Friction Surfacing of Aluminium Alloy 5083 on DH36 Steel Plate
Abstract
:1. Introduction
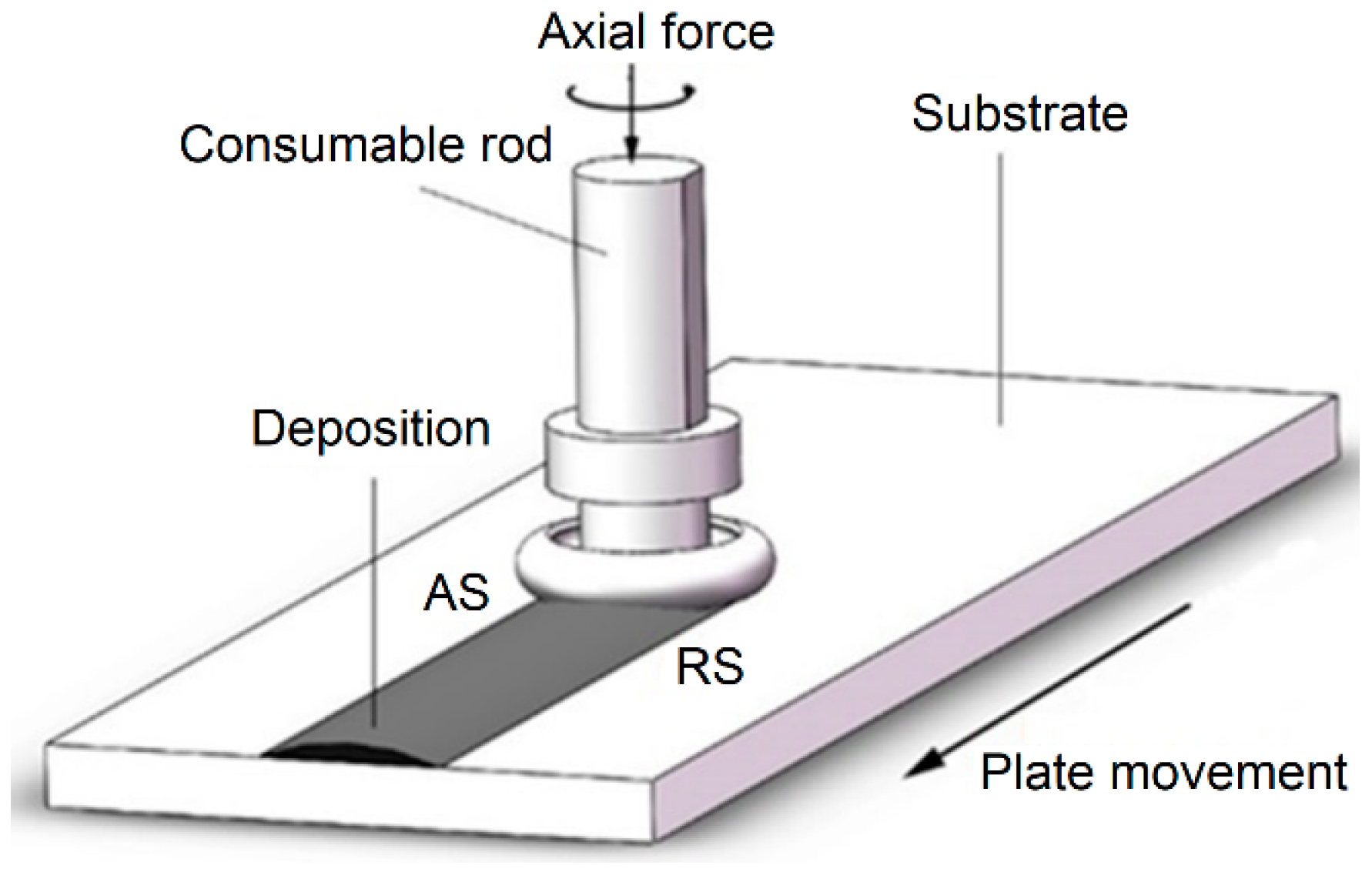
2. Experimental Procedures
3. Results and Discussion
3.1. Surface Morphology
3.2. Thermal Analysis
3.3. Metallographic Analysis
3.4. SEM and EDS Analysis
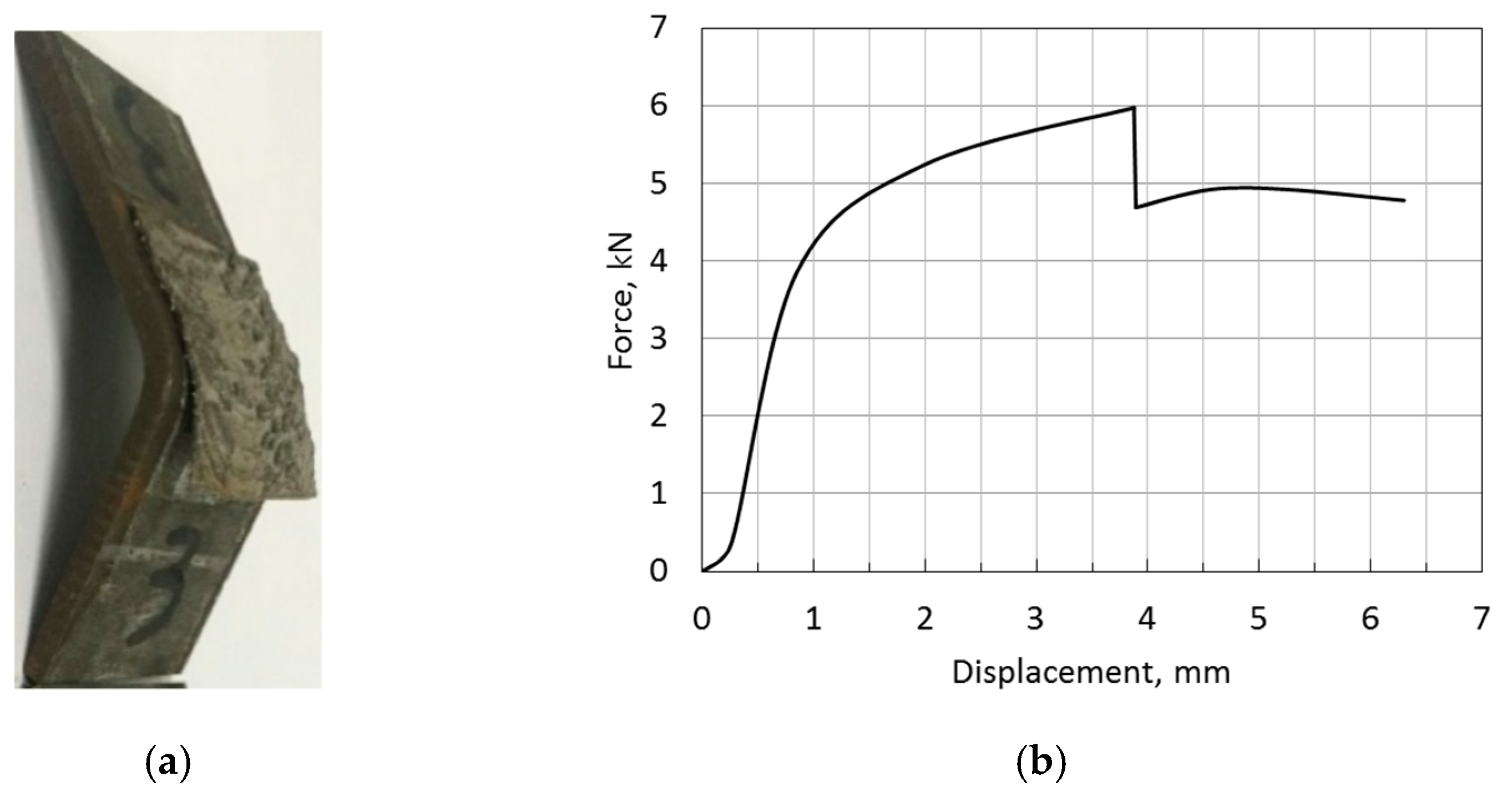
3.5. Bond Strength
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klopstock, H.; Neelands, A.R. An Improved Method of Joining or Welding Metals. UK Patent No. GB572789, 17 October 1941. [Google Scholar]
- Nicholas, E.D. Friction processing technologies. Weld. World 2003, 47, 2–9. [Google Scholar] [CrossRef]
- Dunkerton, S.B.; Thomas, W.M. Repair by Friction Welding. In Proceedings of the TWI Repair and Reclamation Conference, London, UK, 24–25 September 1984. [Google Scholar]
- Ravisekhar, S.; Das, V.C.; Govardhan, D. Friction Surfaced Deposits for Industrial Applications. Mater. Today Proc. 2017, 4, 3796–3801. [Google Scholar] [CrossRef]
- Gandra, J.; Krohn, H.; Miranda, R.M.; Vilaça, P.; Quintino, L.; dos Santos, J.F. Friction surfacing—A review. J. Mater. Process. Technol. 2014, 214, 1062–1093. [Google Scholar] [CrossRef] [Green Version]
- Bedford, G.M.; Vitanov, V.I.; Voutchkov, I.I. On the thermo-mechanical events during friction surfacing of high speed steels. Surf. Coat. Technol. 2001, 141, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Thomas, W.M.; Nicholas, E.D.; Watts, E.R.; Staines, D.G. Friction based welding technology for aluminium. Mater. Sci. Forum 2002, 396, 1543–1548. [Google Scholar] [CrossRef]
