Abstract
A detailed analysis of the dehydrogenation mechanism and reversibility of LiBH4 doped by as-derived Al (denoted Al*) from AlH3 was performed by thermogravimetry (TG), differential scanning calorimetry (DSC), mass spectral analysis (MS), powder X-ray diffraction (XRD), scanning electronic microscopy (SEM), and Fourier transform infrared spectroscopy (FTIR). The results show that the dehydrogenation of LiBH4/Al* is a five-step reaction: (1) LiBH4 + Al → LiH + AlB2 + “Li-Al-B-H” + B2H6 + H2; (2) the decomposition of “Li-Al-B-H” compounds liberating H2; (3) 2LiBH4 + Al → 2LiH + AlB2 + 3H2; (4) LiBH4 → LiH + B + 3/2H2; and (5) LiH + Al → LiAl + 1/2H2. Furthermore, the reversibility of the LiBH4/Al* composite is based on the following reaction: LiH + LiAl + AlB2 + 7/2H2 ↔ 2LiBH4 + 2Al. The extent of the dehydrogenation reaction between LiBH4 and Al* greatly depends on the precipitation and growth of reaction products (LiH, AlB2, and LiAl) on the surface of Al*. A passivation shell formed by these products on the Al* is the kinetic barrier to the dehydrogenation of the LiBH4/Al* composite.
1. Introduction
Hydrogen is recognized as an ideal energy vector with the advantages of high combustion value and zero pollution [1,2,3]. However, the storage of hydrogen is still challenging for its on-board application. Hydrogen energy can be stored in gas, liquid, and solid forms, among which solid hydrogen storage is the safest. Currently, complex metal hydrides are considered as the most promising hydrogen storage materials due to their large hydrogen storage capacities [4,5,6].
Lithium borohydride (LiBH4) has drawn much attention for on-board hydrogen storage due to its theoretical hydrogen storage capacity as high as 18.5 wt.%, which far exceeds the requirements of vehicle hydrogen storage material by the US department of energy [7,8]. Unfortunately, LiBH4 is thermodynamically stable, and dehydrogenation is only initiated when the temperature is above 400 °C under 1 bar H2. The reversibility of LiBH4 is poor, and rehydrogenation requires a temperature over 600 °C under 350 bar H2 [9,10]. Various methods have been developed to improve the dehydrogenation properties and reversibility of LiBH4. Some researchers [11,12,13] found that thermodynamic destabilization of LiBH4 could be achieved by adding reactive hydride composites (RHC) to change its dehydrogenation steps. For instance, Vajo et al. [13] reported that the dehydrogenation reaction enthalpy was much lower than that of the pure LiBH4 by doping with MgH2. The formation of MgB2 during the dehydrogenation reaction destabilized LiBH4, and the reversibility of the LiBH4-MgH2 composite was also better than pure LiBH4. After that, many metal hydrides or complex hydrides have been employed to improve the hydrogen storage properties of LiBH4 [14,15,16,17,18,19,20,21].
According to the theoretical calculation based on phase diagrams, the decomposition temperature of the LiBH4/Al composite was predicted to be significantly lower than that of pure LiBH4 [22,23]. Therefore, Al has been popularly employed as another destabilization agent to improve the hydrogen desorption properties of LiBH4. The Al source can be either a metallic Al or a complex hydrides containing Al [24,25,26]. However, the metallic Al is usually coated with an oxide layer, which greatly limits the improvement of dehydrogenation and the reversibility of LiBH4. Moreover, the utilization of Al-containing hydrides will inevitably introduce the influence of other atoms on the de/rehydrogenation reaction. In order to investigate the mechanism and influence of pure Al on the dehydrogenation and reversibility of LiBH4, an as-prepared Al (denoted Al*) derived from AlH3 was employed as a destabilization agent. The hydrogen desorption properties and mechanism of the LiBH4/Al* composite were studied systematically, along with kinetic investigations using a Sieverts-type apparatus. The Kissinger method was used to calculate the activation energy of the main dehydrogenation step of the LiBH4/Al* composite, and its reversibility was also discussed.
2. Materials and Methods
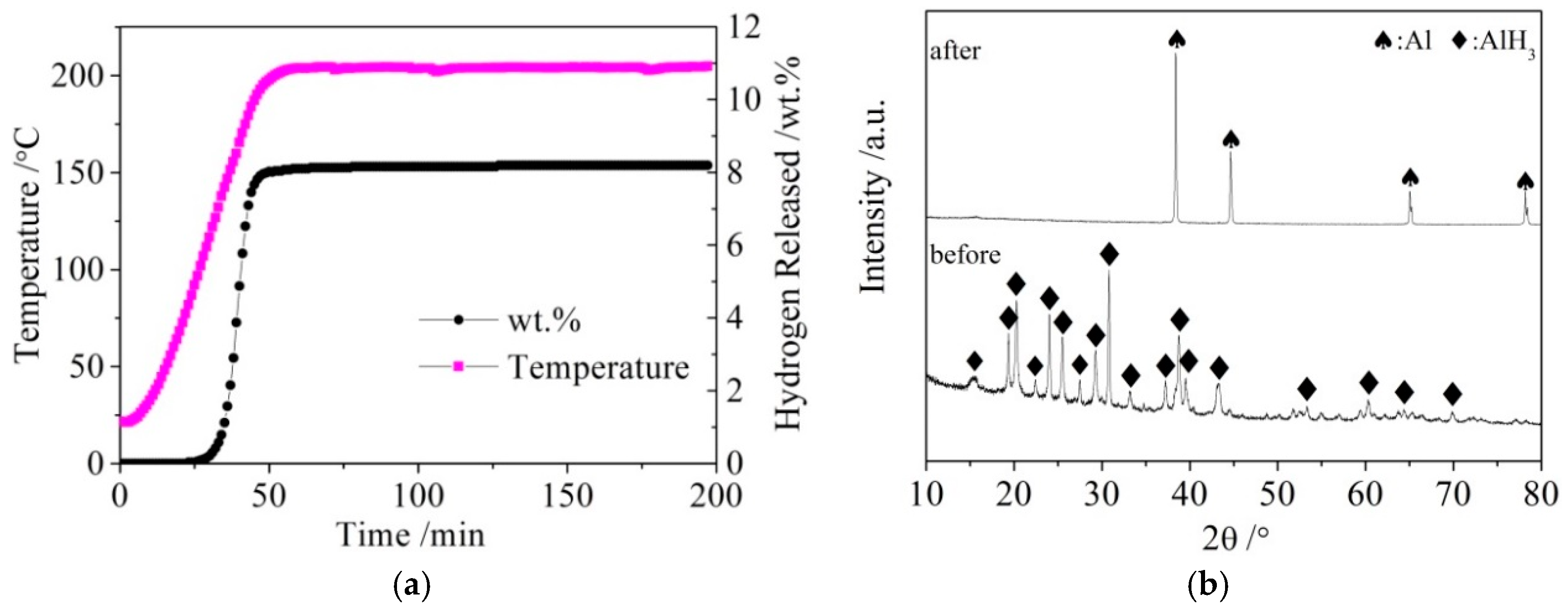
The LiBH4 powder (95% purity; Acros Organics) and Al powder (99% purity; Sinopharm Group) were employed as raw materials. AlH3 was synthesized as follows: LiAlH4 and AlCl3 were dissolved in diethyl ether at a molar ratio of 3:1. After the reaction (1) was fully carried out, the precipitate LiCl was filtered off, and the filtrate containing AlH3 was separated from the mixture. Pure AlH3 was then obtained from the filtrate by drying and de-ethering in vacuum. Finally, AlH3 was completely dehydrogenated to obtain active Al* by heating to 200 °C and holding for 2.5 h. The dehydrogenation curves of AlH3 and X-ray diffraction (XRD) patterns of AlH3 before and after dehydrogenation are shown in Figure 1a,b, respectively.
3LiAlH4 + AlCl3 → 4AlH3 + 3LiCl↓
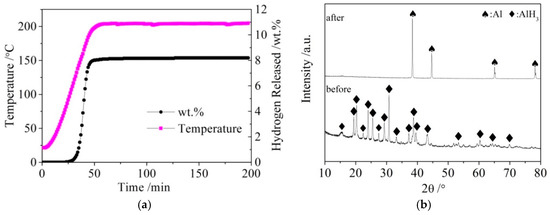
Figure 1.
The dehydrogenation curves (a) of AlH3 and X-ray diffraction (XRD) patterns (b) of AlH3 before and after dehydrogenation.
