Optimization of Quenching and Tempering Parameters for the Precipitation of M7C3 and MC Secondary Carbides and the Removal of the Austenite Retained in Vanadis 10 Tool Steel
Abstract
:1. Introduction
2. Materials and Methods
- The Vickers hardness—the applied load was of 1000 N, while the value of hardness considered in each experiment was the average value obtained from 10 indentations;
- Microstructural variables, such as the percentage of carbides and the amount of martensite present.
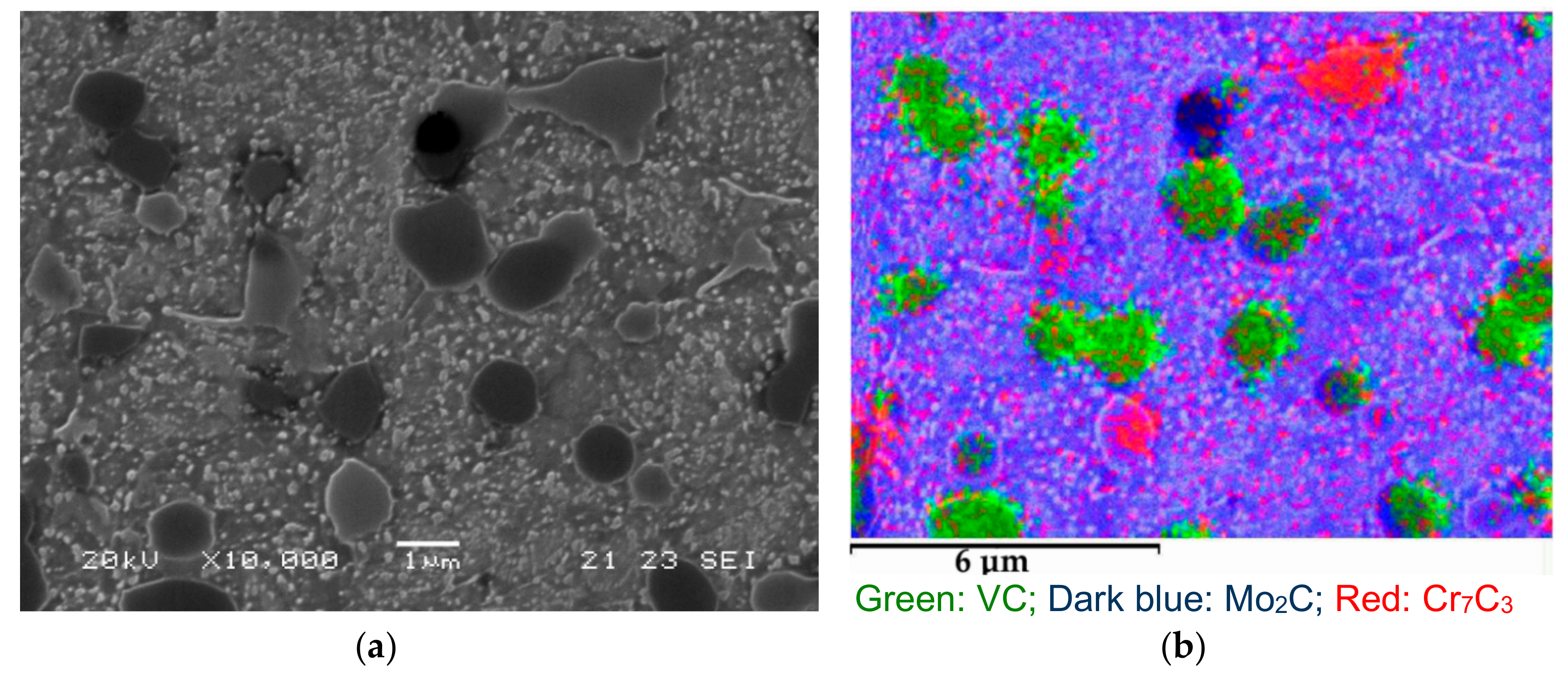
3. Results
4. Conclusions
- The destabilization of austenite is favored by high tempering temperatures (600 °C) and prolonged dwell times at this temperature (at least 4 h). These same conditions favor the removal of the retained austenite and the precipitation of Cr7C3 secondary carbides.
- Prolonged dwell times at the destabilization temperature of austenite (at least 8 h at 1100 °C) as well as high dwell times at the tempering temperature (at least 4 h) favor the precipitation of secondary vanadium carbides.
