Abstract
In view of the fact that Ti–bearing blast furnace primary slag has been explored limitedly and its viscosity–structural property is not fully understood, the phase compositions, viscosity and structure of CaO–8%MgO–Al2O3–SiO2–TiO2–5%FeO slag are investigated by X-ray diffractometer, rotating cylinder method, Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy respectively, considering the effect of Al2O3 and TiO2. The critical temperature that is defined as the temperature below which the viscosity of slag increases quickly, could be explained by the relative amount of perovskite to melilite from phase compositions analysis. The slag viscosity first increases with increasing Al2O3 content from 10 to 15 mass%, and then decreases with the further increase of Al2O3 to 18 mass%. Increasing TiO2 content continuously lowers the viscosity. FTIR and Raman spectra results show that increasing Al2O3 or decreasing TiO2 content leads to complex Si–O and Ti–O networks structure, corresponding to the slag viscosity variation. The effect of weak linkages of Si–O–Al is more dominant when Al2O3exceeds 15 mass%, which results in the decrease of viscosity.
1. Introduction
The blast furnace (BF) smelting of Ti–bearing iron ore occupies an appreciable proportion in hot metal production because of abundant titanomagnetite resources in China [1,2]. In addition, ironmakers use the burden containing TiO2 to protect the hearth refractory due to the formation of titanium carbide and nitride for prolonging the furnace campaign life [3,4,5]. The softening–melting of Ti–bearing materials in BF generates the flowable primary slag whose formation could be also regarded as a process of interaction and reorganization of various phase components [6]. The viscosity of Ti–bearing primary slag could influence many aspects, such as permeability of cohesive zone, mass and heat transfer through metallurgical kinetics and metal–slag separation. The Ti–bearing primary slag assigned to CaO–MgO–Al2O3–SiO2–TiO2–FeO system with high basicity and a significant amount of FeO has different viscous behaviour to the slag in hearth. Recently, the usage of low-cost and high-alumina iron ore is gradually increased because of continuous consumption of the ores rich in iron [7,8]. Al2O3 content in the slag may reach a certain level [9] and the relative amount of TiO2 changes. Therefore, it is important and necessary to understand the effects of Al2O3 and TiO2 on phase compositions and viscosities of Ti–bearing primary slag fundamentally.
The viscosity of CaO–MgO–Al2O3–SiO2–TiO2 system has been investigated by many researchers [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24].There has been a consensus among the studies [11,12,13,14,15,16,17,18] that TiO2was a reducing viscosity agent in the quinary slag with fixed MgO, Al2O3 and basicity. For the effect of binary basicity, most experts reported that viscosity decreased with increasing basicity [17,18,19,20,21,22]. There was also a different view that higher basicity led to higher slag viscosity in the high alumina and medium titania BF slag [23]. Considering the influences of MgO and Al2O3, it was believed that the addition of MgO content can decrease viscosity [13,20,21]. The viscosity increased with increasing Al2O3 content in the studies [21,22,23]. Zhen et al concluded that viscosity of Ti–bearing slag with constant CaO, SiO2 and MgO content increased as Al2O3/TiO2 ratio increased [24]. In the case of CaO–MgO–Al2O3–SiO2–TiO2–FeO slag, work done by Jiao et al showed that viscosity decreased with increasing TiO2 or FeO content [25,26]. Hence, the major studies on Ti–bearing slag are relevant to final slag in BF hearth. Although the practical BF process relates to Ti–bearing primary slag, there is comparatively little data available on its viscosities, especially for the influences of Al2O3 and TiO2.Therefore, the investigation of primary slag is required to enrich and improve the theory of Ti–bearing slag smelting.
In this work, CaO–MgO–Al2O3–SiO2–TiO2–FeO system slag with 8 mass% MgO, 5 mass% FeO and CaO/SiO2 of 1.3, which is related to Ti–bearing primary slag, is synthesized by melting chemical reagents in a Mo crucible at 1823 K with more than 3 h under Ar flow using the viscosity measurement furnace. The effects of Al2O3 and TiO2on viscosity and phase compositions are investigated via rotating method and X-ray diffractometer (XRD), respectively. Furthermore, the slag structure is explored by Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy for better understanding viscous behaviour.
2. Material and Methods
2.1. Preparation of SlagSamples
Several analytically pure chemical reagents, which include TiO2, MgO, Al2O3, SiO2, CaCO3, and FeC2O4·2H2O, are used for preparing the primary slag sample. CaCO3 and FeC2O4·2H2O are consumed for producing CaO and FeO, respectively. All of the materials are dried and weighed according to the designed compositions shown in Table 1. The mass ratio of CaO to SiO2 is fixed at 1.30. In each component of No.1~No.4, contents of FeO, MgO and TiO2 keep constant while Al2O3 content increases from 12 to 18 mass%. The other variable is TiO2 content, changing from 2 to 14 mass%. Apart from FeC2O4·2H2O, other oxides are well mixed in a zirconia ball mill, then pelletized for later use.

