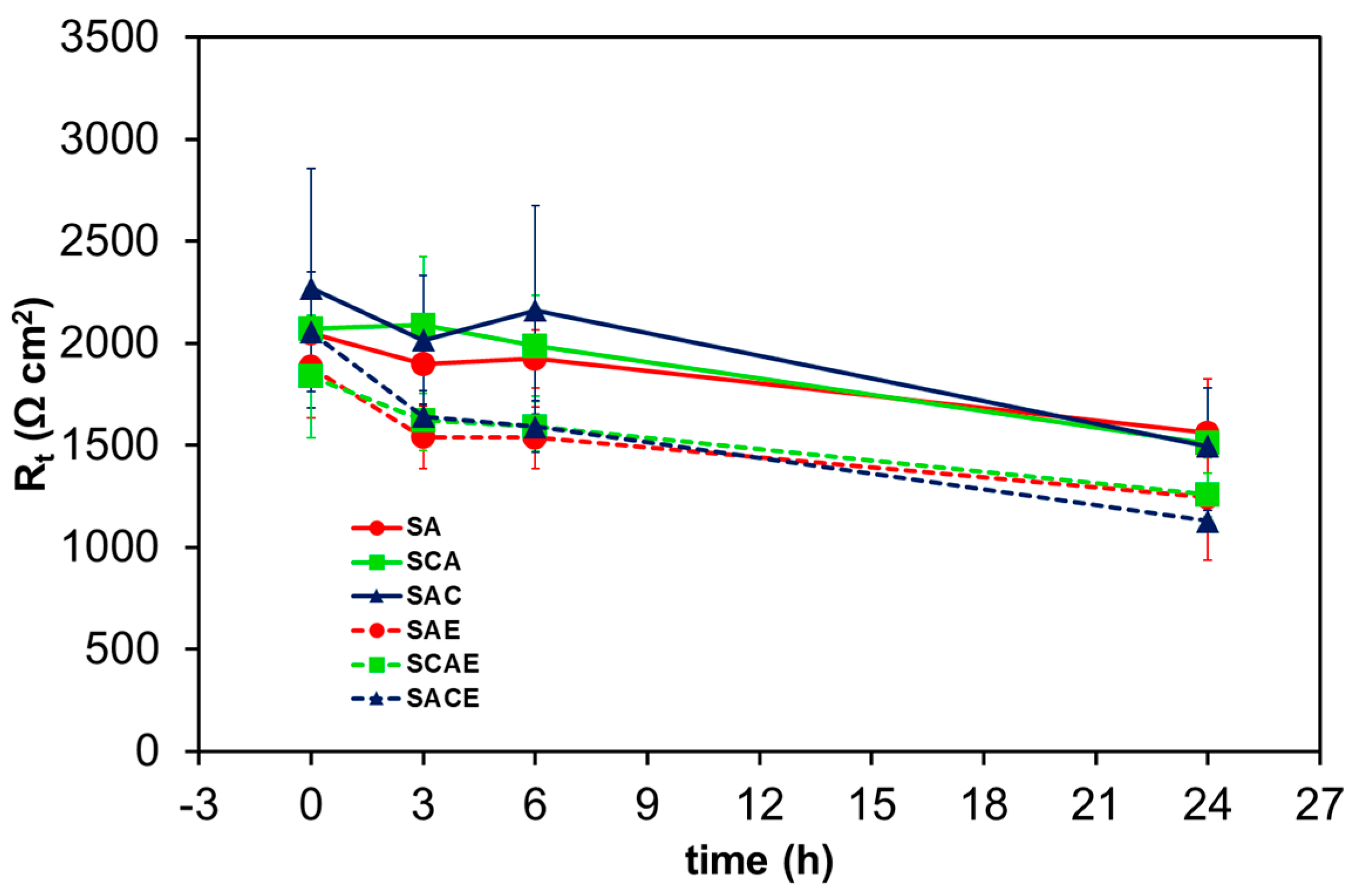

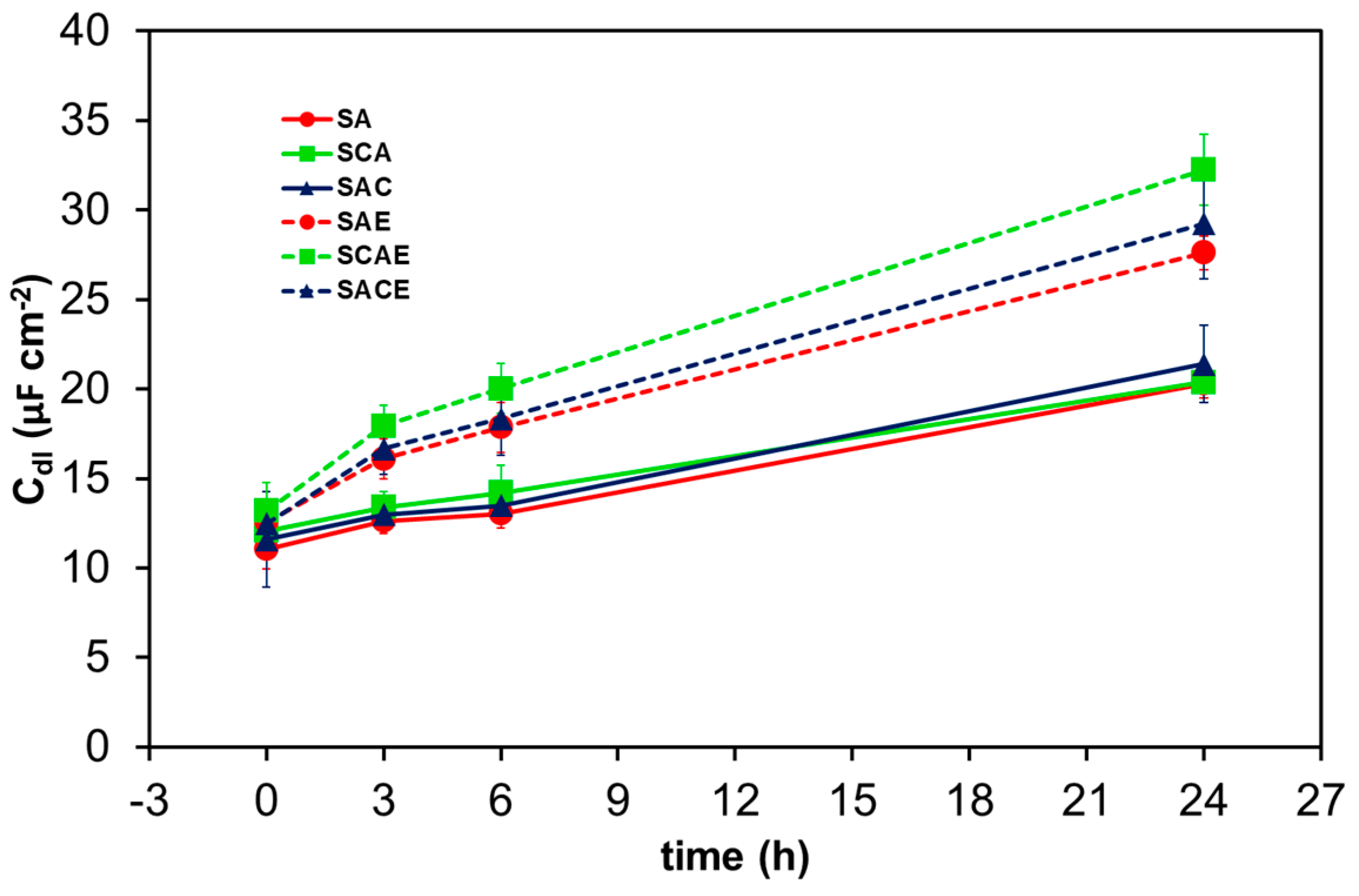


| Sample | Solubilization (S) | Cryogenic Dipping (C) | ECAP (E) | Ageing (A) |
|---|---|---|---|---|
| SA | 1 | - | - | 2 |
| SCA | 1 | 2 | - | 3 |
| SAC | 1 | 3 | 2 | |
| SCAE | 1 | 2 | 4 | 3 |
| SACE | 1 | 3 | 4 | 2 |
| SAE | 1 | - | 3 | 2 |
| Sample | Ecorr (V) | ba (V/decade) | bc (V/decade) | B (V/decade) |
|---|---|---|---|---|
| SA | −0.827 | 0.207 | −0.124 | 0.034 |
| SCA | −0.823 | 0.216 | −0.113 | 0.032 |
| SAC | −0.835 | 0.237 | −0.119 | 0.034 |
| SCAE | −0.832 | 0.170 | −0.158 | 0.035 |
| SACE | −0.840 | 0.179 | −0.132 | 0.033 |
| SAE | −0.819 | 0.156 | −0.127 | 0.030 |
| Sample | Ecorr (V) | ba (V/decade) | bc (V/decade) | B (V/decade) |
|---|---|---|---|---|
| SA | −0.741 | 0.042 | −0.137 | 0.013 |
| SCA | −0.737 | 0.040 | −0.134 | 0.013 |
| SAC | −0.737 | 0.050 | −0.136 | 0.016 |
| SCAE | −0.741 | 0.036 | −0.123 | 0.012 |
| SACE | −0.759 | 0.057 | −0.127 | 0.017 |
| SAE | −0.735 | 0.043 | −0.127 | 0.014 |