- However, given that the majority phase is martensite, the higher hardness values are obtained at a tempering temperature as low as 500 °C. The severity of quenching, corresponding to either air or oil, was shown not to have a significant influence on the final hardness value of the steel regardless of double or triple tempering treatment being applied.
Author Contributions
Funding
Conflicts of Interest
References
- Jurci, P. Cr-V Ledeburitic cold-work tool steels. Materiali in Tehnologije 2011, 45, 383–394. [Google Scholar]
- Pippel, E.; Woltersdorf, J.; Pockl, G.; Lichtenegger, G. Microstructure and nanochemistry of carbide precipitates in high-speed steel S 6-5-2-5. Mater. Charact. 1999, 43, 41–55. [Google Scholar] [CrossRef]
- Talacchia, S.; Amador, J.; Urcola, J. High speed tool steel cut off dies made using powder metallurgy techniques. Revista De Metalurgia 1996, 32, 18–24. [Google Scholar] [CrossRef]
- Nemec, M.; Jurci, P.; Kosnacova, P.; Kucerova, M. Evaluation of structural isotropy of Cr-V ledeburitic steel made by powder metallurgy of rapidly solidified particles. Kovove Materialy Met. Mater. 2016, 54, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Bilek, P.; Sobotova, J.; Jurci, P. Evaluation of the microstructural changes in Cr-V ledeburitic tool steels depending on the austenitization temperature. Materiali in Tehnologije 2011, 45, 489–493. [Google Scholar]
