Erosive Wear Resistance Regarding Different Destabilization Heat Treatments of Austenite in High Chromium White Cast Iron, Alloyed with Mo
Abstract
:1. Introduction
2. Materials and Methods
3. Results
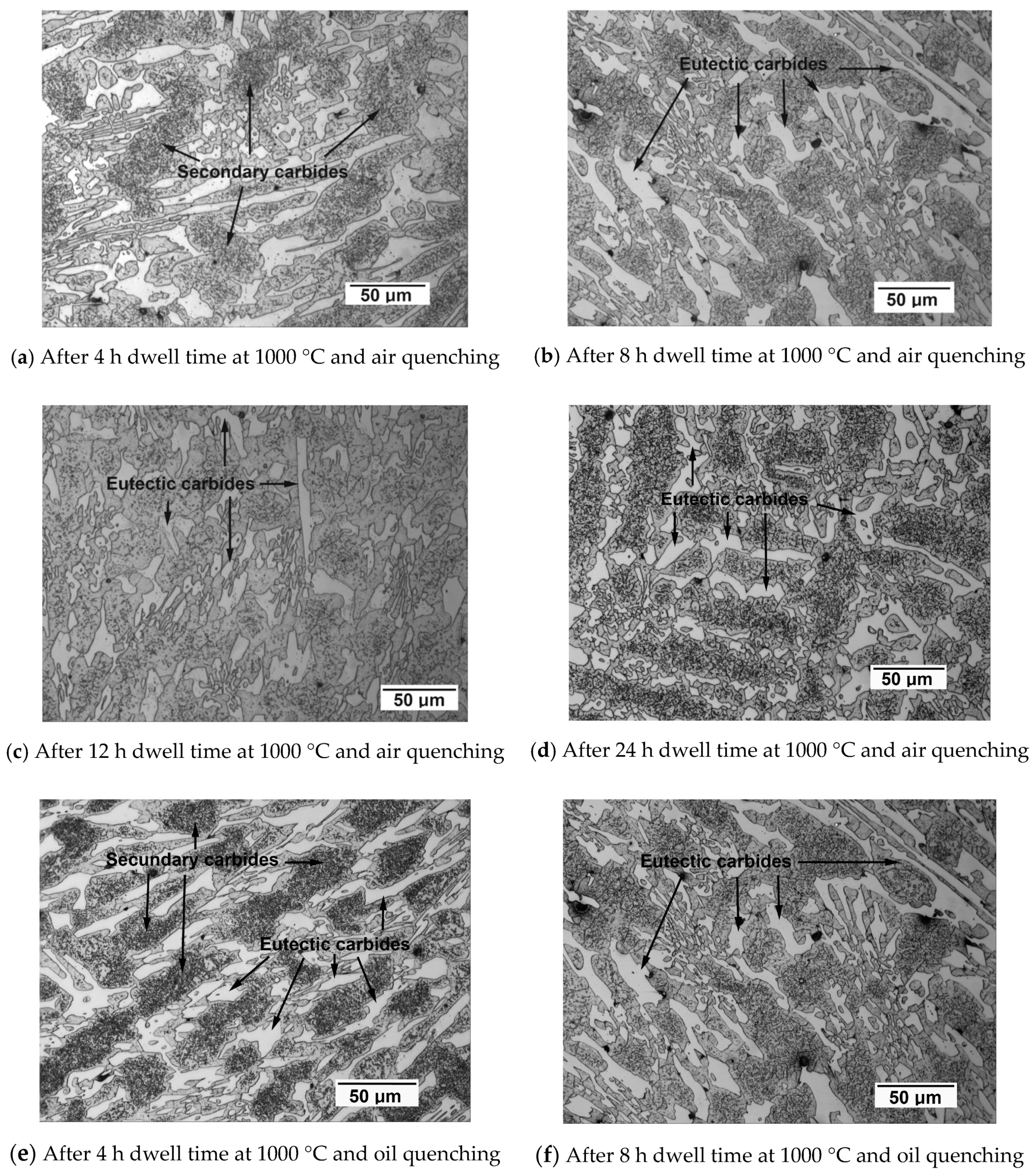
- A greater density of secondary carbides with increasing dwell time at 1000 °C, which leads to an increase in the Ms temperature, thus favoring a decrease in the retained austenite.
- Also, as the dwell time at 1000 °C is increased, the dissolution of those eutectic carbides that have precipitated as a consequence of the non-equilibrium solidification takes place.
- Furthermore, additional precipitation of secondary carbides takes place in the 600–400 °C temperature range when the cooling rate is low (cooling in still air).
- The longer the dwell time at 1000 °C, the greater the percentage of precipitated secondary carbides.