- Padhy, G.K.; Wu, C.S.; Gao, S. Friction stir based welding and processing technologies-processes, parameters, microstructures and applications: A review. J. Mater. Sci. Technol. 2018, 34, 1–38. [Google Scholar] [CrossRef]
- Gandra, J.; Miranda, R.M.; Vilaça, P. Performance analysis of friction surfacing. J. Mater. Process. Technol. 2012, 212, 1676–1686. [Google Scholar] [CrossRef]
- Shinoda, T.; Li, J.Q.; Katoh, Y.; Yashiro, T. Effect of process parameters during friction coating on properties of non-dilution coating layers. Surf. Eng. 1998, 14, 211–216. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Mohanty, B.S. Performance analysis of friction surfacing between two dissimilar materials. Int. J. Innov. Eng. Technol. 2016, 7, 47–58. [Google Scholar]
- Guo, D.; Kwok, C.T.; Chan, S.L.I. Spindle speed in friction surfacing of 316L stainless steel—How it affects the microstructure, hardness and pitting corrosion resistance. Surf. Coat. Technol. 2019, 361, 324–341. [Google Scholar] [CrossRef]
- Vitanov, V.I.; Voutchkov, I.I.; Bedford, G.M. Decision support system to optimise the Frictec (friction surfacing) process. J. Mater. Process. Technol. 2000, 107, 236–242. [Google Scholar] [CrossRef]
- Murugan, B.K.; Balusamy, V.; Padmanaban, R. Process parameter effects in the friction surfacing of MONEL over mild steel. In Proceedings of the IEEE International Conference on Intelligent Systems & Control, Coimbatore, India, 5–6 January 2017. [Google Scholar]