- Jurci, P.; Domankova, M.; Ptacinova, J. Evolution of carbide phases in quenched, sub-zero treated and tempered vanadis 6 ledeburitic tool steel. In Proceedings of the Metal 2014: 23rd International Conference on Metallurgy and Materials, Brno, Czech Republic, 21–23 May 2014; pp. 593–599. [Google Scholar]
- Gonzalez-Pociño, A.; Alvarez-Antolin, F.; Asensio-Lozano, J. Erosive wear resistance regarding diferent destabilization heat treatments of austenite in high chromium white cast iron, alloyed with Mo. Metals 2019, 9, 522. [Google Scholar] [CrossRef]
- Powell, G.L.F.; Bee, J.V. Secondary carbide precipitation in an 18 wt% Cr-1 wt% Mo white iron. J. Mater. Sci. 1996, 31, 707–711. [Google Scholar] [CrossRef]
- Filipovic, M.M. Iron-chromium-carbon-vanadium white cast irons—The microstructure and properties. Hemijska Industrija 2014, 68, 413–427. [Google Scholar] [CrossRef]
- Guitar, M.A.; Suarez, S.; Prat, O.; Guigou, M.D.; Gari, V.; Pereira, G.; Mucklich, F. High chromium cast irons: destabilized-subcritical secondary carbide precipitation and its effect on hardness and wear properties. J. Mater. Eng. Perform. 2018, 27, 3877–3885. [Google Scholar] [CrossRef]
- Efremenko, V.G.; Chabak, Y.G.; Brykov, M.N. Kinetic parameters of secondary carbide precipitation in high-Cr white iron alloyed by Mn-Ni-Mo-V complex. J. Mater. Eng. Perform. 2013, 22, 1378–1385. [Google Scholar] [CrossRef]
- Hui, L.; Han-Guang, F.; Jiang, J.; Jun, W. Effect of heat treatment on microstructure and property of high vanadium wear-resistant alloy. Materialwissenschaft Und Werkstofftechnik 2018, 49, 1485–1493. [Google Scholar] [CrossRef]