- The longer the dwell time at 1000 °C, the greater the percentage of eutectic carbides that dissolve. These eutectic carbides will be those formed as a result of non-equilibrium solidification. It should be noted that the solubility limit of the C in the austenite will increase until 1000 °C is reached.
- Moreover, additional precipitation of secondary carbides in the 600–400 °C temperature range is produced in air-cooled samples. This precipitation tends to offset the dissolution of eutectic carbides.
4. Conclusions
- The longer the dwell time at the destabilization temperature of austenite (1000 °C), the greater the amount of precipitated secondary carbides. However, the percentage of dissolved eutectic carbides is also higher. These dissolved eutectic carbides will have been formed as a result of non-equilibrium solidification. Low cooling rates (in still air) can offset this solution of carbides via the additional precipitation of secondary carbides in the 600–400 °C temperature range.
- The erosive wear resistance depends mainly on the volume fraction of secondary carbides precipitated during the destabilization of austenite. The maximum wear resistance in air cooled samples corresponded to a destabilizing treatment at 1000 °C for 4h and 24 h. However, in those samples quenched in oil, the maximum wear resistances were obtained on those samples destabilized at 1000 °C for 8 h and 12 h.
- There is a sharp decrease exists in the percentage of retained austenite in those treatments with dwell times at 1000 °C equal to or greater than 12 h, reaching minimum values of around 3 wt. %.
- In the oil-quenched samples, the lowest percentage of carbides obtained is 43–46% after dwell times of 4 h and 24 h, reaching a maximum value of 56% when employing a dwell time of 8 h.
- In the air-quenched samples, an increase in hardness is observed with increasing dwell time at the destabilization temperature and as the retained austenite decreases, obtaining a maximum hardness of 961 HV after a dwell time of 24 h. In the oil-quenched samples, however, the maximum hardness was 993 HV after a dwell time of 12 h, which is when an optimum balance is obtained between the percentages of retained austenite and the percentages of precipitated carbides.
Author Contributions
Funding
Conflicts of Interest
References
- Pero-Sanz, J.A. Fundiciones Férreas; Dossat: Madrid, Spain, 1994; p. 154. [Google Scholar]
- Bedolla-Jacuinde, A.; Arias, L.; Hernandez, B. Kinetics of secondary carbides precipitation in a high-chromium white iron. J. Mater. Eng. Perform. 2003, 12, 371–382. [Google Scholar] [CrossRef]
- Powell, G.L.F.; Bee, J.V. Secondary carbide precipitation in an 18 wt.% Cr-1 wt.% Mo white iron. J. Mater. Sci. 1996, 31, 707–711. [Google Scholar] [CrossRef]
- Zhi, X.H.; Xing, J.D.; Gao, Y.M.; Fu, H.G.; Peng, J.Y.; Xiao, B. Effect of heat treatment on microstructure and mechanical properties of a Ti-bearing hypereutectic high chromium white cast iron. Mater. Sci. Eng. A 2008, 487, 171–179. [Google Scholar] [CrossRef]
- Carpenter, S.D.; Carpenter, D.; Pearce, J.T.H. XRD and electron microscope study of a heat treated 26.6% chromium white iron microstructure. Mater. Chem. Phys. 2007, 101, 49–55. [Google Scholar] [CrossRef]
- Karantzalis, A.E.; Lekatou, A.; Diavati, E. Effect of Destabilization Heat Treatments on the Microstructure of High-Chromium Cast Iron: A Microscopy Examination Approach. J. Mater. Eng. Perform. 2009, 18, 1078–1085. [Google Scholar] [CrossRef]
- Kootsookos, A.; Gates, J.D. The role of secondary carbide precipitation on the fracture toughness of a reduced carbon white iron. Mater. Sci. Eng. A 2008, 490, 313–318. [Google Scholar] [CrossRef]