The commercial Al powder was used for comparison with the as-prepared Al* in this study. The LiBH4/Al and LiBH4/Al* composites were synthesized by ball-milling using a QM-3SP4 planetary ball mill (Nanjing Nanda Instrument Plant, Nanjing, China). The ball to powder ratio was 45:1. The milling process was carried out at 400 rpm for 30 min under a 0.1 MPa argon atmosphere. To prevent the temperature from rising too fast during long-term milling, the milling process was paused every 6 min for cooling. All of the samples were handled in a Mikrouna glove box filled with high purity argon (99.999%) and controlled H2O (<0.5 ppm) and O2 (<0.1 ppm) concentrations for preventing contamination.
The morphologies of the as-received Al and as-prepared Al* were observed via field emission scanning electronic microscopy (SEM, Hitachi, Tokyo, Japan). Dehydriding/rehydriding behaviors of the samples were examined using a carefully calibrated Sieverts-type apparatus [27]. For the temperature programmed desorption (TPD) measurements, the samples were heated from room temperature to 600 °C at a rate of 2 °C/min. For the rehydriding measurements, the samples were heated to 400 °C at a rate of 5 °C/min under 8 MPa H2 and held at that temperature for 7 h. The thermal events during dehydrogenation of the samples were investigated by thermogravimetry/differential scanning calorimeter (TG/DSC, Netzsch, Ahlden, Germany). For the isothermal hydrogen desorption measurements, the samples were rapidly heated to a set temperature (i.e., 100 °C, 350 °C, 500 °C, and 600 °C) and held for 3h under argon flowing at 50 mL/min. For the non-isothermal dehydrogenation (i.e., the temperature programmed desorption, TPD) measurements, the samples were heated gradually from room temperature to 600 °C with a heating rate of 5 °C/min. The hydrogen desorption spectra were collected synchronously using a mass spectrometer (MS, Netzsch, Ahlden, Germany). The phase of the as-prepared samples and the dehydrogenation product of them at various temperatures were identified by X-ray diffraction (XRD, PANalytical, Almelo, Netherlands) and Fourier transform infrared spectroscopy (FTIR, Bruker, Basel, Switzerland). During XRD measurements, the samples were sealed with a polypropylene membrane to avoid exposure to any moisture or oxygen.
3. Results and Discussion
3.1. Dehydrogenation Mechanism of the LiBH4/Al* Composite
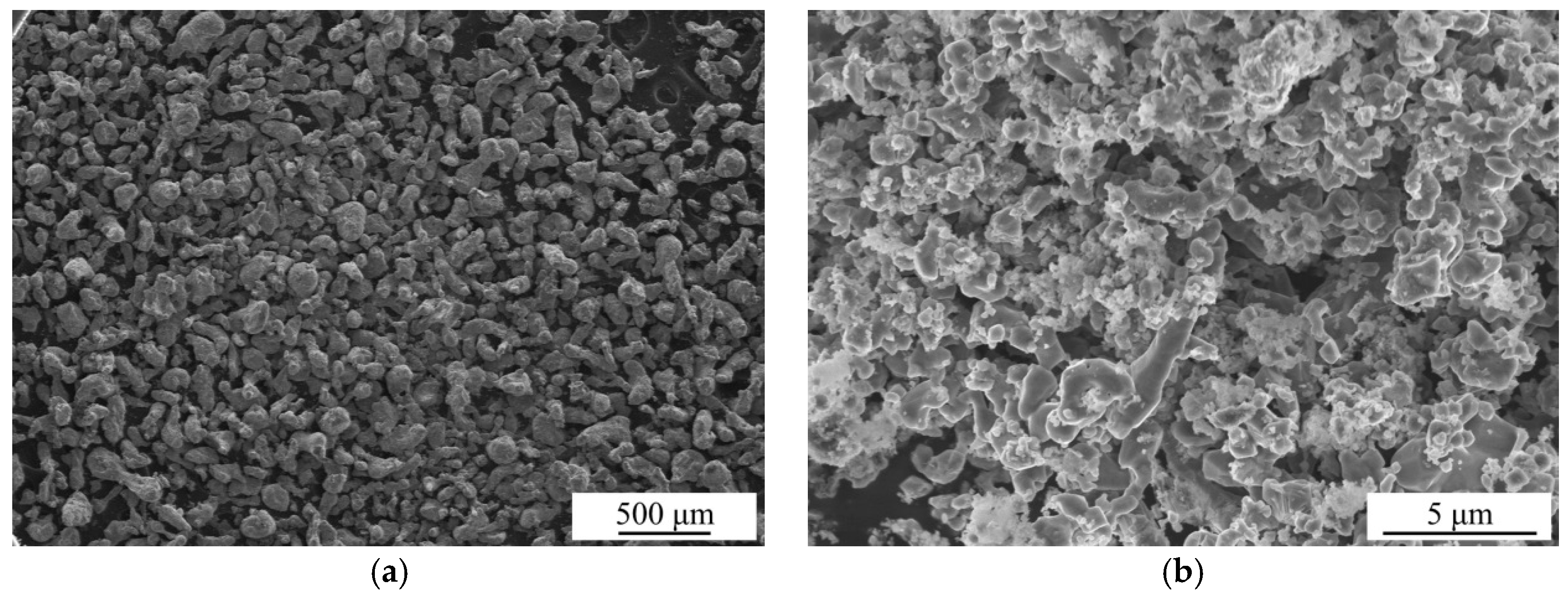
The SEM images of the as-received Al particles and as-prepared Al* particles are shown in Figure 2. It can be seen that the particle size of the as-received Al is about 100 μm, while the particle size of active Al* derived from AlH3 is about 1% of this. A sharp reduction in the particle size means a significant increase in the specific surface area.
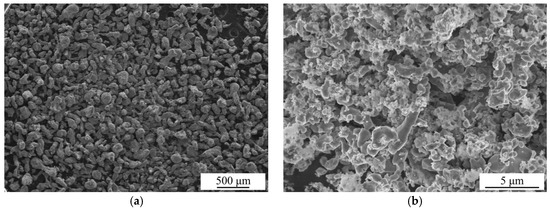
Figure 2.
Scanning electronic microscopy (SEM) images of the as-received Al particles (a) and as-prepared Al* particles (b).
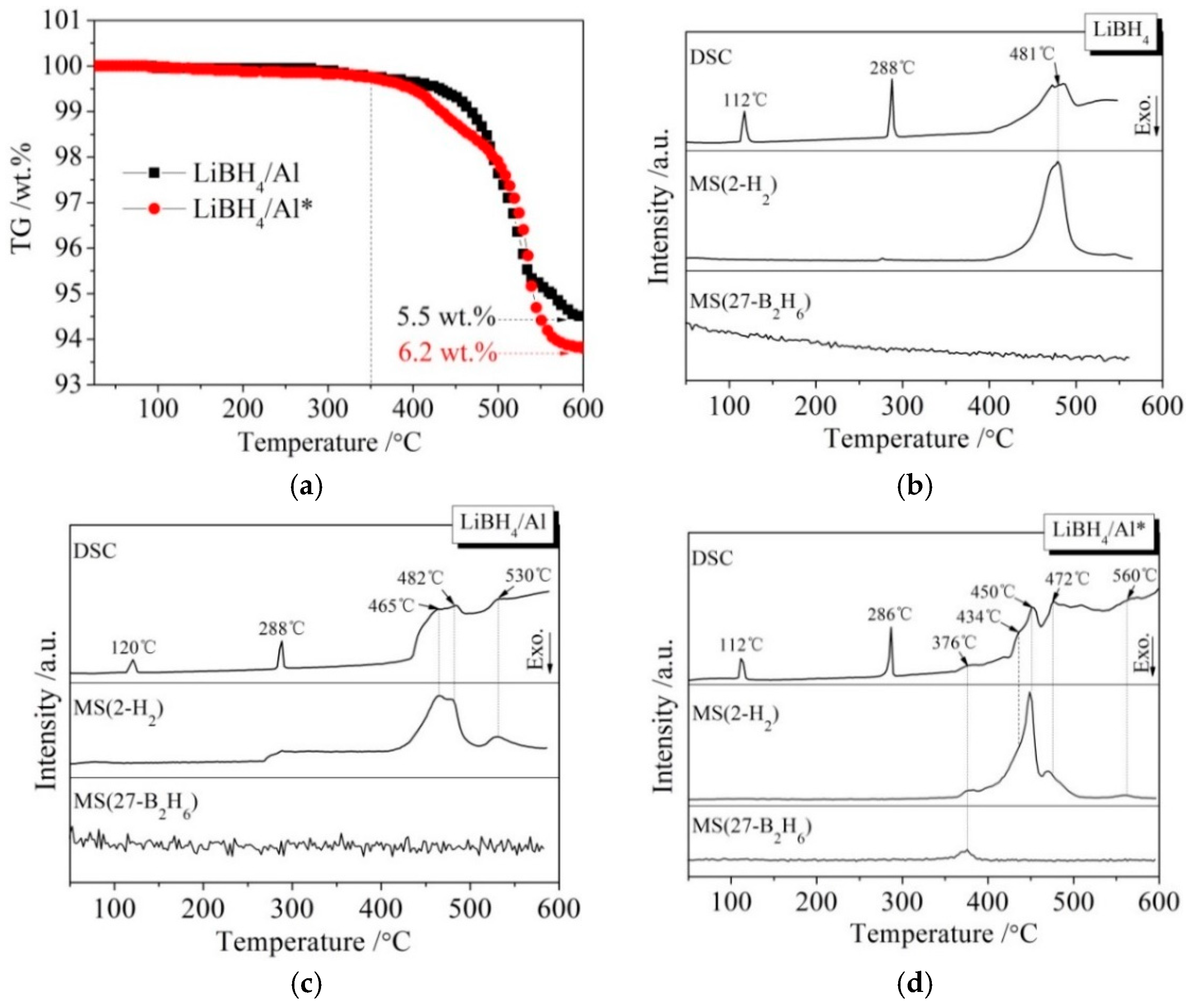
Figure 3 presents different simultaneous signals for the dehydrogenation of LiBH4/Al and LiBH4/Al* samples: the thermogravimetry (TG) signal, DSC signal, and hydrogen signal are plotted over the temperature. It can be seen from Figure 3a that the dehydrogenation curves of the LiBH4/Al and LiBH4/Al* composites are almost the same before 350 °C, and they both liberate about 0.1 wt.% of H2. After being heated to 350 °C, the dehydrogenation rate of LiBH4/Al* is clearly faster than that of LiBH4/Al. Finally, the total dehydrogenation amounts of LiBH4/Al and LiBH4/Al* samples at 600 °C reached 5.5 wt.% and 6.2 wt.%, respectively. This can be ascribed to the Al* derived from AlH3 having larger specific surface area, and the oxide-free surface of Al* possessing higher chemical reactivity. Therefore, the dehydrogenation reaction of LiBH4/Al* is more sufficient than LiBH4/Al.