- Durica, J.; Ptacinova, J.; Hudakova, M.; Kusy, M.; Jurci, P. Microstructure and Hardness of cold work vanadis 6 steel after subzero treatment at-140 degrees C. Adv. Mater. Sci. Eng. 2018, 7. [Google Scholar] [CrossRef]
- Yan, F.; Shi, H.S.; Fan, J.F.; Xu, Z. An investigation of secondary carbides in the spray-formed high alloyed Vanadis 4 steel during tempering. Mater. Charact. 2008, 59, 883–889. [Google Scholar] [CrossRef]
- Wilmes, S.; Zwick, G. Effecto of niobium and vanadium as an alloying element in toll steels with high chromium content. In Proceedings of the Sixth International Tooling Conference, Karlstad, Sweden, 10–13 September 2002; pp. 269–287. [Google Scholar]
- Jurci, P.; Domankova, M.; Hudakova, M.; Ptacinova, J.; Pasak, M.; Palcek, P. Characterization of microstructure and tempering response of conventionally quenched, short- and long-time sub-zero treated PM Vanadis 6 ledeburitic tool steel. Mater. Charact. 2017, 134, 398–415. [Google Scholar] [CrossRef]
- Pero-Sanz, J.A. Aceros; Dossat: Madrid, Spain, 2004; p. 558. [Google Scholar]
- Vilar, R.; Colaco, R.; Almeida, A. Laser surface treatment of tool steels. Laser Processing: Surface Treatment and Film Deposition; Woodhead Publishing: Sawston, UK, 2012; Volume 307, pp. 453–478. [Google Scholar]
- Jurci, P.; Domankova, M.; Ptacinova, J.; Pasak, M.; Kusy, M.; Priknerova, P. Investigation of the Microstructural Changes and Hardness Variations of Sub-Zero Treated Cr-V Ledeburitic Tool Steel Due to the Tempering Treatment. J. Mater. Eng. Perform. 2018, 27, 1514–1529. [Google Scholar] [CrossRef]