- Voutchkov, I.; Jaworski, B.; Vitanov, V.I.; Bedford, G.M. An integrated approach to friction surfacing process optimisation. Surf. Coat. Technol. 2001, 141, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Rafi, H.K.; Ram, G.D.J.; Phanikumar, G.; Prasad Rao, K. Friction surfaced tool steel (H13) coatings on low carbon steel: A study on the effects of process parameters on coating characteristics and integrity. Surf. Coat. Technol. 2010, 205, 232–242. [Google Scholar] [CrossRef]
- Rafi, H.K.; Ram, G.D.J.; Phanikumar, G.; Prasad Rao, K. Friction surfacing of austenitic stainless steel on low carbon steel: Studies on the effects of traverse speed. In Proceedings of the World Congress on Engineering, London, UK, 30 June–2 July 2010. [Google Scholar]
- Tokisue, H.; Katoh, K.; Asahina, T.; Usiyama, T. Mechanical properties of 5052/2017 dissimilar aluminum alloys deposit by friction surfacing. Mater. Trans. 2006, 47, 874–882. [Google Scholar] [CrossRef]
- E290-14. Standard Test Methods for Bend Testing of Material for Ductility; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Vilaça, P.; Gandra, J.; Vidal, C. Linear Friction Based Processing Technologies for Aluminum Alloys: Surfacing, Stir Welding and Stir Channeling. In Alum. Aluminium Alloys—New Trends in Fabrication and Applications; Ahmad, Z., Ed.; InTech: London, UK, 2012; pp. 159–197. [Google Scholar]























| Si | Mg | Cu | Mn | Zn | Ti | Cr | Fe |
|---|---|---|---|---|---|---|---|
| 0.98 | 4.0 | 0.10 | 0.70 | 0.25 | 0.15 | 0.10 | 0.40 |
| C | Si | Mn | P | S | Nb | Al | N |
|---|---|---|---|---|---|---|---|