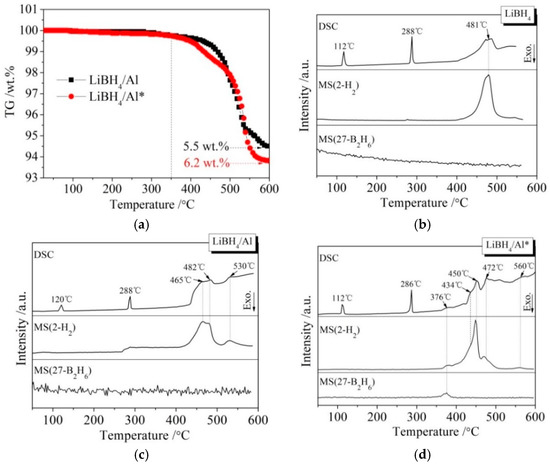
Figure 3.
Thermogravimetry (TG) curves (a) of LiBH4/Al and LiBH4/Al* samples and differential scanning calorimetry/mass spectrometry (DSC/MS) curves of LiBH4 (b), LiBH4/Al (c), and LiBH4/Al* (d) samples.
The DSC/MS curves of LiBH4, LiBH4/Al, and LiBH4/Al* are displayed in Figure 3b–d, respectively. The endothermic peak in the DSC curve of pure LiBH4 at 112 °C corresponds to the crystal transformation from an orthorhombic phase (o-LiBH4) to a hexagonal phase (h-LiBH4), while the endothermic peak at 288 °C corresponds to the melting of h-LiBH4 [28]. LiBH4 is dehydrogenated in the temperature range of 400 to 550 °C, and the dehydrogenation rate reached a maximum at 481 °C according to Figure 3b. Therefore, the endothermic peak at this temperature is ascribed to the decomposition of LiBH4 based on Reaction (2).
LiBH4 → LiH + B + 3/2H2↑
There are three endothermic peaks at 465, 482, and 530 °C in the DSC curve of the LiBH4/Al sample (Figure 3c). Each endothermic peak corresponds to a hydrogen evolution peak in the MS curve. The endothermic peak at 482 °C is in good agreement with that of the decomposition of LiBH4 mentioned above. Compared with Figure 3b, the new endothermic peaks at 465 and 530 °C should be related to the reaction of LiBH4 and the added Al. According to the work of other researchers [29,30,31], the endothermic peak at 465 °C is ascribed to the LiBH4 reaction with Al forming LiH, AlB2, and liberating H2 (Reaction (3)), and the endothermic peak at 530 °C is attributed to the reaction of LiH with Al to form LiAl alloy and H2 (Reaction (4)).
2LiBH4 + Al → 2LiH + AlB2 + 3H2↑
LiH + Al → LiAl + 1/2H2↑
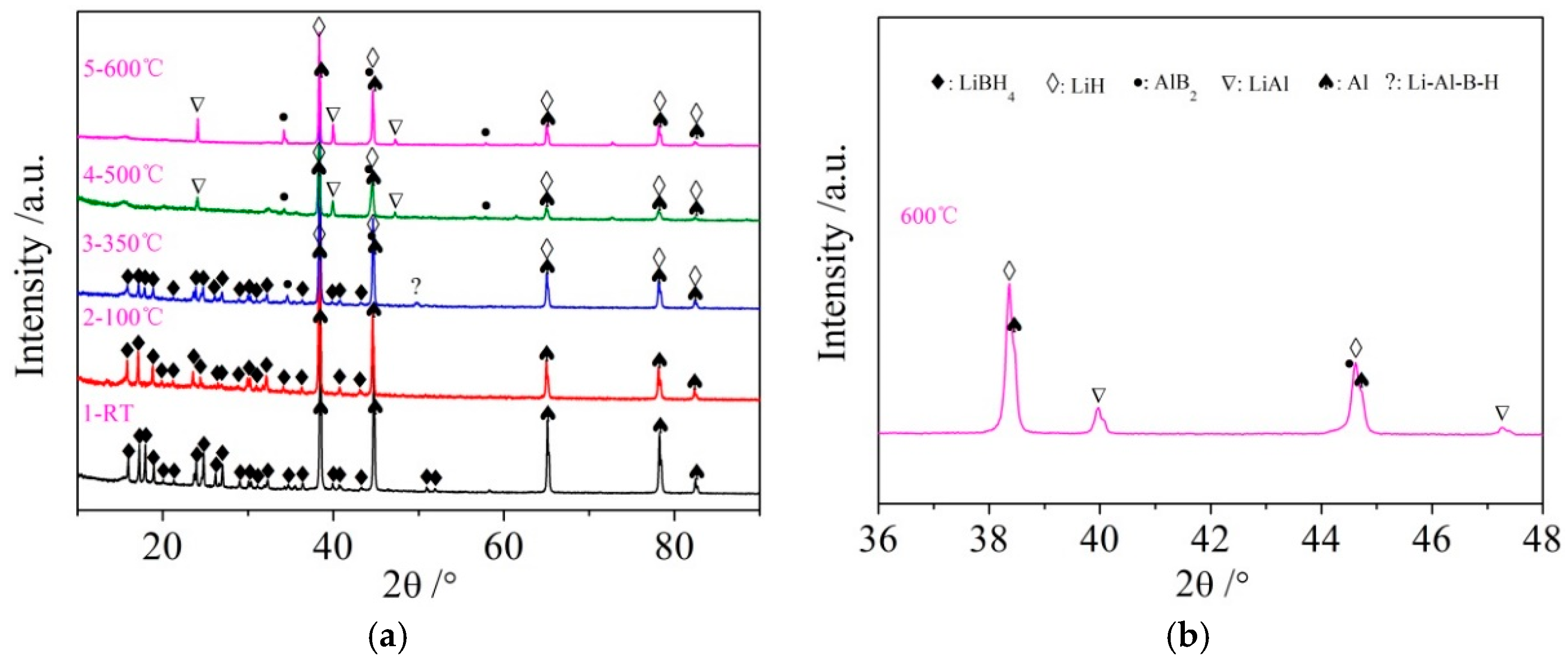
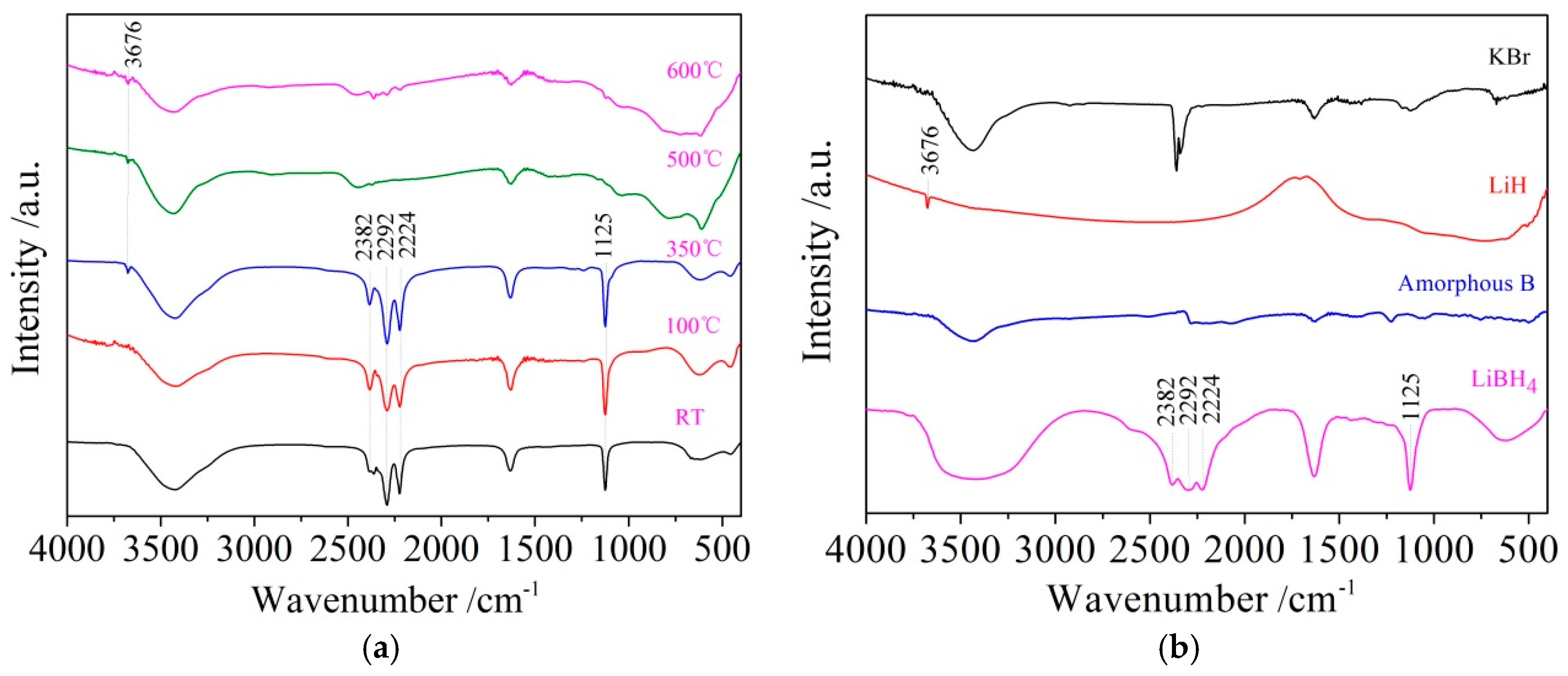
It can be seen from Figure 3d that the dehydrogenation behavior of LiBH4/Al* is more sophisticated than that of LiBH4/Al. There is a tiny endothermic peak appearing at 376 °C in the DSC curve of the LiBH4/Al* composite, accompanied by a small amount of H2 and B2H6 desorption reflected in the MS curve. What is more, there are four endothermic peaks of dehydrogenation locate at 434, 450, 472, and 560 °C. In order to investigate the mechanism of these thermal events, XRD and FTIR analyses were conducted on the solid products of the LiBH4/Al* sample at different dehydrogenation temperatures (e.g., 100, 350, 500, and 600 °C). The results are shown in Figure 4; Figure 5, respectively. It can be seen from Figure 4a that no new phase was detected when the sample was heated to 100 °C. The shrinkage of the diffraction peaks of LiBH4 is related to its crystal transformation. When the sample was heated to 350 °C, some tiny diffraction peaks of AlB2 and an unknown phase appeared. The unknown phase, marked “?”, was also reported by other researchers and considered to be compounds with components of Li-Al-B [32,33]. Combined with the FTIR spectra in Figure 5a, the diffraction peaks of LiH overlapping with the diffraction peaks of Al can also be found in Figure 4a at this stage. This indicates that LiBH4 had started to react with Al* to form LiH, AlB2, and compounds containing Li-Al-B. At the same time, B2H6 and H2 were released, and the rate reached a peak at 376 °C according to Figure 3d. Therefore, the further decrease of the diffraction intensity of LiBH4 at 350 °C (Figure 4a) can be attributed to its melting and dehydrogenation. When the sample was heated to 500 °C, the