- Sobotova, J.; Jurci, P.; Dlouhy, I. The effect of subzero treatment on microstructure, fracture toughness, and wear resistance of Vanadis 6 tool steel. Mater. Sci. Eng. 2016, 652, 192–204. [Google Scholar] [CrossRef]
- Jurci, P.; Domankova, M.; Caplovic, L.; Ptacinova, J.; Sobotova, J.; Salabova, P.; Prikner, O.; Sustarsic, B.; Jenko, D. Microstructure and hardness of sub-zero treated and no tempered P/M Vanadis 6 ledeburitic tool steel. Vacuum 2015, 111, 92–101. [Google Scholar] [CrossRef]
- Prat-Bartés, A.; Tort-Martorell, X.; Grima-Cintas, P.; Pozueta-Fernández, L.; Solé-Vidal, I. MétodosEstadísticos, 2nd ed.; UPC: Barcelona, Spain, 2004; p. 376. [Google Scholar]
- Wei, S.Z.; Zhu, J.H.; Xu, L.J. Effects of vanadium and carbon on microstructures and abrasive wear resistance of high speed steel. Tribol. Int. 2006, 39, 641–648. [Google Scholar] [CrossRef]
- Antolin, J.F.A.; Garrote, L.F.; Lozano, J.A. Application of Rietveld Refinement to the correlation of the microstructure evolution of white cast irons with 18 and 25%-wt. Cr after oil quench and successive temper treatments, with abrasive wear and bending testing. Revista De Metalurgia 2018, 54, 11. [Google Scholar] [CrossRef]


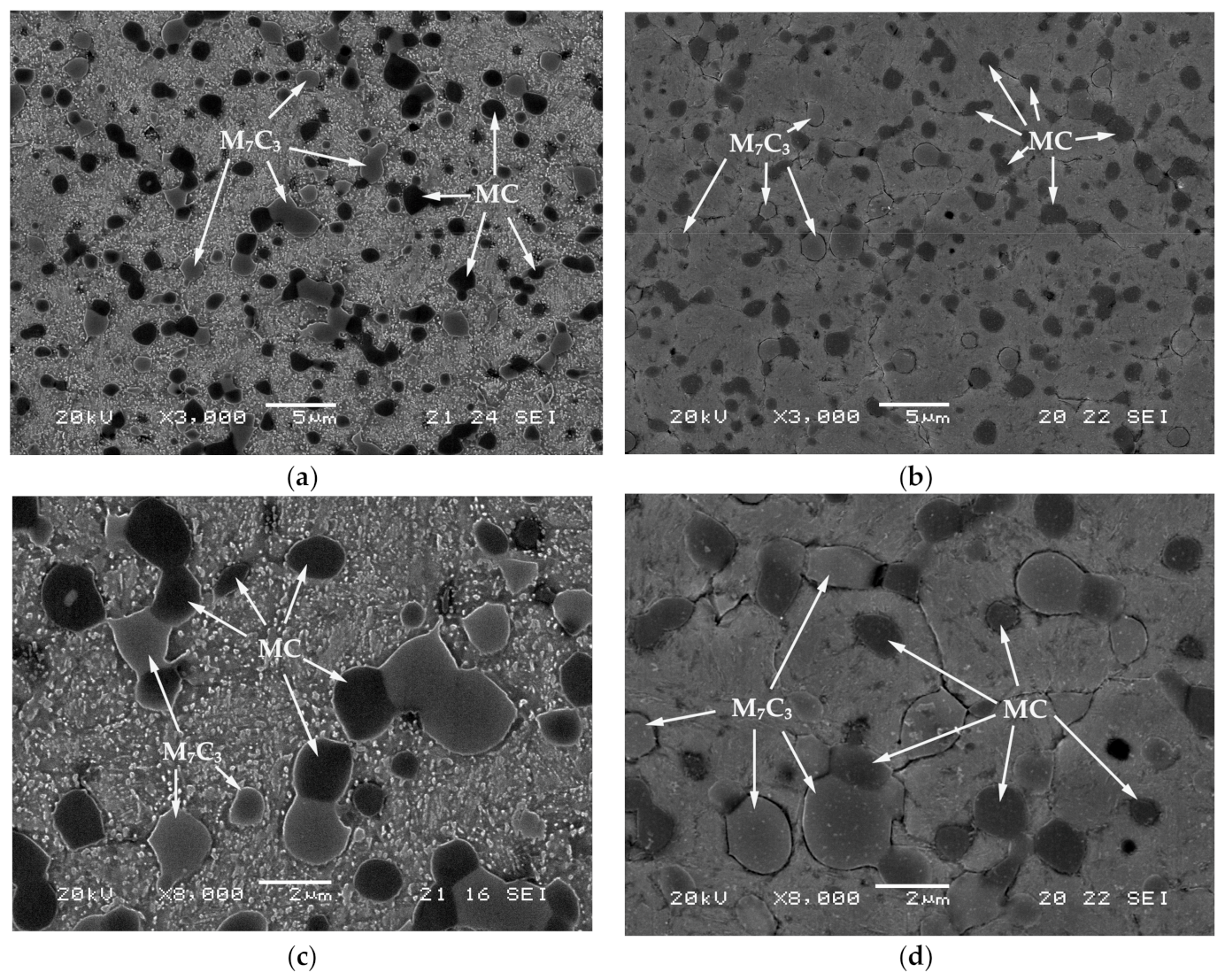
| C | Si | Mn | Cr | Mo | V |
|---|---|---|---|---|---|
| 2.9 | 0.5 | 0.5 | 8 | 1.5 | 9.8 |
| Factors | Levels | |||
|---|---|---|---|---|
| Code | Description of the Factors | Unit | −1 Level | +1 Level |