Table 1.
Chemical compositions of designed Ti–bearing primary slags (mass%).
2.2. Viscosity Measurement
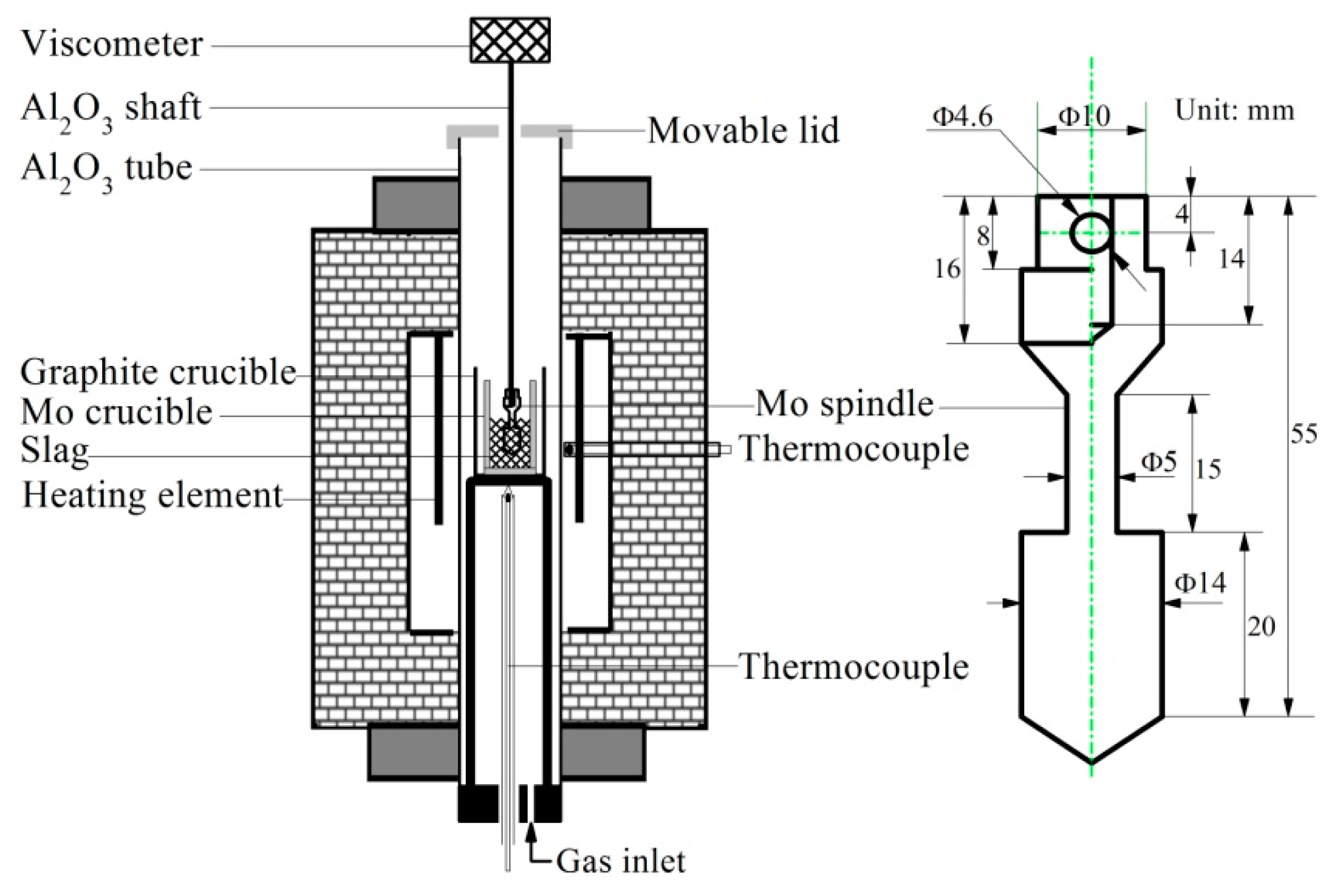
The viscosity measurement is conducted by a melt physical property comprehensive testing equipment (RTW–10, Northeastern University, Shenyang, China) shown in Figure 1. Mo spindle and crucible are selected touching the slag. Mo crucible is installed in a high–purity graphite crucible for better protecting the slag. The thermocouple near the crucible is used for monitoring the slag sample temperature which should follow the furnace temperature. The programmable viscometer is designed for viscosity measurement at the given shear rates and it is calibrated with castor oil of known viscosities at different temperatures before experiments. The known viscosities of castor oil are 0.986, 0.651 and 0.451 Pa·s, corresponding to 20, 25 and 30 °C, respectively. During the calibration, the castor oil is filled in the Mo crucible with the same sizes for slag viscosity measurement until the depth is 40 mm.