LiBH4 could not be detected by the XRD analysis (Figure 4a), and the vibrational peaks of B–H stretching (2382, 2292, and 2224 cm−1) and bending (1125 cm−1) disappeared (Figure 5a), indicating that LiBH4 had been completely consumed in dehydrogenation reactions at 376, 434, 450, and 472 °C (Figure 3d). What is more, the diffraction peaks of LiAl appeared, and the diffraction intensity of LiH and AlB2 slightly increased, while the peaks of Al weakened, and the peaks of compounds containing Li-Al-B disappeared. Combined with the analyses of LiBH4 and LiBH4/Al samples, it can be reasonably assumed that the main dehydrogenation peak of LiBH4/Al* at 450 °C is attributed to the reaction of LiBH4 and Al to form LiH, AlB2, and H2 based on Reaction (3). The reaction temperature was lower than that of the LiBH4/Al sample probably because the particle size of active Al* derived from AlH3 is much smaller than that of as-purchased Al, and the oxide-free surface of Al* possesses higher chemical reactivity. An easier atomic diffusion and shorter diffusion lengths led to less activation energy required for the reaction. The dehydrogenation peak at 472 °C is ascribed to the self-decomposition of LiBH4 forming LiH, B, and H2 based on Reaction (2). However, the diffraction peaks of B were not found in the XRD examination, and this may be because B was in an amorphous state. Therefore, the dehydrogenation peak at 434 °C is probably related to the decomposition of the unknown compounds, which can be denoted as “Li-Al-B-H”. Furthermore, the dehydrogenation peak at 376 °C is believed to be attributed to Reaction (5). In addition, AlB2 is generally considered to be a product that makes the dehydrogenation system reversible, while B2H6 is a toxic gas, which may be a problem for the future application of the LiBH4/Al* system. Finally, the appearance of LiAl indicates that LiH had begun to react with Al* to form LiAl and liberate H2 (Reaction (4)) before 500 °C.
LiBH4 + Al → LiH + AlB2 + “Li-Al-B-H” + B2H6↑+ H2↑
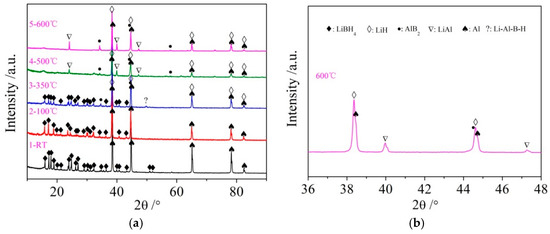
Figure 4.
XRD patterns (a) of the LiBH4/Al* sample obtained at different temperatures (room temperature, 100, 350, 500, and 600 °C) and expanded XRD pattern of LiBH4/Al* sample of 600 °C at a 2θ range of 36–48° (b).
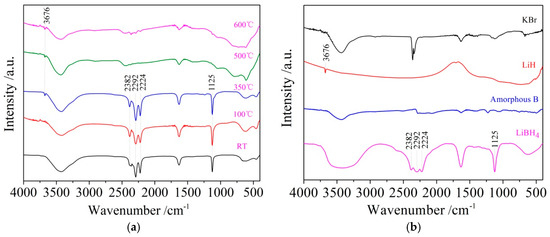
Figure 5.
Fourier transform infrared spectroscopy (FTIR) patterns of the LiBH4/Al* sample (a) obtained at different temperatures (room temperature, 100, 350, 500, and 600 °C) and reference substances (b) including KBr, LiH, amorphous B, and LiBH4.
Compared with the XRD patterns at 500 °C, no new phase was detected when the LiBH4/Al* sample was heated to 600 °C. The increase of the relative diffraction intensity of LiAl implies that the reaction of LiH with Al* continued from 500 to 600 °C. The dehydrogenation rate of this reaction reached a peak at 560 °C according to Figure 3d. The existence of LiH and Al suggests that the LiBH4/Al* system still dehydrogenated incompletely even at 600 °C. In fact, the dehydrogenation amount of the LiBH4/Al* sample is far from the theoretical value (7.2 wt.%) according to Figure 3a, indicating that there exist some kinetic barriers in the dehydrogenation reaction of the LiBH4/Al* composite. Moreover, the physical barrier is probably the reaction products from the previous step, which surround the Al* particles and preventing Al* from coming into contact with other reactants.