| A | Tempering temperature | °C | 500 | 600 |
| B | Dwell time at 1100 °C | h | 4 | 8 |
| C | Quench cooling medium | - | air | oil |
| D | Number of temperings | - | 2 | 3 |
| E | Tempering time | h | 2 | 4 |
| No. | A | B | C | D | E | Restricted Confounding Pattern |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | +1 | +1 | A+BD+CE B+AD C+AE D+AB E+AC BC+DE BE+CD |
| 2 | +1 | −1 | −1 | −1 | −1 | |
| 3 | −1 | +1 | −1 | −1 | +1 | |
| 4 | +1 | +1 | −1 | +1 | −1 | |
| 5 | −1 | −1 | +1 | +1 | −1 | |
| 6 | +1 | −1 | +1 | −1 | +1 | |
| 7 | −1 | +1 | +1 | −1 | −1 | |
| 8 | +1 | +1 | +1 | +1 | +1 |
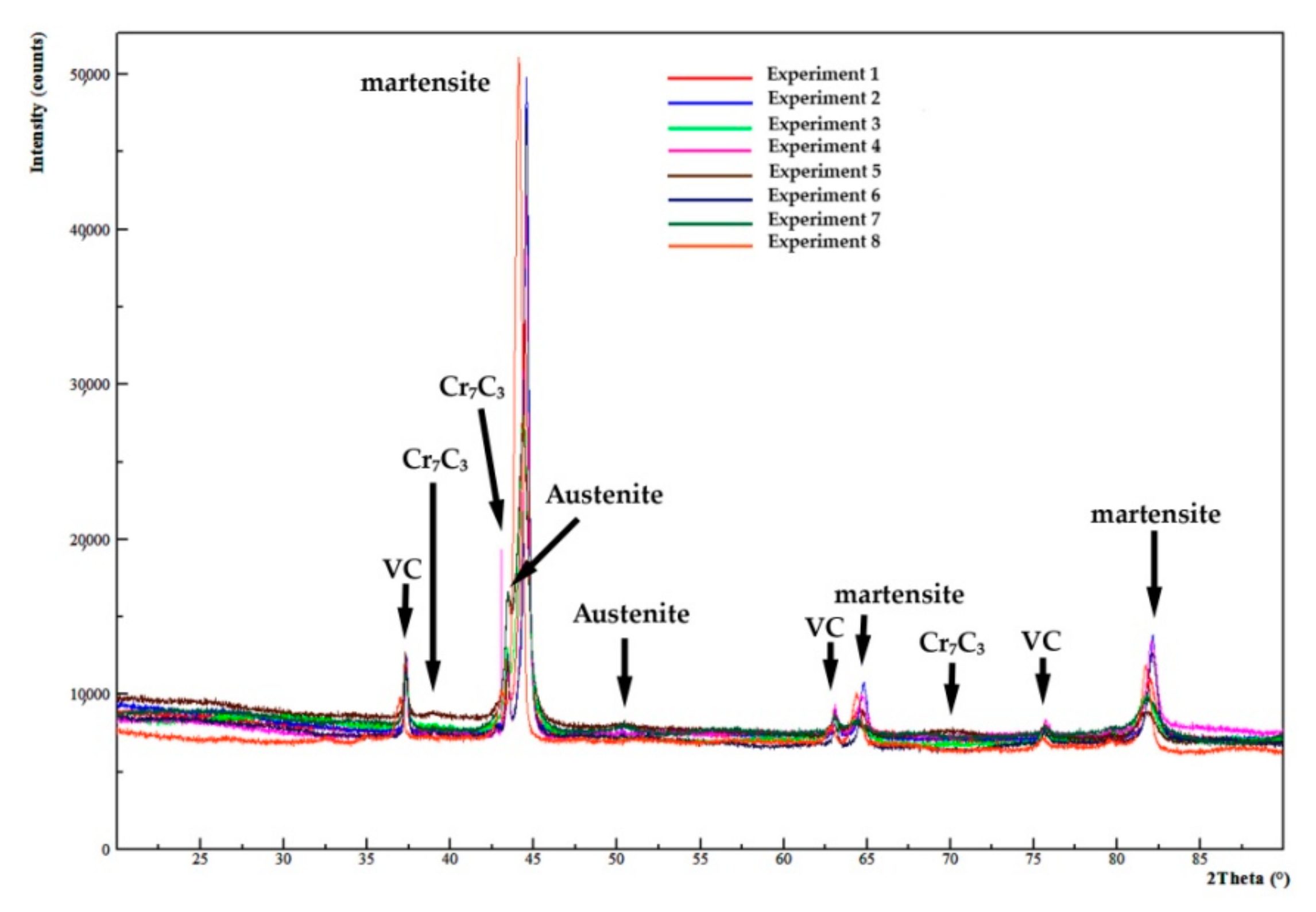
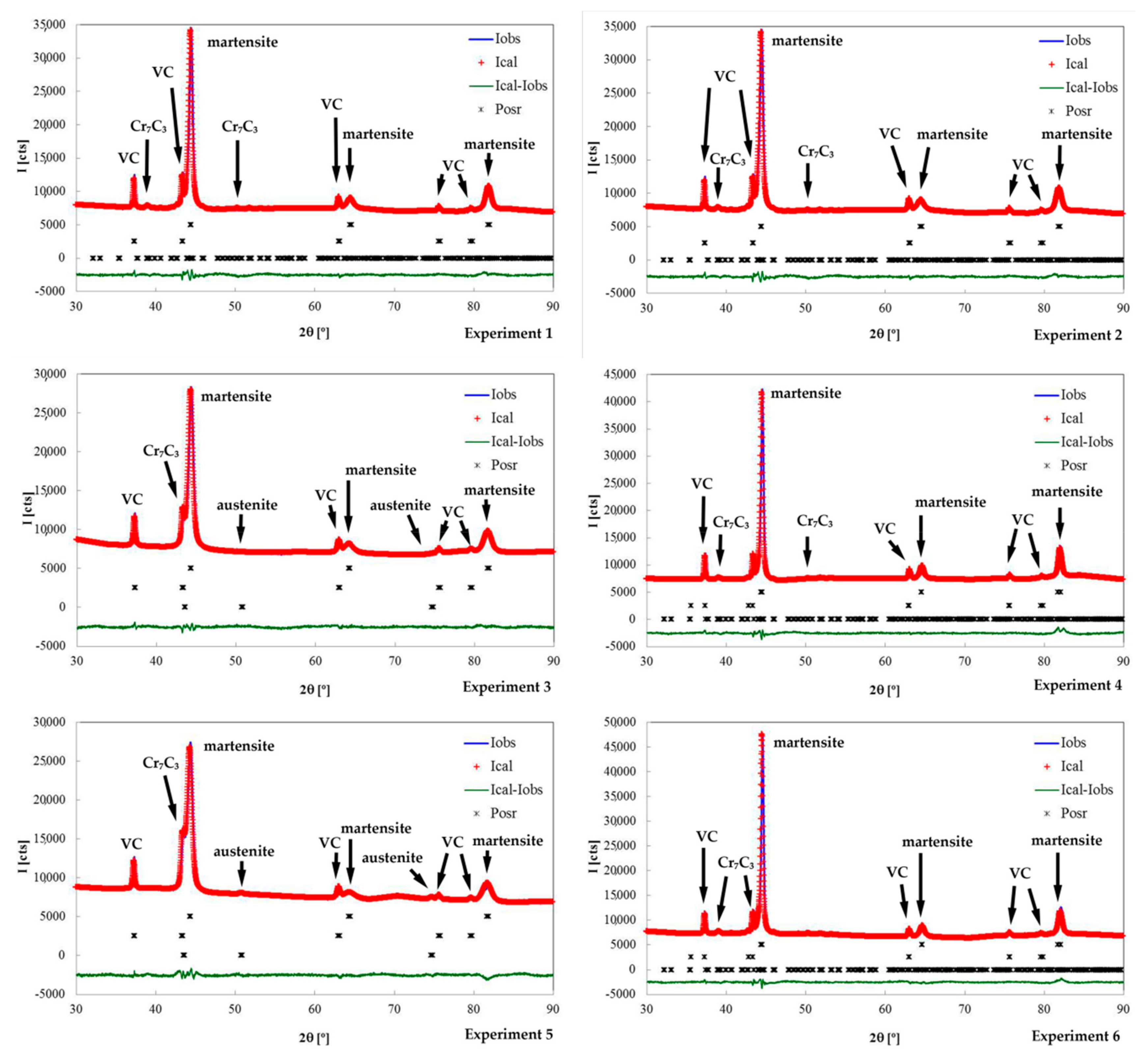
| Experiment | Rietveld Fitting | Phases | a (Å) | b (Å) | c (Å) | wt.% | Esd. |
|---|---|---|---|---|---|---|---|
| 1 | Rwp = 9.21 Chi2 = 1.91 | Martensite | 2.878 | 76.90 | ±1.33 | ||
| Austenite | - | - | |||||
| VC | 4.162 | 15.10 | ±0.55 | ||||
| Cr7C3 | 4.504 | 7.033 | 12.214 | 8.00 | ±1.05 | ||
| 2 | Rwp = 9.81 Chi2 = 2.28 | Martensite | 2.873 | 74.14 | ±1.09 | ||
| Austenite | - | - | |||||
| VC | 4.163 | 15.24 | ±0.53 | ||||
| Cr7C3 | 4.504 | 7.033 | 12.214 | 10.62 | ±0.91 | ||
| 3 | Rwp = 8.35 Chi2 = 1.51 | Martensite | 2.884 | 78.19 | ±1.49 | ||
| Austenite | 3.588 | 1.46 | ±0.49 | ||||
| VC | 4.168 | 18.95 | ±0.77 | ||||
| Cr7C3 | 4.504 | 7.033 | 12.214 | 1.40 | ±0.28 | ||
| 4 | Rwp = 10.9 Chi2 = 2.37 | Martensite | 2.874 | 76.86 | ±1.23 | ||
| Austenite | - | - | |||||
| VC | 4.162 | 15.56 | ±0.54 | ||||