- Efremenko, V.; Shimizu, K.; Chabak, Y. Effect of Destabilizing Heat Treatment on Solid-State Phase Transformation in High-Chromium Cast Irons. Metall. Mater. Trans. A 2013, 44, 5434–5446. [Google Scholar] [CrossRef]
- Liu, Q.; Shibata, H.; Hedstrom, P.; Joonsson, P.G.; Nakajima, K. Dynamic Precipitation Behavior of Secondary M7C3 Carbides in Ti-alloyed High Chromium Cast Iron. Isij Int. 2013, 53, 1237–1244. [Google Scholar] [CrossRef] [Green Version]
- Wiengmoon, A.; Pearce, J.T.H.; Chairuangsri, T. Relationship between microstructure, hardness and corrosion resistance in 20 wt. % Cr, 27 wt. % Cr and 36 wt. % Cr high chromium cast irons. Mater. Chem. Phys. 2011, 125, 739–748. [Google Scholar] [CrossRef]
- Bedolla-Jacuinde, A.; Guerra, F.V.; Mejia, I.; Zuno-Silva, J.; Rainforth, M. Abrasive wear of V-Nb-Ti alloyed high-chromium white irons. Wear 2015, 332, 1006–1011. [Google Scholar] [CrossRef]
- Lai, J.P.; Pan, Q.L.; Sun, Y.W.; Xiao, C.A. Effect of Si Content on the Microstructure and Wear Resistance of High Chromium Cast Iron. ISIJ Int. 2018, 58, 1532–1537. [Google Scholar] [CrossRef]
- Antolin, J.F.A.; Garrote, L.F.; Lozano, J.A. Application of Rietveld Refinement to the correlation of the microstructure evolution of white cast irons with 18 and 25%-wt. Cr after oil quench and successive temper treatments, with abrasive wear and bending testing. Rev. Metal. 2018, 54, 11. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, J.; Fan, H.Y.; Yang, H.S.; Liu, H.H.; Shen, B.L. Effects of high temperature and cryogenic treatment on the microstructure and abrasion resistance of a high chromium cast iron. J. Mater. Process. Technol. 2009, 209, 3236–3240. [Google Scholar] [CrossRef]
- Liu, H.H.; Wang, J.; Yang, H.S.; Shen, B.L. Effects of cryogenic treatment on microstructure and abrasion resistance of CrMnB high-chromium cast iron subjected to sub-critical treatment. Mater. Sci. Eng. A 2008, 478, 324–328. [Google Scholar] [CrossRef]
- Filipovic, M.M. Iron-chromium-carbon-vanadium white cast irons—The microstructure and properties. Hemijska Industrija 2014, 68, 413–427. [Google Scholar] [CrossRef]
- Guitar, M.A.; Suarez, S.; Prat, O.; Guigou, M.D.; Gari, V.; Pereira, G.; Mucklich, F. High Chromium Cast Irons: Destabilized-Subcritical Secondary Carbide Precipitation and Its Effect on Hardness and Wear Properties. J. Mater. Eng. Perform. 2018, 27, 3877–3885. [Google Scholar] [CrossRef]
- Jia, X.S.; Hao, Q.G.; Zuo, X.W.; Chen, N.L.; Rong, Y.H. High hardness and toughness of white cast iron: The proposal of a novel process. Mater. Sci. Eng. A 2014, 618, 96–103. [Google Scholar] [CrossRef]
- Gasan, H.; Erturk, F. Effects of a Destabilization Heat Treatment on the Microstructure and Abrasive Wear Behavior of High-Chromium White Cast Iron Investigated Using Different Characterization Techniques. Metall. Mater. Trans. A 2013, 44, 4993–5005. [Google Scholar] [CrossRef]
- Liu, H.H.; Wang, J.; Shen, B.L.; Yang, H.S.; Gao, S.J.; Huang, S.J. Effects of deep cryogenic treatment on property of 3Cr13Mo1V1.5 high chromium cast iron. Mater. Des. 2007, 28, 1059–1064. [Google Scholar] [CrossRef]
- Yang, H.S.; Wang, J.; Shen, B.L.; Liu, H.H.; Gao, S.J.; Huang, S.J. Effect of cryogenic treatment on the matrix structure and abrasion resistance of white cast iron subjected to destabilization treatment. Wear 2006, 261, 1150–1154. [Google Scholar] [CrossRef]
- Inthidech, S.; Sricharoenchai, P.; Matsubara, Y. Effect of molybdenum content on subcritical heat treatment behaviour of hypoeutectic 16 and 26 wt-% chromium cast irons. Int. J. Cast Met. Res. 2012, 25, 257–263. [Google Scholar] [CrossRef]
- Cetinkaya, C. An investigation of the wear behaviours of white cast irons under different compositions. Mater. Des. 2006, 27, 437–445. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, P.; Wang, K.; Li, H.Z.; Gong, M.Y.; Tong, W.P. Microstructure and mechanical properties of a Mo alloyed high chromium cast iron after different heat treatments. Vacuum 2018, 156, 59–67. [Google Scholar] [CrossRef]