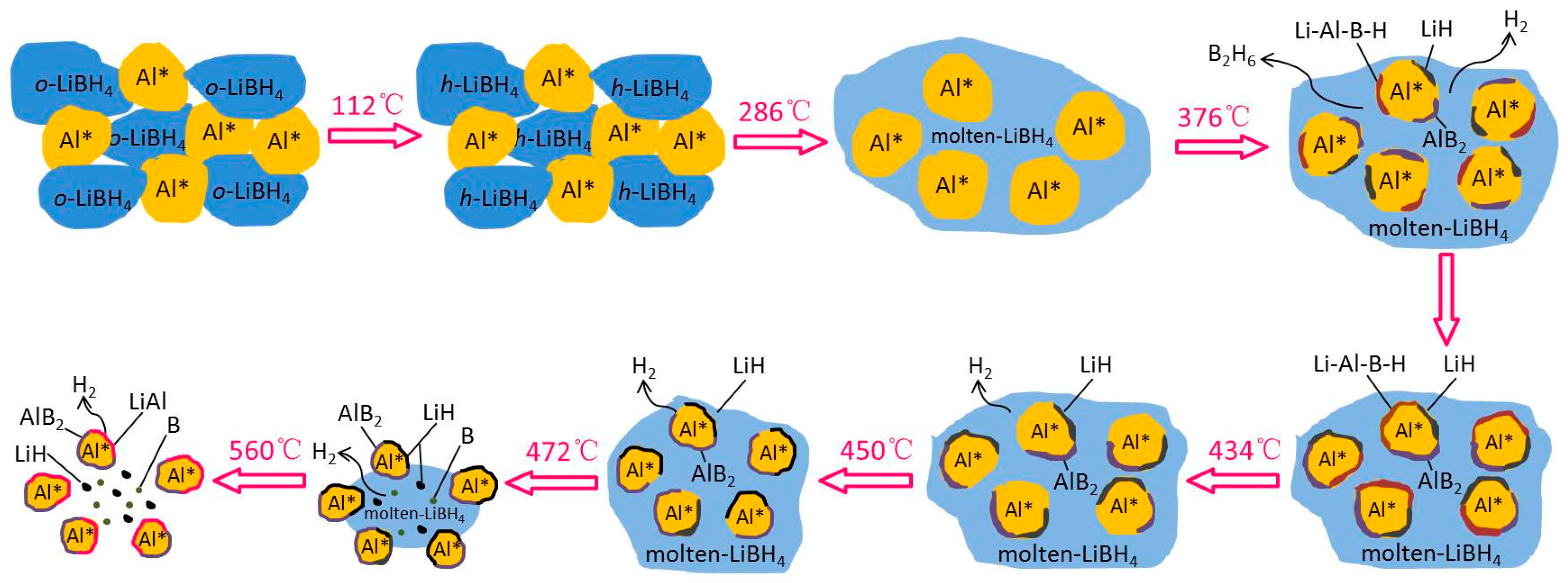
The whole hydrogen desorption process of the LiBH4/Al* sample, which is schematically shown in Figure 6, can be concluded as follows: While heating in the crucible, LiBH4 first transformed from an orthorhombic phase (o-LiBH4) to a hexagonal phase (h-LiBH4) at 112 °C and melted at 288 °C. Then, the molten LiBH4 reacted with Al* to form LiH, AlB2, and “Li-Al-B-H” compounds while releasing B2H6 and H2 based on Reaction (5) at 376 °C. As the LiH, AlB2, and “Li-Al-B-H” compounds were supposed to nucleate and grow on the surface of Al*, the reaction stopped when Al* was completely wrapped by these reaction products to form a passivation shell. When the temperature rose to 434 °C, the decomposition of the “Li-Al-B-H” compounds liberated a certain amount of H2, and the encapsulated Al* exposed some new surfaces. Thus, the main dehydrogenation reaction of LiBH4 and Al* occurred at 450 °C to form LiH, AlB2, and H2 based on reaction (3). Similarly, the reaction stopped when the surface of Al* was completely wrapped by LiH and AlB2. Therefore, the excess molten LiBH4 underwent self-decomposition to form LiH, B, and H2 (Reaction (2)) at 472 °C. That boron (B) was not detected in the XRD examination may be because B was in the amorphous state. Finally, the product LiH reacted with Al* to form LiAl alloy and H2 based on Reaction (4) when the sample was heated to 560 °C. The actual dehydrogenation amount of the LiBH4/Al* sample did not reach the theoretical value since there were still uncontacted and unreacted LiH and Al* at 600 °C.
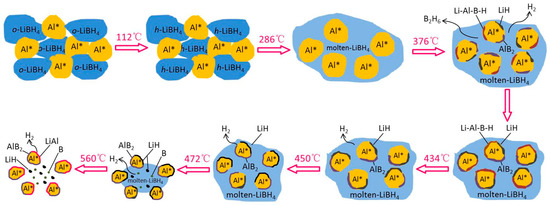
Figure 6.
Schematic diagram of the dehydrogenation process of the LiBH4/Al* sample.
3.2. Kinetic Properties of the Dehydrogenation of the LiBH4/Al* Composite
The kinetic properties of the dehydrogenation of the LiBH4/Al* composite were studied using the Kissinger method, which assumes that the apparent activation energy (Ea) of dehydrogenation reaction is determined by Equation (6).
ln(β/Tm2) = −Ea/RTm + C
In this equation, β is the heating rate in thermal analysis, and Tm represents the absolute temperature at the maximum reaction rate. Moreover, R is the universal gas constant, and C also represents a constant. Therefore, the Ea of the dehydrogenation reaction of the LiBH4/Al* composite can be obtained from the slope of a linearly fitted line in the ln(β/Tm2)-Tm−1 spectrum.