| Cr7C3 | 4.504 | 7.033 | 12.214 | 7.58 | ±0.98 | ||
| 5 | Rwp = 10.1 Chi2 = 2.13 | Martensite | 2.883 | 69.70 | ±1.38 | ||
| Austenite | 3.588 | 12.56 | ±0.57 | ||||
| VC | 4.164 | 15.64 | ±0.74 | ||||
| Cr7C3 | 4.504 | 7.033 | 12.214 | 2.10 | ±0.37 | ||
| 6 | Rwp = 9.24 Chi2 = 2.07 | Martensite | 2.874 | 74.57 | ±1.10 | ||
| Austenite | - | - | |||||
| VC | 4.165 | 14.46 | ±0.53 | ||||
| Cr7C3 | 4.504 | 7.033 | 12.214 | 10.97 | ±0.90 | ||
| 7 | Rwp = 10.5 Chi2 = 2.28 | Martensite | 2.880 | 71.45 | ±1.33 | ||
| Austenite | 3.588 | 12.08 | ±0.54 | ||||
| VC | 4.159 | 14.46 | ±0.73 | ||||
| Cr7C3 | 4.504 | 7.033 | 12.214 | 2.01 | ±0.34 | ||
| 8 | Rwp = 10.5 Chi2 = 2.16 | Martensite | 2.870 | 75.95 | ±1.11 | ||
| Austenite | - | - | |||||
| VC | 4.148 | 19.21 | ±0.77 | ||||
| Cr7C3 | 4.504 | 7.033 | 12.214 | 4.84 | ±0.61 |
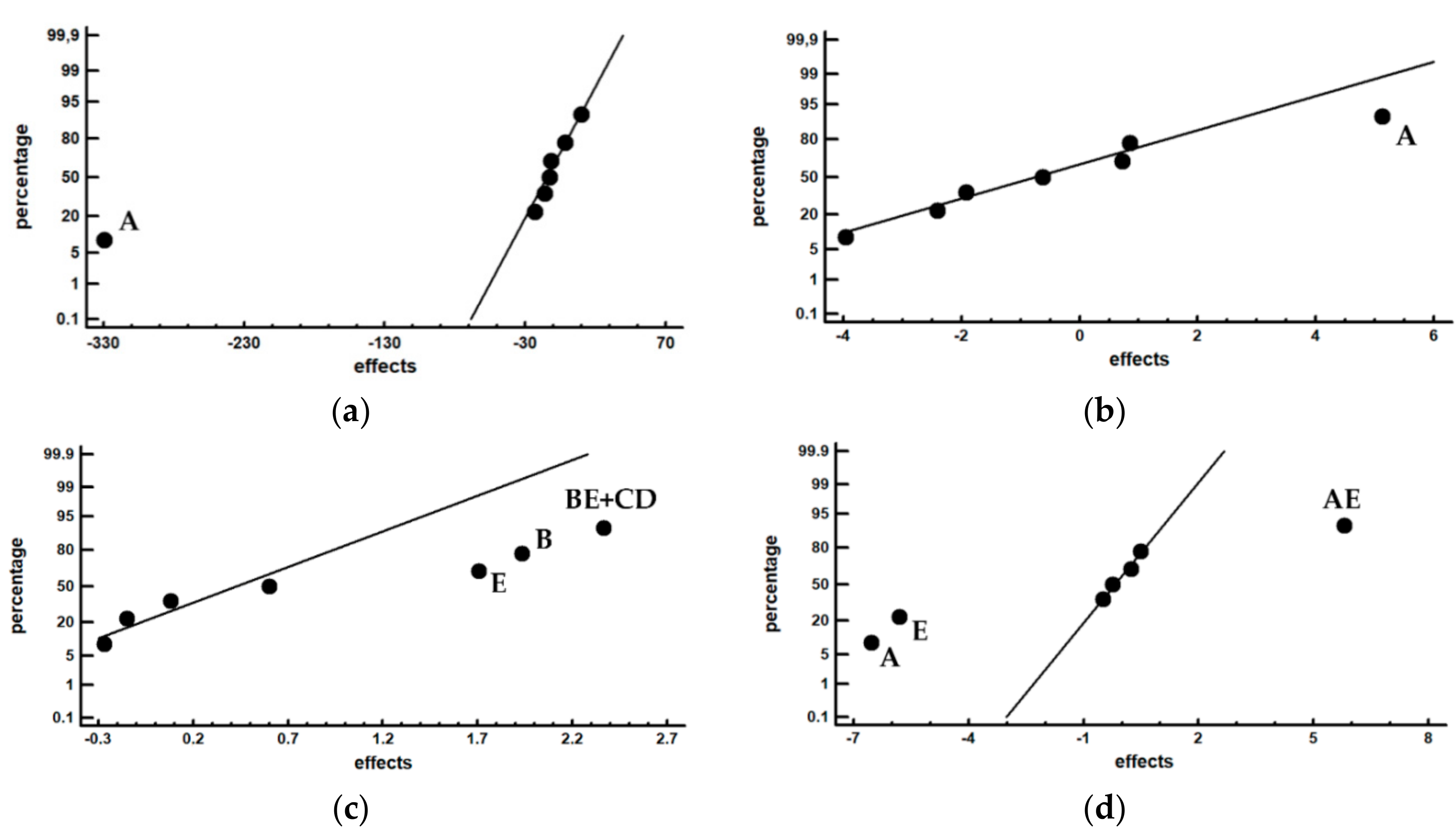
| (a) Hardness | |||||
| Experiment | HV100 | Effect | Effect | ||
| 1 | 836 | 673 | Average | ||
| 2 | 500 | −329.5 | A+BC+CE | ||
| 3 | 825 | −11.5 | B+AD+CF | ||
| 4 | 534 | −1.5 | C+AE+BF | ||
| 5 | 861 | 10 | D+AB+EF | ||
| 6 | 518 | −16 | E+AC+DF | ||
| 7 | 829 | −23 | F+BC+DE | ||
| 8 | 481 | −12.5 | AF+BE+CD | ||
| (b) Carbides | |||||
| Experiment | Cr7C3 | VC | Effect | ||
| (wt.%) | Effect | (wt.%) | Effect | ||
| 1 | 8.00 | 5.94 | 15.10 | 16.08 | Average |
| 2 | 10.62 | 5.12 | 15.24 | 0.08 | A+BC+CE |
| 3 | 1.40 | −3.96 | 18.95 | 1.93 | B+AD+CF |
| 4 | 7.58 | −1.92 | 15.56 | −0.27 | C+AE+BF |
| 5 | 2.10 | −0.62 | 15.64 | 0.60 | D+AB+EF |