- Oh, H.; Lee, S.; Jung, J.Y.; Ahn, S. Correlation of microstructure with the wear resistance and fracture toughness of duocast materials composed of high-chromium white cast iron and low-chromium steel. Metall. Mater. Trans. A 2001, 32, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Scandian, C.; Boher, C.; de Mello, J.D.B.; Rezai-Aria, F. Effect of molybdenum and chromium contents in sliding wear of high-chromium white cast iron: The relationship between microstructure and wear. Wear 2009, 267, 401–408. [Google Scholar] [CrossRef]
- Wiengmoon, A.; Khantee, J.; Pearce, J.T.H.; Chairuangsri, T. Effect of pre-annealing heat treatment on destabilization behavior of 28 wt. % Cr-2.6 wt. % C high-chromium cast iron. In Proceedings of the 7th Global Conference on Materials Science and Engineering (CMSE2018), Xi’an, China, 1–4 November 2018. [Google Scholar]
- Fairhust, W.; Rohrig, K. Abrasion resistant high chromium cast irons. Foundry Trade J. 1974, 136, 685–698. [Google Scholar]
- Wang, J.; Li, C.; Liu, H.H.; Yang, H.S.; Shen, B.L.; Gao, S.J.; Huang, S.J. The precipitation and transformation of secondary carbides in a high chromium cast iron. Mater. Charact. 2006, 56, 73–78. [Google Scholar] [CrossRef]
- Efremenko, V.G.; Chabak, Y.G.; Brykov, M.N. Kinetic Parameters of Secondary Carbide Precipitation in High-Cr White Iron Alloyed by Mn-Ni-Mo-V Complex. J. Mater. Eng. Perform. 2013, 22, 1378–1385. [Google Scholar] [CrossRef]







| C | Si | Mn | Cr | Mo | S | P |
|---|---|---|---|---|---|---|
| 3.0 | 1.2 | 0.8 | 18.2 | 2.0 | 0.009 | 0.024 |
| Quenching (Destabilization Heat Treatment at 1000 °C) | Rietveld Fitting | Phases | Bragg R-Factor | Volume % | Esd. | |
|---|---|---|---|---|---|---|
| Air | 4 h | Rwp = 9.66 Chi2 = 2.37 | α’ | 1.5 | 36.59 | ±094 |
| γ | 4.76 | 8.24 | ±0.41 | |||
| M7C3 | 2.72 | 55.17 | ±1.51 | |||
| 8 h | Rwp = 11 Chi2 = 2.77 | α’ | 1.04 | 35.48 | ±0.91 | |
| γ | 7.86 | 9.94 | ±0.44 | |||
| M7C3 | 4.5 | 54.58 | ±1.47 | |||
| 12 h | Rwp = 10.2 Chi2 = 2.62 | α’ | 4.39 | 45.63 | ±1.07 | |
| γ | 5.54 | 2.61 | ±0.29 | |||
| M7C3 | 3.71 | 51.76 | ±1.50 | |||
| 24 h | Rwp = 10.7 Chi2 = 2.74 | α’ | 5.34 | 44.30 | ±1.1 | |
| γ | 6.15 | 2.83 | ±0.3 | |||
| M7C3 | 4.58 | 52.87 | ±1.54 | |||
| Oil | 4 h | Rwp = 11.3 Chi2 = 2.77 | α’ | 3.96 | 50.09 | ±1.49 |
| γ | 8.28 | 6.59 | ±0.42 | |||
| M7C3 | 8.82 | 43.33 | ±1.63 | |||
| 8 h | Rwp = 10.9 Chi2 = 1.99 | α’ | 3.43 | 36.53 | ±1.17 | |
| γ | 3.85 | 6.94 | ±0.57 | |||
| M7C3 | 4.69 | 56.52 | ±1.80 | |||
| 12 h | Rwp = 11.7 Chi2 = 2.67 | α’ | 8.16 | 44.84 | ±1.39 | |
| γ | 8.9 | 2.26 | ±0.45 | |||
| M7C3 | 8.12 | 52.90 | ±1.84 | |||
| 24 h | Rwp = 11.6 Chi2 = 2.45 | α’ | 6.44 | 51.35 | ±1.57 | |
| γ | 9.1 | 2.22 | ±0.48 | |||
| M7C3 | 8.47 | 46.43 | ±1.81 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Pociño, A.; Alvarez-Antolin, F.; Asensio-Lozano, J. Erosive Wear Resistance Regarding Different Destabilization Heat Treatments of Austenite in High Chromium White Cast Iron, Alloyed with Mo. Metals 2019, 9, 522. https://doi.org/10.3390/met9050522
Gonzalez-Pociño A, Alvarez-Antolin F, Asensio-Lozano J. Erosive Wear Resistance Regarding Different Destabilization Heat Treatments of Austenite in High Chromium White Cast Iron, Alloyed with Mo. Metals. 2019; 9(5):522. https://doi.org/10.3390/met9050522
Chicago/Turabian StyleGonzalez-Pociño, Alejandro, Florentino Alvarez-Antolin, and Juan Asensio-Lozano. 2019. "Erosive Wear Resistance Regarding Different Destabilization Heat Treatments of Austenite in High Chromium White Cast Iron, Alloyed with Mo" Metals 9, no. 5: 522. https://doi.org/10.3390/met9050522





