Re-Melting Nb–Si-Based Ultrahigh-Temperature Alloys in Ceramic Mold Shells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Master Alloy Preparation
2.2. Mold Shells Preparation
2.3. Re-Melting Operation
2.4. Inspection of the Samples
3. Results
3.1. Microstructure and Phase Composition of the Master Alloy
3.2. Macroscopic Features of the Samples after Re-Melting
3.3. Microstructure and Composition of the Re-Melted Alloy
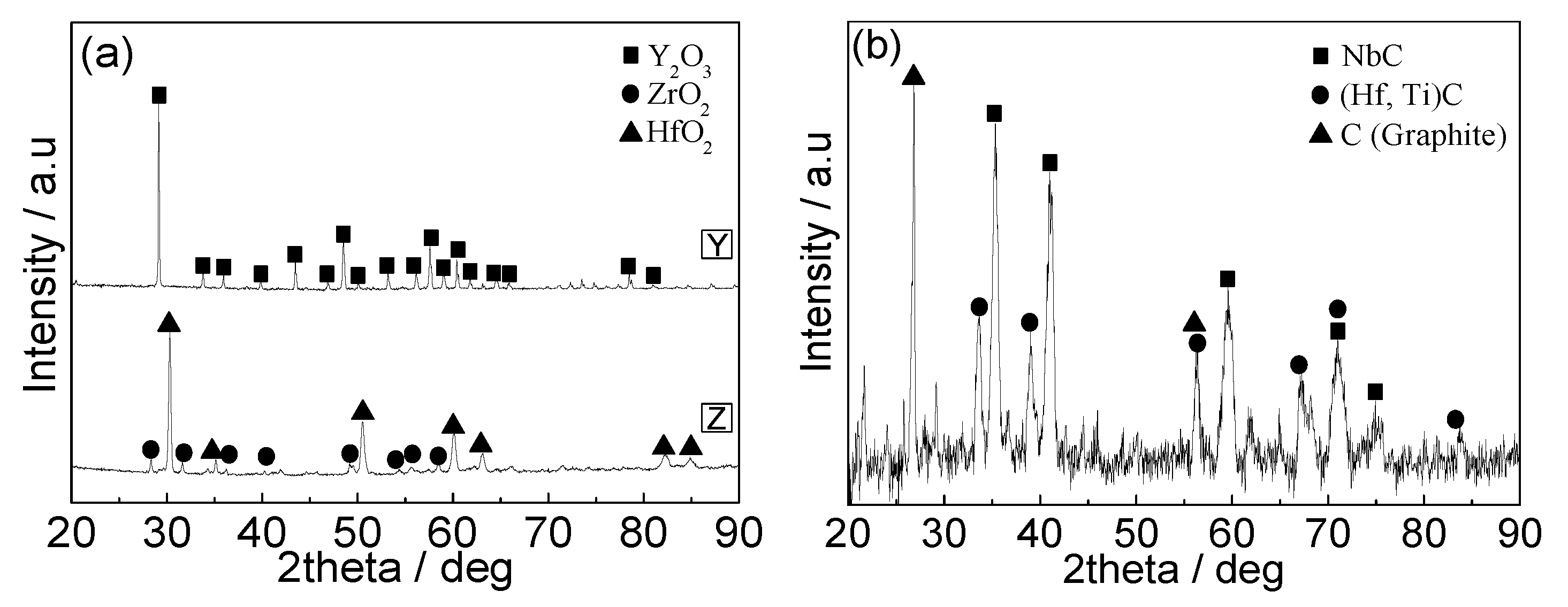
3.4. Interfaces between the Alloy and the Ceramic Mold Shells after Re-Melting
4. Discussion
4.1. Formation of a Carbides Layer on the Surface of the Alloy Re-Melted in the Furnace with the Graphite Heating Element
4.2. Formation of the Microstructure of the Alloy Re-Melted in the Furnace with the Graphite Heating Element
4.3. Interactions between the Mold Shells and the Alloy Re-Melted in the Furnace with the Graphite Heating Element
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bewlay, B.P.; Jackson, M.R.; Zhao, J.C.; Subramanian, P.R. A review of very-high-temperature Nb-silicide-based composites. Metall. Mater. Trans. 2003, 34, 2043–2052. [Google Scholar] [CrossRef]
- Zhao, J.C.; Westbrook, J.H. Ultrahigh-temperature materials for jet engines. MRS Bull. 2003, 28, 622–630. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Jackson, M.R.; Zhao, J.C.; Subramanian, P.R.; Mendiratta, M.G.; Lewandowski, J.J. Ultrahigh-temperature Nb-Silicide-based composites. MRS Bull. 2003, 28, 646–653. [Google Scholar] [CrossRef]
- Fei, T.; Yu, Y.X.; Zhou, C.G.; Sha, J.B. The deformation and fracture modes of fine and coarsened Nbss phase in a Nb-20Si-24Ti-2Al-2Cr alloy with a Nbss/Nb5Si3 microstructure. Mater. Des. 2017, 116, 92–98. [Google Scholar] [CrossRef]
- Shi, S.X.; Zhu, L.G.; Zhang, H.; Sun, Z.M. Toughening of α-Nb5Si3 by Ti. J. Alloy Compd. 2016, 689, 296–301. [Google Scholar] [CrossRef]
- Guan, K.; Jia, L.N.; Kong, B.; Yuan, S.N.; Zhang, H. Study of the fracture mechanism of Nbss/Nb5Si3 in situ composite: Based on a mechanical characterization of interfacial strength. Mat. Sci. Eng. A-Struct. 2016, 663, 98–107. [Google Scholar] [CrossRef]
- Cockeram, B.; Saqib, M.; Srinivasan, R.; Weiss, I. Role of Nb3Si in high tempereture deformation of a cast Nb-10%Si in-situ composite. Scri. Mater. 1992, 26, 749–754. [Google Scholar] [CrossRef]
- Sekido, N.; Miura, S.; Mitarai, Y.Y.; Kimura, Y.; Mishima, Y. Dislocation character and operative slip systems in α-Nb5Si3 tested at 1673 K. Intermetallics 2010, 18, 841–845. [Google Scholar] [CrossRef]