| 6 | 10.97 | 0.72 | 14.46 | 1.70 | E+AC+DF |
| 7 | 2.01 | 0.85 | 14.46 | −0.15 | F+BC+DE |
| 8 | 4.84 | −2.4 | 19.21 | 2.36 | AF+BE+CD |
| (c) Retained Austenite | |||||
| Experiment | Austenite | Effect | |||
| (wt.%) | Effect | ||||
| 1 | - | 3.26 | Average | ||
| 2 | - | −6.52 | A+BC+CE | ||
| 3 | 1.46 | 0.24 | B+AD+CF | ||
| 4 | - | 5.79 | C+AE+BF | ||
| 5 | 12.56 | −0.24 | D+AB+EF | ||
| 6 | - | −5.79 | E+AC+DF | ||
| 7 | 12.08 | −0.48 | F+BC+DE | ||
| 8 | - | 0.48 | AF+BE+CD | ||
| AE | −1 | +1 |
|---|---|---|
| −1 | 12.3 | 0.7 |
| +1 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Pociño, A.; Alvarez-Antolin, F.; Asensio-Lozano, J. Optimization of Quenching and Tempering Parameters for the Precipitation of M7C3 and MC Secondary Carbides and the Removal of the Austenite Retained in Vanadis 10 Tool Steel. Metals 2019, 9, 627. https://doi.org/10.3390/met9060627
Gonzalez-Pociño A, Alvarez-Antolin F, Asensio-Lozano J. Optimization of Quenching and Tempering Parameters for the Precipitation of M7C3 and MC Secondary Carbides and the Removal of the Austenite Retained in Vanadis 10 Tool Steel. Metals. 2019; 9(6):627. https://doi.org/10.3390/met9060627
Chicago/Turabian StyleGonzalez-Pociño, Alejandro, Florentino Alvarez-Antolin, and Juan Asensio-Lozano. 2019. "Optimization of Quenching and Tempering Parameters for the Precipitation of M7C3 and MC Secondary Carbides and the Removal of the Austenite Retained in Vanadis 10 Tool Steel" Metals 9, no. 6: 627. https://doi.org/10.3390/met9060627