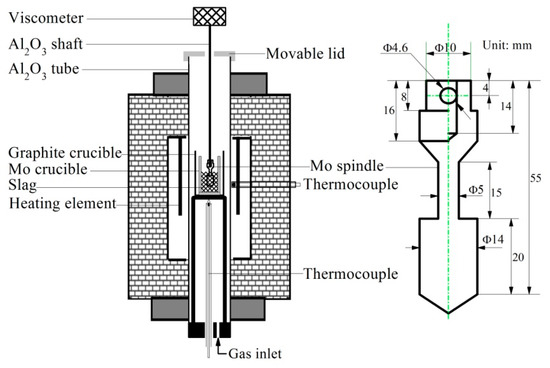
Figure 1.
Experimental apparatus for viscosity measurement.
FeC2O4·2H2O is first put into the Mo crucible (40 mm inner diameter and 80 mm depth). As temperature rises, FeC2O4·2H2O graduallydecomposes, softens and melts. Meanwhile, the pelletized ball mentioned in Section 2.1 is carefully added. The furnace temperature is heated to 1823 K, held for more than 3 hours, and then drops to 1773 K. At the moment, the rotating spindle is soaked in molten slag and stirs the slag for 30 min. About 140 g slag (~40 mm depth) is eventually obtained for viscosity measurement on cooling style from 1773 K at every 25 K interval. The equilibration time is 25 min at each temperature. After viscosity measurement, the primary slag is reheated to 1773 K and kept for 60 min, and then poured into water, dried, and crushed by a disk mill for chemical compositions analysis and next phases and structure investigation. In the whole process, a constant high purity Ar gas flow (0.5 L/min) is introduced for protection. The FeO content in every slag sample analyzed by the titration method is given in Table 1, showing little change in FeO content and good protection during experiment.
2.3. Phase Compositions and Structure Investigation
Two parts (each about 5 g) of the post-experimental slag powder are re-melted at 1773 K and kept for 60 min under Ar gas flow using small Mo crucibles formed by punching (29 mm inner diameter, 4.5 mm depth and 0.1 mm thickness). One is slowly cooled to the ambient temperature with 10 K/min in the furnace and examined by XRD (SmartLab SE, Rigaku, Tokyo, Japan) for phases investigation. The other is rapidly quenched in water for structure analysis using XRD, FTIR (Thermo Scientific Nicolet IS5, Nicolet, Madison, WI, USA) and Raman