- Cheng, X.; Yuan, C.; Green, N.R.; Withey, P.A. Sintering mechanisms of Yttria with different additives. Ceram. Int. 2013, 39, 4791–4799. [Google Scholar] [CrossRef]
- Cheng, X.; Yuan, C.; Shevchenko, D.; Withey, P. The influence of mold pre-heat temperature and casting size on the interaction between a Ti-46Al-8Nb-1B alloy and the mold comprising an Al2O3 face coat. Mater. Chem. Phys. 2014, 146, 295–302. [Google Scholar] [CrossRef]
- Guo, H.S.; Guo, X.P. Microstructure evolution and room temperature fracture toughness of an integrally directionally solidified Nb–Ti–Si based ultrahigh temperature alloy. Scri. Mater. 2011, 64, 637–640. [Google Scholar] [CrossRef]
- Ma, L.M.; Yuan, S.; Cui, R.; Tang, X.; Li, Y.; Gao, M.; Zhang, H. Interactions between Nb-silicide based alloy and yttria mold during directional solidification. Int. J. Refract. Met. Hard Meter. 2012, 30, 96–101. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.P. Improvement on sintering property of face coat of yttria mold shells for investment casting. Ceram. Int. 2019, 45, 5297–5305. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X.P. Microstructure, mechanical properties and oxidation resistance of Nb suicide based ultrahigh temperature alloys with Hf addition. Mat. Sci. Eng. A-Struct. 2015, 645, 88–98. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.P.; Qiao, Y.Q. Interactions between Nb-Si based ultrahigh temperature alloy and yttria matrix mold shells. Mater. Des. 2017, 116, 461–471. [Google Scholar] [CrossRef]
- Smith, J.F.; Carlson, O.N.; Deavillez, R.R. The niobium-carbon system. J. Nucl. Mater. 1987, 148, 1–16. [Google Scholar] [CrossRef]
- Gusev, A.I. Phase diagrams of the pseudobinary TiC-NbC, TiC-TaC, ZrC-NbC, ZrC-TaC, and HfC-TaC carbide systems. Russ. J. Phys. Chem. 1985, 59, 336–340. [Google Scholar]
- Okamoto, H. The C-Hf(carbon-hafnium) system. Bull. Alloy Phase Diagrams 1990, 11, 396–403. [Google Scholar] [CrossRef]
- Kohler, F. Estimation of the thermodynamic data for a ternary system from the corresponding binary system. Monatsh. Chem. 1960, 91, 738–740. [Google Scholar] [CrossRef]
- Miedema, A.R.; Châtel, P.F.; Boer, F.R. Cohesion in alloys—Fundamentals of a semi-empirical model. Physica. B 1980, 100, 1–28. [Google Scholar] [CrossRef]
- Mizutani, N.; Tajima, Y.; Kato, M. Phase relations in the system Y2O3-TiO2. J. Am. Ceram. Soc. 1976, 59, 168. [Google Scholar] [CrossRef]