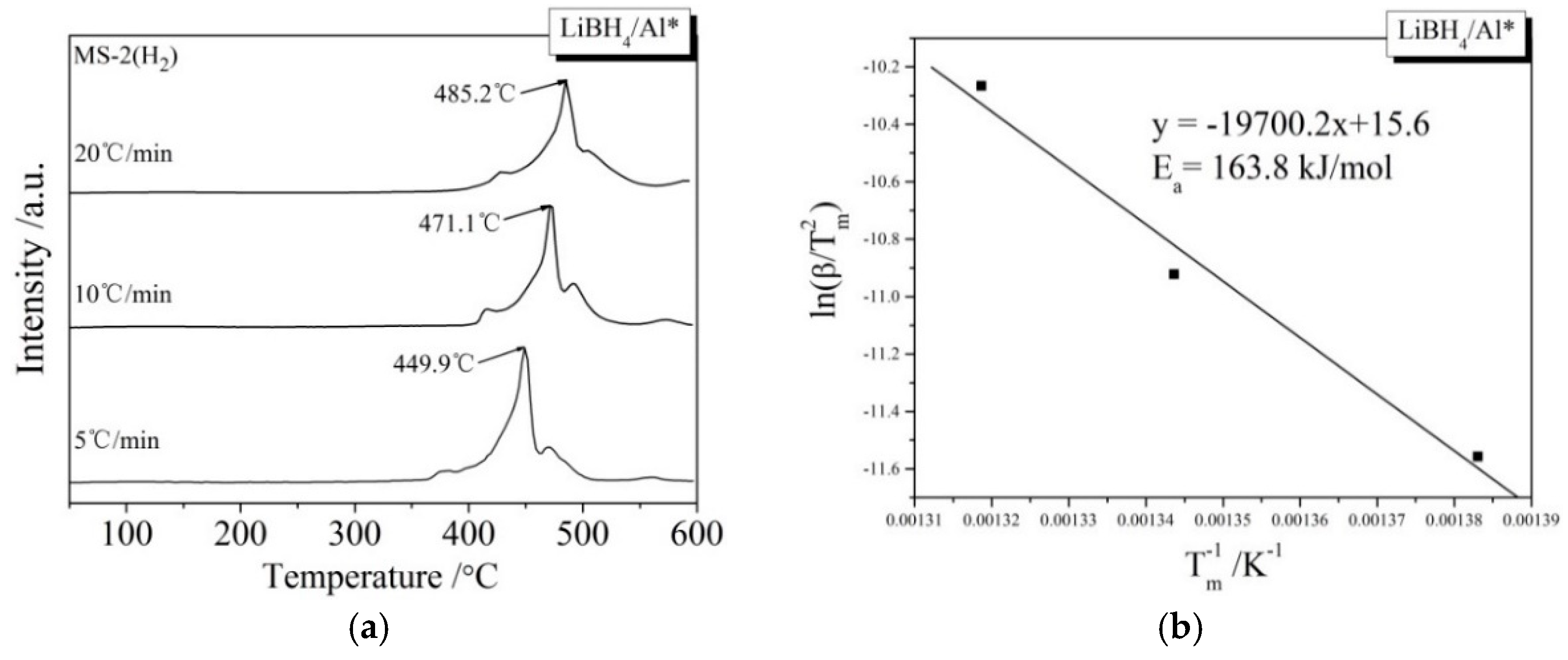
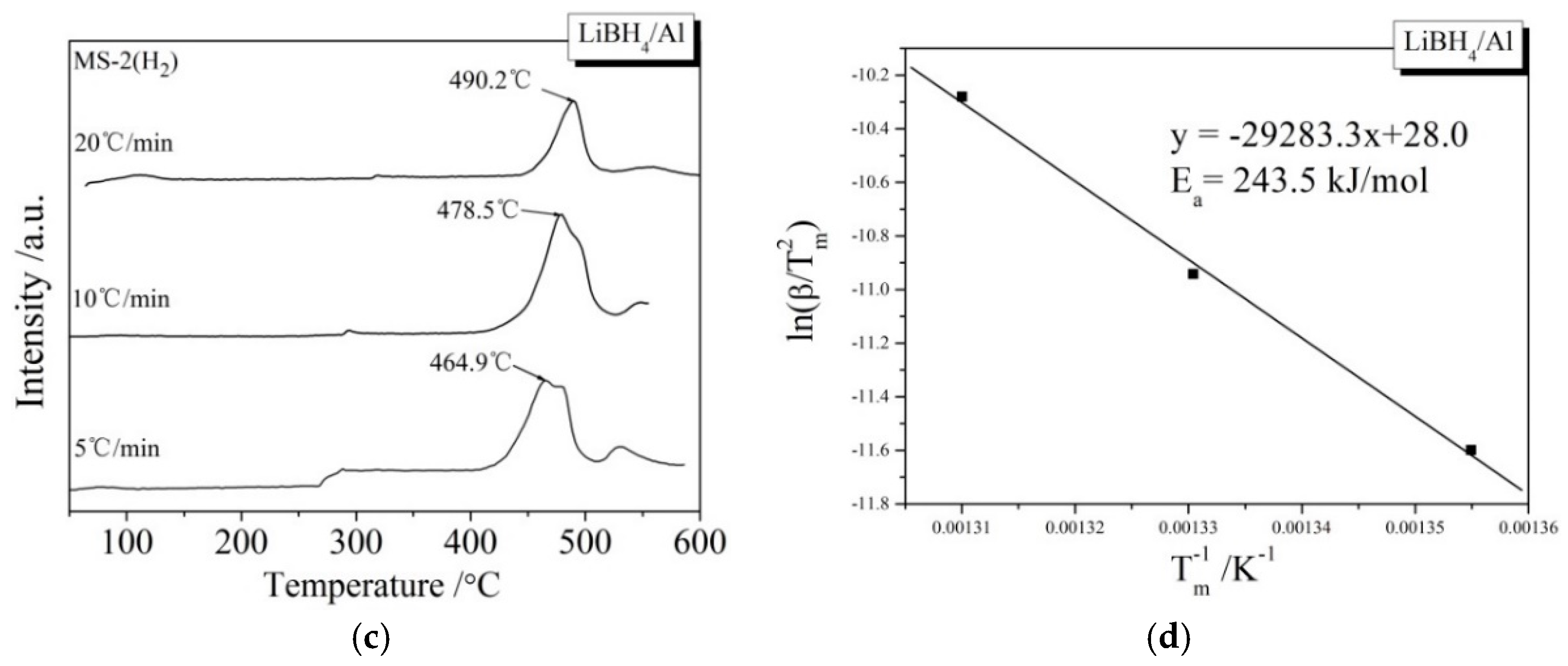
During the kinetic investigations, the LiBH4/Al and LiBH4/Al* samples were heated to 600 °C at the rates of 5, 10, and 20 °C/min, respectively. The MS curves at various heating rates and the Kissinger spectra reflecting the Ea of the main dehydrogenation reaction are shown in Figure 7. It can be seen that the temperatures for the maximum dehydrogenation rate of LiBH4/Al* at the heating rates of 5, 10, and 20 °C/min are 449.9, 471.1, and 485.2 °C, respectively. These are all lower than that of LiBH4/Al at the same heating rates. The Ea of the main dehydrogenation reaction of LiBH4/Al* is calculated to be 163.8 kJ/mol, while that of LiBH4/Al is 243.5 kJ/mol. This is in good agreement with the previous analysis that the smaller particle size and higher chemical reactivity of Al* can reduce the activation energy and improve the kinetic properties of the dehydrogenation reaction.
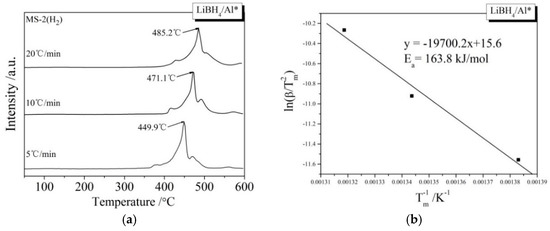
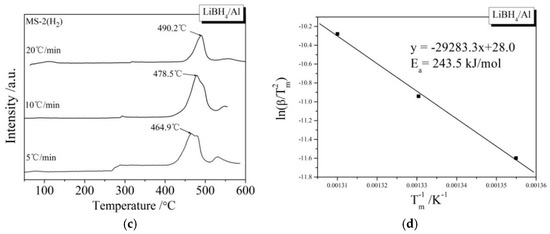
Figure 7.
MS curves (a,c) of LiBH4/Al* and LiBH4/Al samples at different heating rates (5, 10, and 20 °C/min) and Kissinger spectra (b,d) of the main dehydrogenation reaction of the LiBH4/Al* and LiBH4/Al samples.
3.3. Reversibility of the LiBH4/Al* Composite
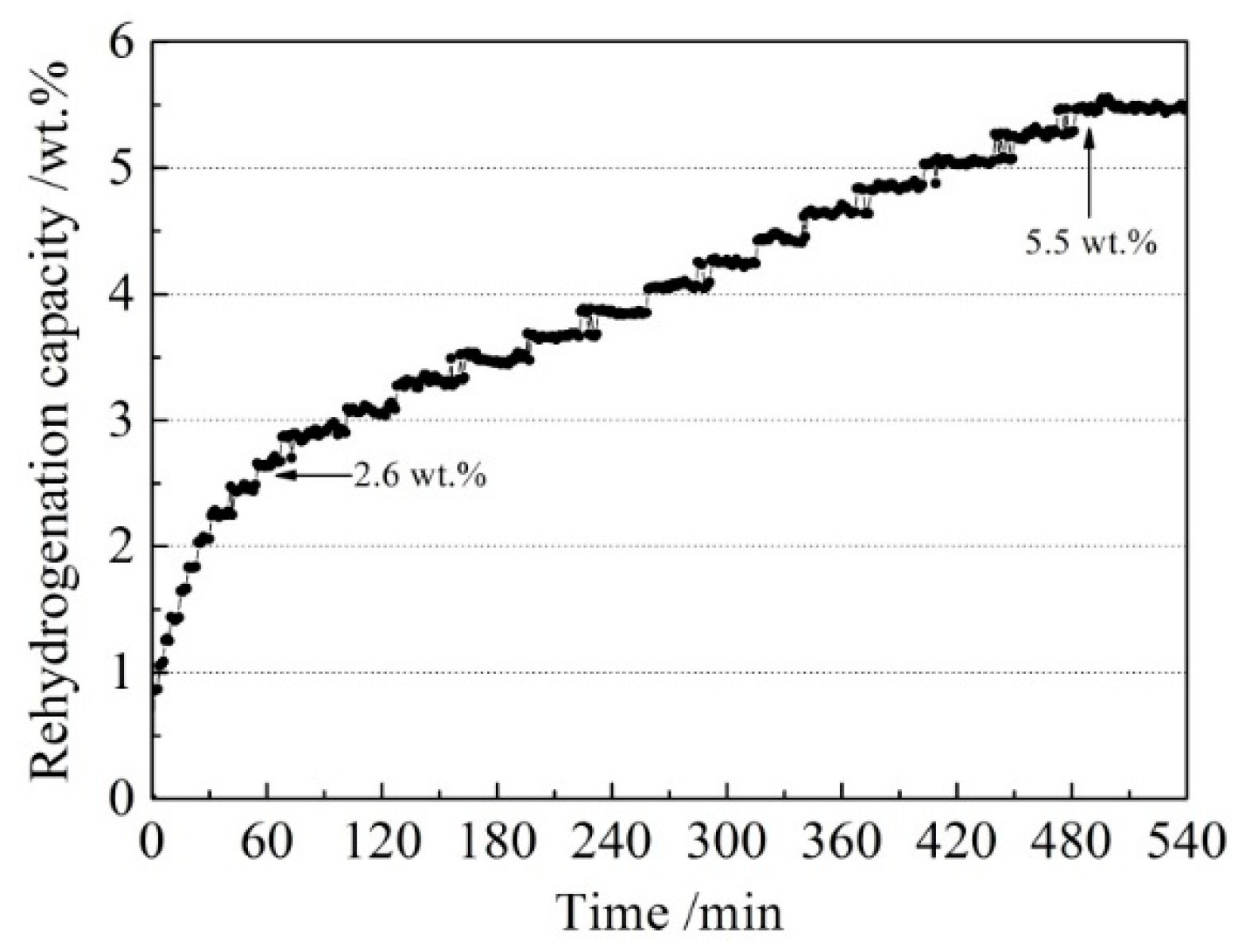
In order to investigate the reversibility of the LiBH4/Al* composite, a rehydrogenation test was carried out under 8 MPa H2 at 400 °C. The rehydrogenation curve of the sample is shown in Figure 8. It can be seen that the dehydrogenated LiBH4/Al* sample absorbed 2.6 wt.% of hydrogen in the first 60 min. Then it entered a stable hydrogen absorption stage and reached saturation after 480 min. The total rehydrogenation capacity was 5.5 wt.%. Compared with the harsh rehydrogenation conditions reported by other researchers [9,10], the doping of active Al* derived from AlH3 effectively improved the reversible hydrogen storage properties of LiBH4.