spectroscopy (XploRA PLUS, Horiba Scientific, Edison, NJ, USA). Potassium bromide (KBr) tablet method is adopted to attain FTIR transmitting spectra. The ratio of sample to KBr is 1:150. The spectrum for each slag sample is an average of 32 scans, which deducts the spectrum of blank KBr tablet. Raman spectroscopy is performed on samples by a laser confocal micro-Raman spectrometer, of which the excitation wavelength is 532 nm. The recorded spectral range is 100–2000 cm−1.
3. Results and Discussion
3.1. The Critical Temperatures
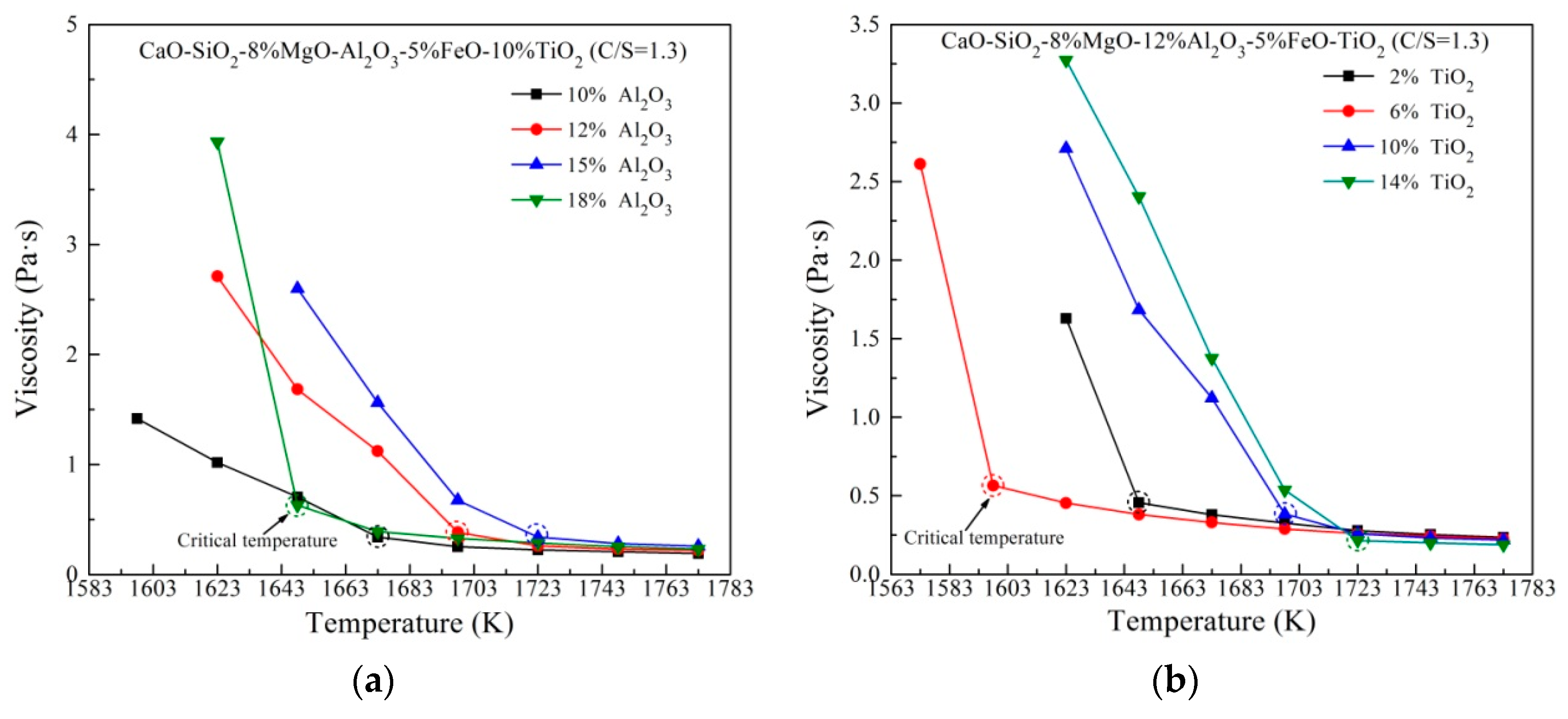
Figure 2 shows the viscosity variation of slag containing different Al2O3 and TiO2 content against temperature. As expected, viscosity decreases with increasing temperature. It is noteworthy that the additions of Al2O3 and TiO2 result in various critical temperatures of slags. The critical temperature (CT) is defined as the temperature below which the slag viscosity increases quickly and marked as the dotted circle. A low critical temperature represents a wide thermostable operation region, which is advantage to actual production. The highest CT with changing Al2O3 content is observed at 15 mass% Al2O3, after which the CT drops dramatically with further rise of Al2O3. On the other hand, with increasingTiO2 content, the CT first decreases, and then increases.
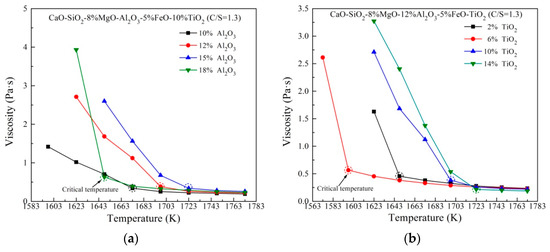
Figure 2.
Viscosity of slag containing different (a) Al2O3 content and (b) TiO2 content against temperature.
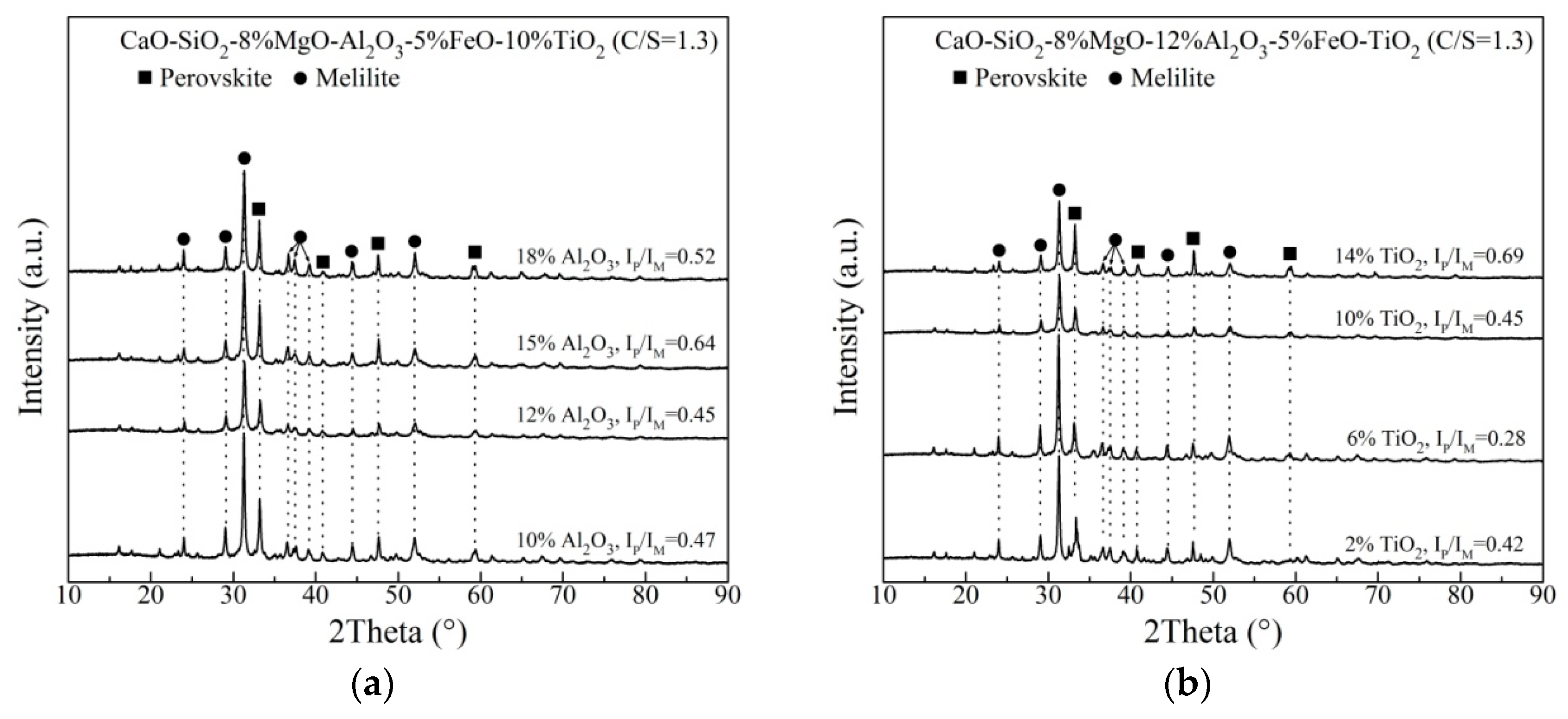