- Mah, T.I.; Petry, M.D. Eutectic composition in the pseudobinary of Y4Al2O9 and Y2O3. J. Am. Ceram. Soc. 1992, 75, 2006–2009. [Google Scholar] [CrossRef]
- Lakiza, S.M.; Lopato, L.M. Stable and metastable phase relations in the system alumina-zirconia-yttria. J. Am. Ceram. Soc. 1997, 80, 893–902. [Google Scholar] [CrossRef]











| Zones | Compositions/% | |||||||
|---|---|---|---|---|---|---|---|---|
| Nb | Ti | Si | Hf | Cr | Al | O | C | |
| 1 | 28.87 | 11.96 | 9.66 | 1.53 | 2.51 | 1.70 | 14.17 | 29.60 |
| Re-normalization | 51.48 | 21.17 | 17.28 | 2.63 | 4.41 | 3.02 | - | - |
| 2 | 25.10 | 9.65 | 6.55 | 0.78 | 2.06 | 1.51 | 14.38 | 39.96 |
| Re-normalization | 54.98 | 21.14 | 14.35 | 1.71 | 4.51 | 3.31 | - | - |
| 3 | 21.84 | 8.99 | 5.25 | 0.29 | 2.34 | 0.91 | 7.13 | 53.25 |
| Re-normalization | 55.12 | 22.70 | 13.25 | 0.73 | 5.91 | 2.29 | - | - |
| 4 | 23.37 | 7.94 | 6.50 | 0.33 | 2.78 | 1.63 | 7.09 | 50.36 |
| Re-normalization | 54.92 | 18.66 | 15.28 | 0.78 | 6.53 | 3.83 | - | - |
| 5 | 22.20 | 9.33 | 4.88 | 0.47 | 2.25 | 1.12 | 8.73 | 51.02 |
| Re-normalization | 55.16 | 23.18 | 12.12 | 1.17 | 5.59 | 2.78 | - | - |
| 6 | 25.27 | 8.24 | 6.95 | 0.39 | 2.71 | 1.47 | 7.47 | 47.51 |
| Re-normalization | 56.12 | 18.30 | 15.43 | 0.87 | 6.02 | 3.26 | - | - |
| Mold Shells | Furnace Heating Element | Phase Composition | Position | Thickness/μm | Formation Mode |
|---|---|---|---|---|---|
| Y | Tungsten | HfO2 + Y2O3 | On the interface | 1.5 | Interface reaction |
| Z | Tungsten | HfO2 | On the interface | 58.1 | Interface reaction |
| Y | Graphite | Y2O3 + Y–Al and Y–Ti composite oxides | In Y mold shell adjacent to interface | - | Infiltration reaction |
| Z | Graphite | ZrO2 + Zr–Ti composite oxides | In Z mold shell adjacent to interface | - | Infiltration reaction |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Guo, X. Re-Melting Nb–Si-Based Ultrahigh-Temperature Alloys in Ceramic Mold Shells. Metals 2019, 9, 721. https://doi.org/10.3390/met9070721
Wang Y, Guo X. Re-Melting Nb–Si-Based Ultrahigh-Temperature Alloys in Ceramic Mold Shells. Metals. 2019; 9(7):721. https://doi.org/10.3390/met9070721
Chicago/Turabian StyleWang, Yin, and Xiping Guo. 2019. "Re-Melting Nb–Si-Based Ultrahigh-Temperature Alloys in Ceramic Mold Shells" Metals 9, no. 7: 721. https://doi.org/10.3390/met9070721
APA StyleWang, Y., & Guo, X. (2019). Re-Melting Nb–Si-Based Ultrahigh-Temperature Alloys in Ceramic Mold Shells. Metals, 9(7), 721. https://doi.org/10.3390/met9070721