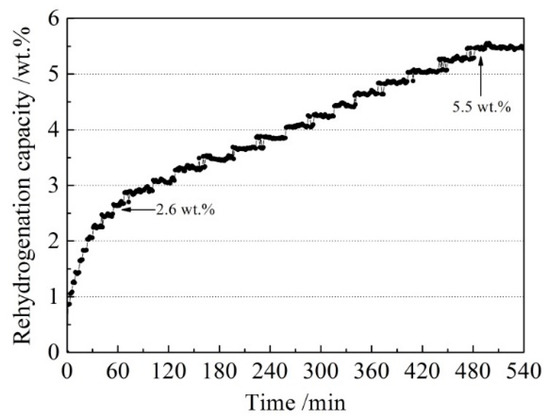
Figure 8.
The rehydrogenation curve of the dehydrogenated LiBH4/Al* sample under 8 MPa H2 at 400 °C.
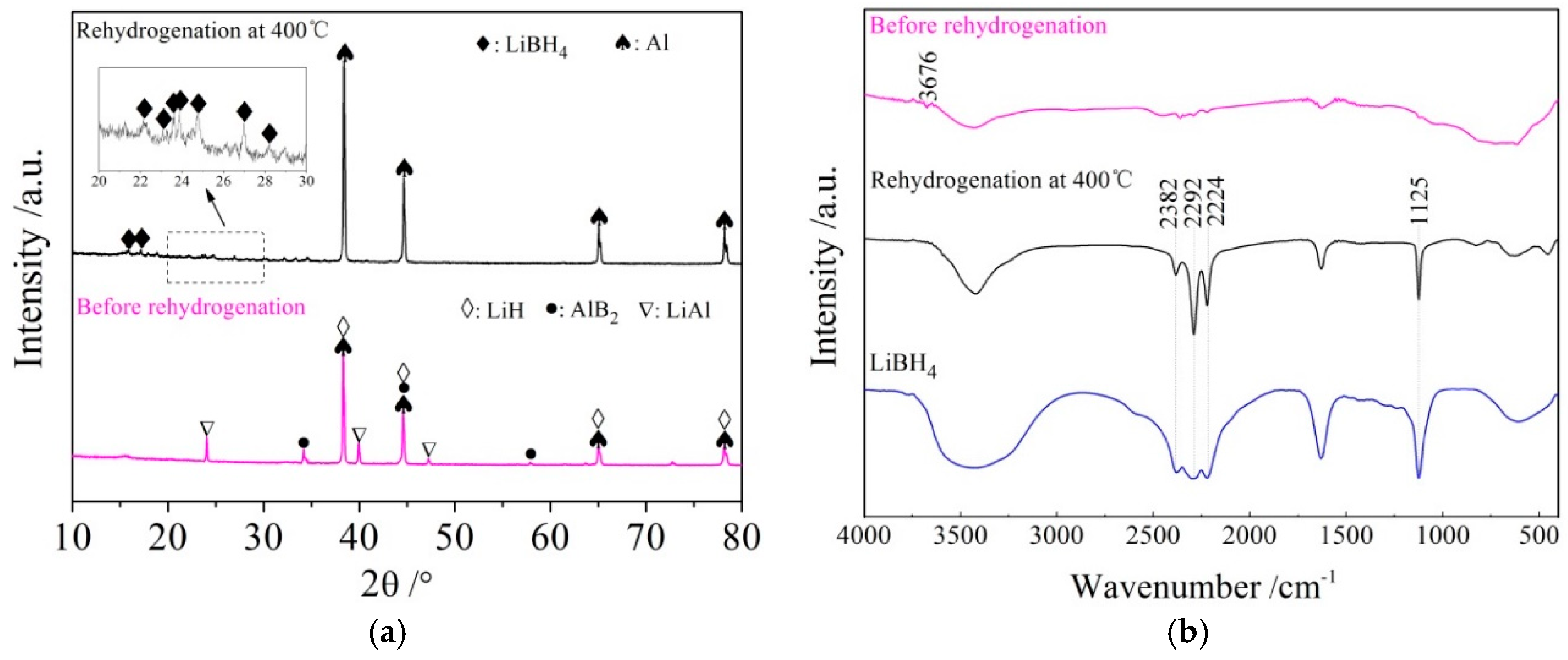
The rehydrogenation mechanism exploration was conducted using XRD and FTIR analysis of the rehydrided products of the LiBH4/Al* sample, and the results are shown in Figure 9a,b, respectively. It can be seen from the XRD patterns that the diffraction peaks of LiAl, LiH, and AlB2 disappeared, while the diffraction peaks of LiBH4 reappeared, and the diffraction intensity of Al increased after rehydrogenation. Furthermore, the vibrational peaks of B–H stretching (2382, 2292, and 2224 cm−1) and bending (1125 cm−1) were also detected in the FTIR spectra. Therefore, the re-formation of LiBH4 can be confirmed during the rehydrogenation process. Based on the above analysis, it can be safely concluded that the rehydrogenation process of LiBH4/Al* is based on Reaction (7).
LiH + LiAl + AlB2 + 7/2H2 ↔ 2LiBH4 + 2Al
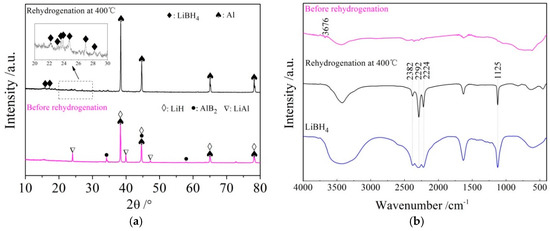
Figure 9.
XRD patterns (a) and FTIR spectra (b) of LiBH4/Al* sample before and after rehydrogenation.
4. Conclusions
The dehydrogenation of LiBH4 doped by active Al* derived from AlH3 results from a five-step reaction: (1) LiBH4 + Al → LiH + AlB2 + “Li-Al-B-H” + B2H6 + H2; (2) the decomposition of “Li-Al-B-H” compounds liberating H2; (3) 2LiBH4 + Al → 2LiH + AlB2 + 3H2; (4) LiBH4 → LiH + B + 3/2H2; and (5) LiH + Al → LiAl + 1/2H2. Furthermore, the reversibility of the LiBH4/Al* composite is based on the following reaction: LiH + LiAl + AlB2 + 7/2H2 ↔ 2LiBH4 + 2Al.