XRD analysis of the slowly cooled, slag samples with varying Al2O3 and TiO2 content are shown in Figure 3. The phases in the Ti–bearing primary slags are melilite and perovskite. The diffraction peak intensities of melilite and perovskite change with adding Al2O3 and TiO2. For the XRD pattern of each slag, the backgrounds is determined and subtracted through Highscore Plus Software (Version 3.0e, PANalytical, Almelo, The Netherlands). Subsequently, the strongest peaks of perovskite and melilite are found and their intensities are recorded as IP and IM, respectively. IP/IM can be calculated and listed in Figure 3. IP/IM is defined as the ratio of the strongest diffraction intensity of perovskite and melilite, indicating the relative amount of perovskite to melilite. From Figure 3a, as Al2O3 content increases, IP/IM first increases to the maximum value at 15 mass% Al2O3, and then decreases on the whole. Figure 3b shows that when TiO2 content increases, IP/IM first decreases and then increases, exhibiting a minimum value at 6 mass% TiO2. The melting temperature of perovskite is 1970 °C. Comparatively, the melting temperature of melilite is much lower. It has been reported that the slag with more content of high–melting–point substance has the higher melting temperature and stronger crystallization capacity, which would result the higher CT [13,19,27,28,29,30]. Therefore, the variation of CT could be explained by the investigation of phase compositions.
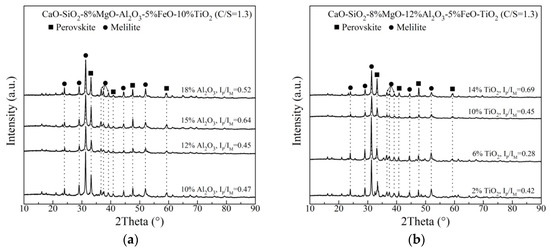
Figure 3.
Analysis of the slowly cooled slag samples with (a) varying Al2O3 content and (b) varying TiO2 content.
3.2. Effects of Al2O3 and TiO2 on Viscosity
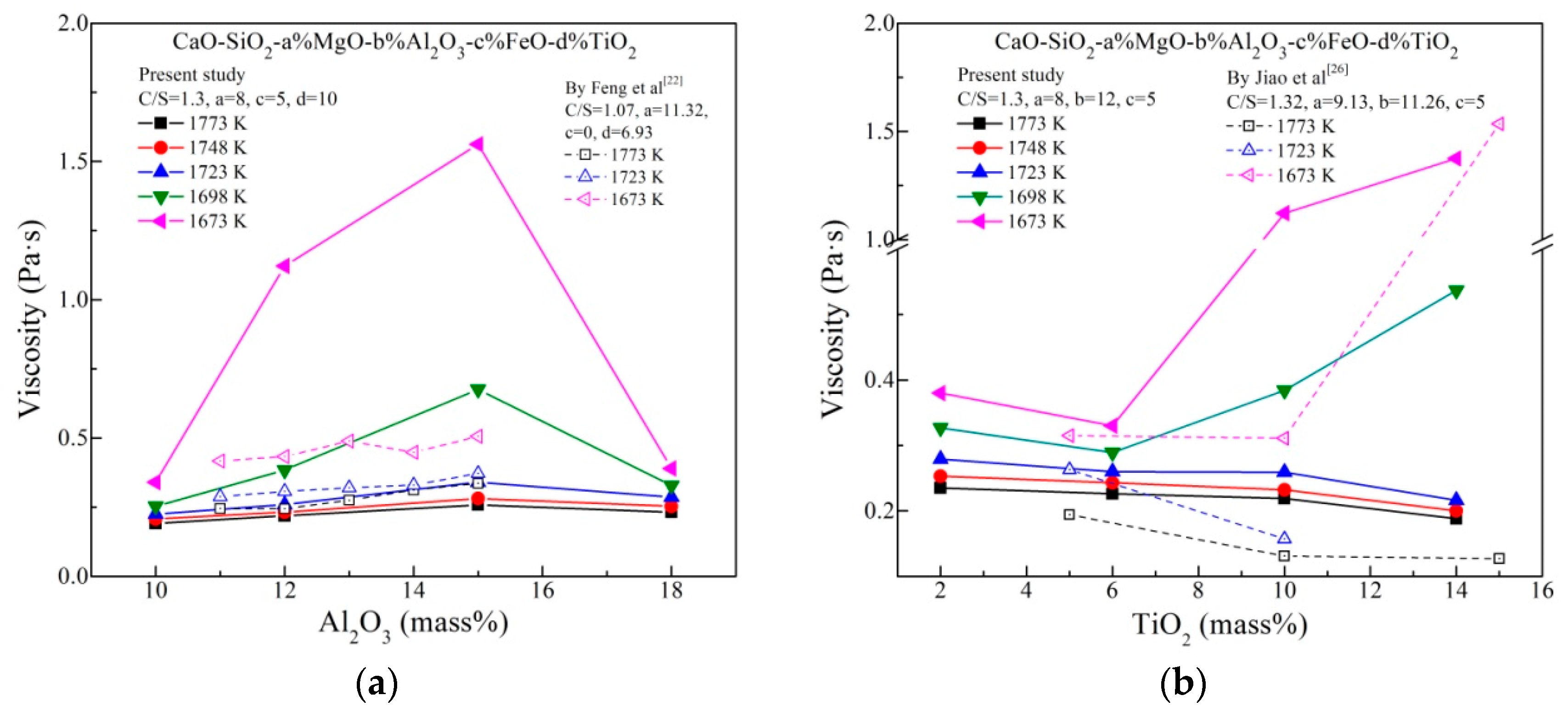
Figure 4 shows the effects of Al2O3 and TiO2 content on viscosity at various temperatures. As depicted in Figure 4a, the slag viscosity initially increases, reaches the maximum at 15 mass% Al2O3 and again drops afterwards with increasing Al2O3 content. Obviously, the effect of Al2O3 is more slight at higher temperature. This is because the relative wide