| Examined Sites | Al | Mg | Si | Mn | Pb | Fe |
|---|---|---|---|---|---|---|
| # 1 | 6.41 | 1.22 | - | - | 92.37 | - |
| # 2 | 97.77 | 1.35 | 0.88 | - | - | - |
| # 3 | 59.15 | 0.39 | 8.28 | 15.87 | - | 16.31 |
| # 4 | 15.62 | 0.20 | 26.32 | 0.19 | 57.67 | - |
| # 5 | 50.45 | 0.35 | 6.33 | 19.89 | 0.10 | 22.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viceré, A.; Roventi, G.; Paoletti, C.; Cabibbo, M.; Bellezze, T. Corrosion Behavior of AA6012 Aluminum Alloy Processed by ECAP and Cryogenic Treatment. Metals 2019, 9, 408. https://doi.org/10.3390/met9040408
Viceré A, Roventi G, Paoletti C, Cabibbo M, Bellezze T. Corrosion Behavior of AA6012 Aluminum Alloy Processed by ECAP and Cryogenic Treatment. Metals. 2019; 9(4):408. https://doi.org/10.3390/met9040408
Chicago/Turabian StyleViceré, Annamaria, Gabriella Roventi, Chiara Paoletti, Marcello Cabibbo, and Tiziano Bellezze. 2019. "Corrosion Behavior of AA6012 Aluminum Alloy Processed by ECAP and Cryogenic Treatment" Metals 9, no. 4: 408. https://doi.org/10.3390/met9040408