| 0.11 | 0.37 | 1.48 | 0.014 | 0.004 | 0.02 | 0.02 | 0.002 |
| Test No. | Rotation Speed (rpm) | Travel Speed (mm/min) | Feed Rate (mm/min) |
|---|---|---|---|
| 1 | 900 | 80 | 15 |
| 2 | 900 | 80 | 20 |
| 3 | 900 | 80 | 25 |
| 4 | 900 | 60 | 20 |
| 5 | 900 | 80 | 20 |
| 6 | 900 | 100 | 20 |
| 7 | 600 | 80 | 20 |
| 8 | 900 | 80 | 20 |
| 9 | 1200 | 80 | 20 |
| Test No. | Width of Deposition (mm) | Thickness of Deposition (mm) |
|---|---|---|
| 1 | 26.7 | 1 |
| 2 | 26.5 | 1 |
| 3 | 25.5 | 1 |
| 4 | 26.5 | 1.1 |
| 6 | 25.6 | 1 |
| 7 | 31.4 | 2.2 |
| 9 | 23.0 | 1 |
| Element | Weight Percent, wt % | Atomic Percent, at % | |
|---|---|---|---|
| Point A | Al | 61.90 | 76.62 |
| Fe | 36.51 | 21.84 | |
| others | 1.59 | - | |
| Point B | Al | 62.32 | 77.11 |
| Fe | 37.21 | 22.24 | |
| others | 0.47 | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Qin, W.; Galloway, A.; Toumpis, A. Friction Surfacing of Aluminium Alloy 5083 on DH36 Steel Plate. Metals 2019, 9, 479. https://doi.org/10.3390/met9040479
Li H, Qin W, Galloway A, Toumpis A. Friction Surfacing of Aluminium Alloy 5083 on DH36 Steel Plate. Metals. 2019; 9(4):479. https://doi.org/10.3390/met9040479
Chicago/Turabian StyleLi, Hongjun, Wei Qin, Alexander Galloway, and Athanasios Toumpis. 2019. "Friction Surfacing of Aluminium Alloy 5083 on DH36 Steel Plate" Metals 9, no. 4: 479. https://doi.org/10.3390/met9040479
APA StyleLi, H., Qin, W., Galloway, A., & Toumpis, A. (2019). Friction Surfacing of Aluminium Alloy 5083 on DH36 Steel Plate. Metals, 9(4), 479. https://doi.org/10.3390/met9040479