spacing and weak interionic attraction between cations and anions. In such a slag system with much basic oxides, there are sufficient cations for charge compensation [27], which is beneficial to the existence of [AlO4]5−–tetrahedrons. [AlO4]5− is believed to be a network former increasing the complexity of networks structure. Therefore, the increase of viscosity could be attributed to the acid characteristics of Al2O3, similar to the work by Feng et al that showed the Ti–bearing slag viscosity increased with increasing the Al2O3 content from 11 to 15% [22]. However, the Al2O3 content more than 15 mass% results to a decrease in the viscosity. Al2O3 incorporates into the silicate slag and forms Si–O–Al linkages. The Si–O–Al linkages are weaker than Si–O–Si bonds, which means that the migration of structural units will be easy if excessiveAl2O3 is added. This effect may be more dominant to some degree in the Al2O3 content exceeding 15 mass% within the slags, resulting the viscosity to be decreased. It should be further confirmed by spectra analysis.
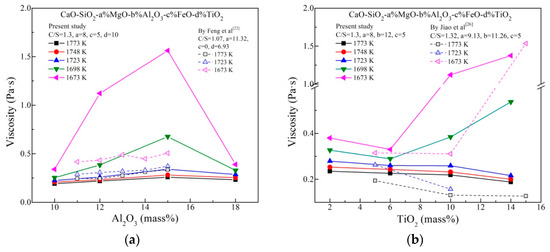
Figure 4.
Effects of (a) Al2O3 content and (b) TiO2 content on viscosity at various temperatures.
From Figure 4b, when the temperature is lower than 1698 K, TiO2 content exceeding 6 mass% will increase the viscosity. This is because of the formation of solid perovskite speculated from phase compositions. Above 1698 K, the viscosity decreases by adding TiO2, which supports that TiO2 is a reducing viscosity agent. It is proposed that TiO2 may be a basic oxide and could break the slag networks structure in some reports [11,13,15,26]. The decrease of viscosity with increasing TiO2 could be attributed to the simplification of networks structure, which will be certified by structure analysis.