The hydrogen desorption kinetics of LiBH4 were effectively improved by doping with active Al* derived from AlH3. Higher dehydrogenation capacity, lower activation energy, and better reversibility of LiBH4/Al* can be achieved due to the larger specific surface area and higher chemical reactivity of Al*. The extent of the dehydrogenation reaction between LiBH4 and Al* greatly depended on the precipitation and growth of reaction products (LiH, AlB2, and LiAl) on the surface of the Al*. A passivation shell formed by these products on the Al* is the kinetic barrier to the dehydrogenation of the LiBH4/Al* composite. Therefore, future work should focus on cracking this barrier to further improve the hydrogen storage properties of the LiBH4/Al* composite.
Author Contributions
Conceptualization, Q.H. and D.Z.; methodology, Q.H. and D.D.; data curation, M.X. and X.W.; formal analysis, X.W.; investigation, M.X. and X.J.; resources, Q.H.; supervision, Q.H.; project administration, Q.H.; funding acquisition, Q.H.; writing-original draft preparation, X.W.; writing-review and editing, Q.H. and D.Z.
Funding
This work was funded by the Zhejiang Provincial Natural Science Foundation of China (Grant Nos. LQ16E010002, LY18E010003), and the National Natural Science Foundation of China (Grant Nos. 51501100, 51801112, 51704001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonisation. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Colbertaldo, P.; Agustin, S.B.; Campanari, S.; Brouwer, J. Impact of hydrogen energy storage on California electric power system: Towards 100% renewable electricity. Int. J. Hydrogen Energy 2019, 44, 9558–9576. [Google Scholar] [CrossRef]
- Zhao, G.; Nielsen, E.R.; Troncoso, E.; Hyde, K.; Romeo, J.S.; Diderich, M. Life cycle cost analysis: A case study of hydrogen energy application on the Orkney Islands. Int. J. Hydrogen Energy 2019, 44, 9517–9528. [Google Scholar] [CrossRef]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- Puszkiel, J.; Garroni, S.; Milanese, C.; Gennari, F.; Klassen, T.; Dornheim, M.; Pistidda, C. Tetrahydroborates: Development and potential as hydrogen storage medium. Inorganics 2017, 5, 74. [Google Scholar] [CrossRef]
- Wu, R.; Ren, Z.; Zhang, X.; Lu, Y.; Li, H.; Gao, M.; Pan, H.; Liu, Y. Nanosheet-like Lithium Borohydride Hydrate with 10 wt.% of Hydrogen Release at 70 °C as a Chemical Hydrogen Storage Candidate. J. Phys. Chem. Lett. 2019, 10, 1872–1877. [Google Scholar] [CrossRef]
- DOE, U.S. Targets for Onboard Hydrogen Storage Systems for Light-Duty Vehicles; US Department of Energy, Office of Energy Efficiency and Renewable Energy and The Freedom CAR and Fuel Partnership: Washington, DC, USA, 2009.
- Orimo, S.I.; Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Towata, S.I.; Züttel, A. Dehydriding and rehydriding reactions of LiBH4. J. Alloys Compd. 2005, 404, 427–430. [Google Scholar] [CrossRef]
- Liu, X.; Peaslee, D.; Jost, C.Z.; Baumann, T.F.; Majzoub, E.H. Systematic pore-size effects of nanoconfinement of LiBH4: Elimination of diborane release and tunable behavior for hydrogen storage applications. Chem. Mater. 2011, 23, 1331–1336. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives-synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef]
- Von Colbe, J.B.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.; Guzik, M.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef]
- Chaudhary, A.L.; Li, G.; Matsuo, M.; Orimo, S.I.; Deledda, S.; Sørby, M.H.; Hauback, B.C.; Pistidda, C.; Klassen, T.; Dornheim, M. Simultaneous desorption behavior of M borohydrides and Mg2FeH6 reactive hydride composites (M = Mg, then Li, Na, K, Ca). Appl. Phys. Lett. 2015, 107, 073905. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Gennari, F.C. Reversible hydrogen storage in metal-doped Mg-LiBH4 composites. Scr. Mater. 2009, 60, 667–670. [Google Scholar] [CrossRef]
- Mao, J.F.; Guo, Z.P.; Liu, H.K.; Yu, X.B. Reversible hydrogen storage in titanium-catalyzed LiAlH4-LiBH4 system. J. Alloys Compd. 2009, 487, 434–438. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Gennari, F.C.; Larochette, P.A.; Ramallo-López, J.M.; Vainio, U.; Karimi, F.; Pranzas, P.K.; Troiani, H.; Pistidda, C.; Jepsen, J.; Tolkiehn, M.; et al. Effect of Fe additive on the hydrogenation-dehydrogenation properties of 2LiH + MgB2/2LiBH4+MgH2 system. J. Power Sources 2015, 284, 606–616. [Google Scholar] [CrossRef]
- Noritake, T.; Aoki, M.; Towata, S.; Ninomiya, A.; Nakamori, Y.; Orimo, S. Crystal structure analysis of novel complex hydrides formed by the combination of LiBH4 and LiNH2. Appl. Phys. A 2006, 83, 277–279. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, X.; Feidenhans’l, R.; Vegge, T. Destabilized LiBH4-NaAlH4 Mixtures Doped with Titanium Based Catalysts. J. Phys. Chem. C 2008, 112, 18244–18248. [Google Scholar] [CrossRef]
- Sofianos, M.V.; Chaudhary, A.L.; Paskevicius, M.; Sheppard, D.A.; Humphries, T.D.; Dornheim, M.; Buckley, C.E. Hydrogen storage properties of eutectic metal borohydrides melt-infiltrated into porous Al scaffolds. J. Alloys Compd. 2019, 775, 474–480. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Q. The reactions in LiBH4-NaNH2 hydrogen storage system. Int. J. Hydrogen Energy 2011, 36, 9733–9742. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, S. Effect of Al on the dehydrogenation of LiBH4 from first-principles calculations. Int. J. Hydrogen Energy 2017, 42, 6181–6188. [Google Scholar]
- Cho, Y.W.; Shim, J.H.; Lee, B.J. Thermal destabilization of binary and complex metal hydrides by chemical reaction: A thermodynamic analysis. Calphad 2006, 30, 65–69. [Google Scholar] [CrossRef]
- Yu, X.; Xia, G.; Guo, Z.; Liu, H. Dehydrogenation/rehydrogenation mechanism in aluminum destabilized lithium borohydride. Int. J. Mater. Res. 2009, 24, 2720–2727. [Google Scholar] [CrossRef]
- Kato, S.; Bielmann, M.; Ikeda, K.; Orimo, S.I.; Borgschulte, A.; Züttel, A. Surface changes on AlH3 during the hydrogen desorption. Appl. Phys. Lett. 2010, 96, 051912. [Google Scholar] [CrossRef]
- Carrillo-Bucio, J.; Tena-García, J.; Suárez-Alcántara, K. Dehydrogenation of surface-oxidized mixtures of 2LiBH4+ Al/Additives (TiF3 or CeO2). Inorganics 2017, 5, 82. [Google Scholar] [CrossRef]
- Kwak, Y.J.; Song, M.Y. How to Analyse Metal Hydride Decomposition Temperatures Using a Sieverts’ Type Hydriding-Dehydriding Apparatus and Hydrogen-Storage Characteristics for an MgH2-Based Alloy. Mater. Sci. 2018, 24, 24–28. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.M.C.E.P.; Sudan, P.H.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356, 515–520. [Google Scholar] [CrossRef]
- Kang, X.D.; Wang, P.; Ma, L.P.; Cheng, H.M. Reversible hydrogen storage in LiBH4 destabilized by milling with Al. Appl. Phys. A 2007, 89, 963–966. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Q.; Zhang, J.; Liu, S.S.; Sun, L.X. The dehydrogenation reactions and kinetics of 2LiBH4-Al composite. J. Phys. Chem. C 2009, 113, 18424–18430. [Google Scholar] [CrossRef]
- Friedrichs, O.; Kim, J.W.; Remhof, A.; Buchter, F.; Borgschulte, A.; Wallacher, D.; Cho, Y.W.; Fichtner, M.; Ohb, K.H.; Züttel, A. The effect of Al on the hydrogen sorption mechanism of LiBH4. Phys. Chem. Chem. Phys. 2009, 11, 1515–1520. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Utke, R. Confined LiBH4-LiAlH4 in nanopores of activated carbon nanofibers. Int. J. Hydrogen Energy 2015, 40, 7083–7092. [Google Scholar] [CrossRef]
- Hansen, B.R.; Ravnsbæk, D.B.; Reed, D.; Book, D.; Gundlach, C.; Skibsted, J.; Jensen, T.R. Hydrogen storage capacity loss in a LiBH4-Al composite. J. Phys. Chem. C 2013, 117, 7423–7432. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).