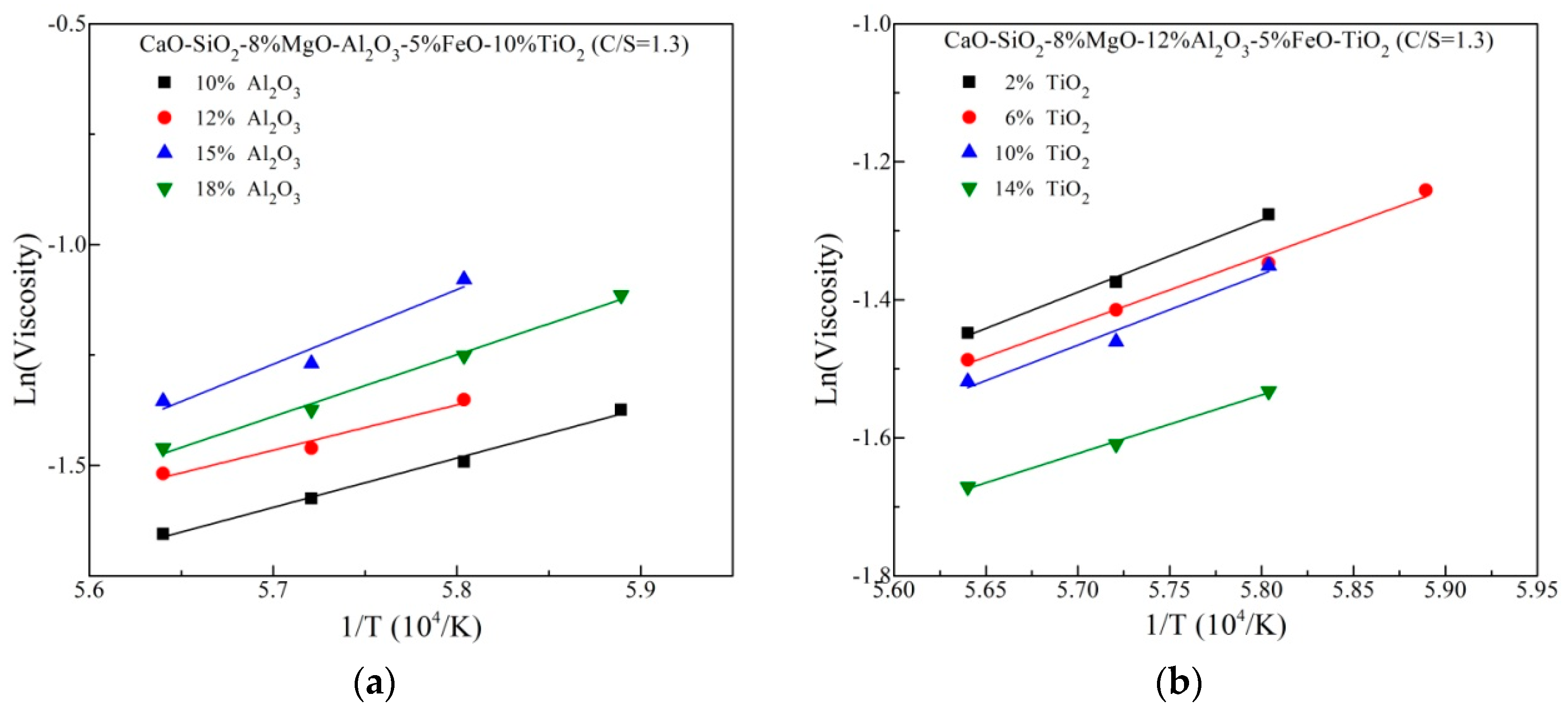
The viscosity variation with compositions at high temperature could be interpreted via the viscosity activation energy (Eη) simply, which represents the energy barrier for viscous flow running and indirectly suggests a change in the structure. According to the well-know Arrhenius law, the natural logarithm of viscosity against reciprocal of temperature is described in Figure 5. Eη is calculated from the linear slope, listed in Table 2. It is observed that the variation of Eη is an increasing trend but the maximum value of Eη is achieved at 15 mass% Al2O3. This is basically in agreement with the above viscosity trend. For increasing TiO2 content, Eη exhibits a decreasing trend overall. This suggests more simple structural units with low flowing energy barrier generate in the slags, contributing the decreased viscosity.
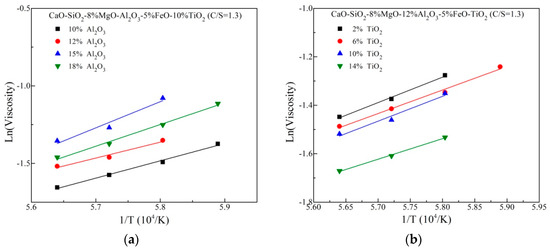
Figure 5.
Natural logarithm of viscosity against reciprocal of temperature (a) differentAl2O3 content (b) different TiO2 content.

Table 2.
Viscosity activation energy of slags.
3.3. Structure Analysis by FTIR and Raman
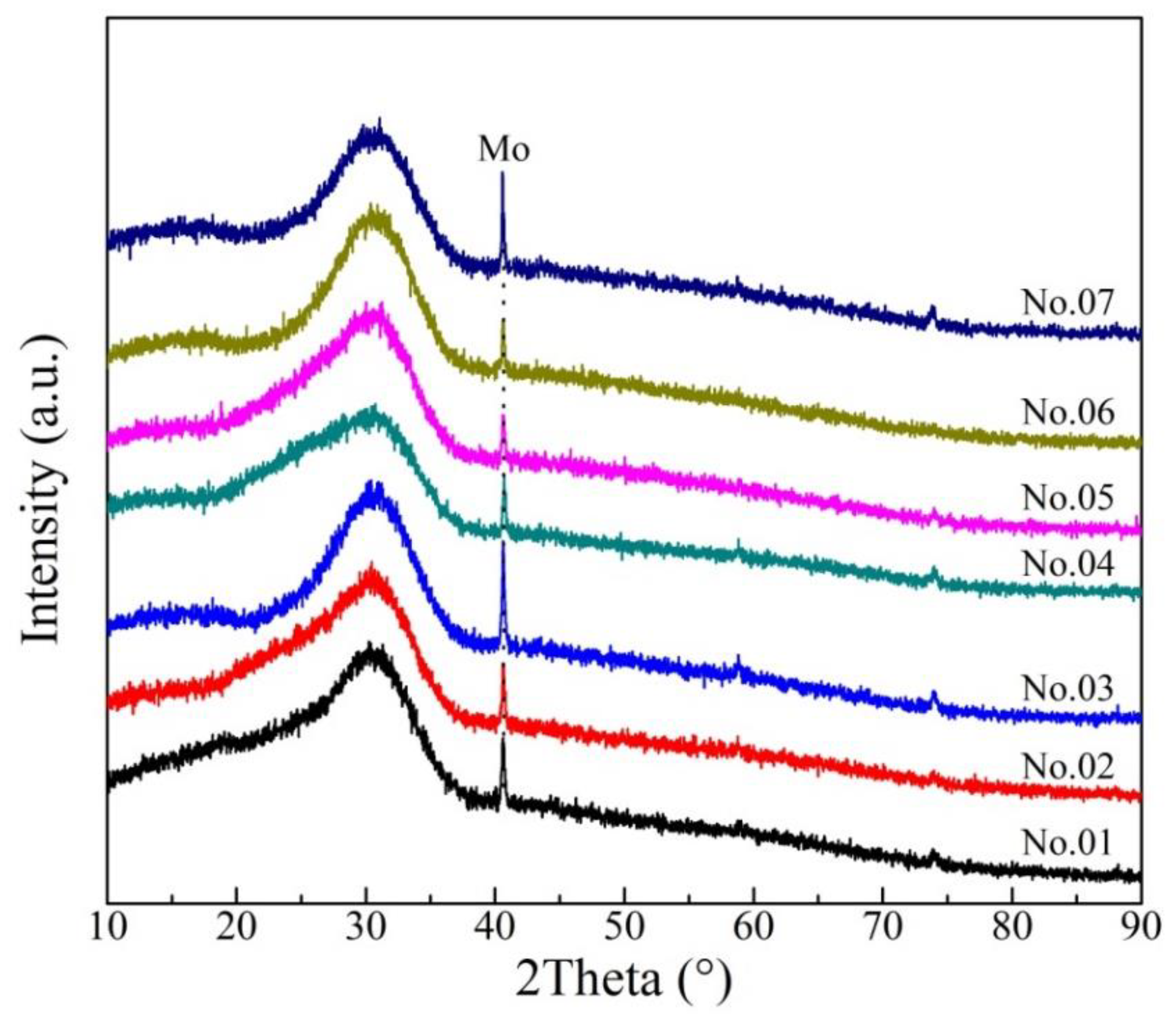
The XRD results of rapidly quenched slags at 1773 K are shown in Figure 6, indicating that there is no characteristic peaks of minerals. The diffraction peak due to Mo is caused by incomplete separation between rapidly quenched slag and Mo crucible. It is proved that the samples are amorphous and homogeneous and maintain the high temperature state.
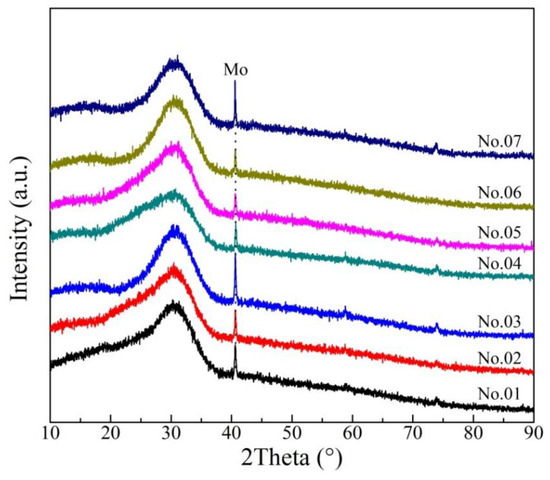
Figure 6.
Patterns of the rapidly quenched slag samples.
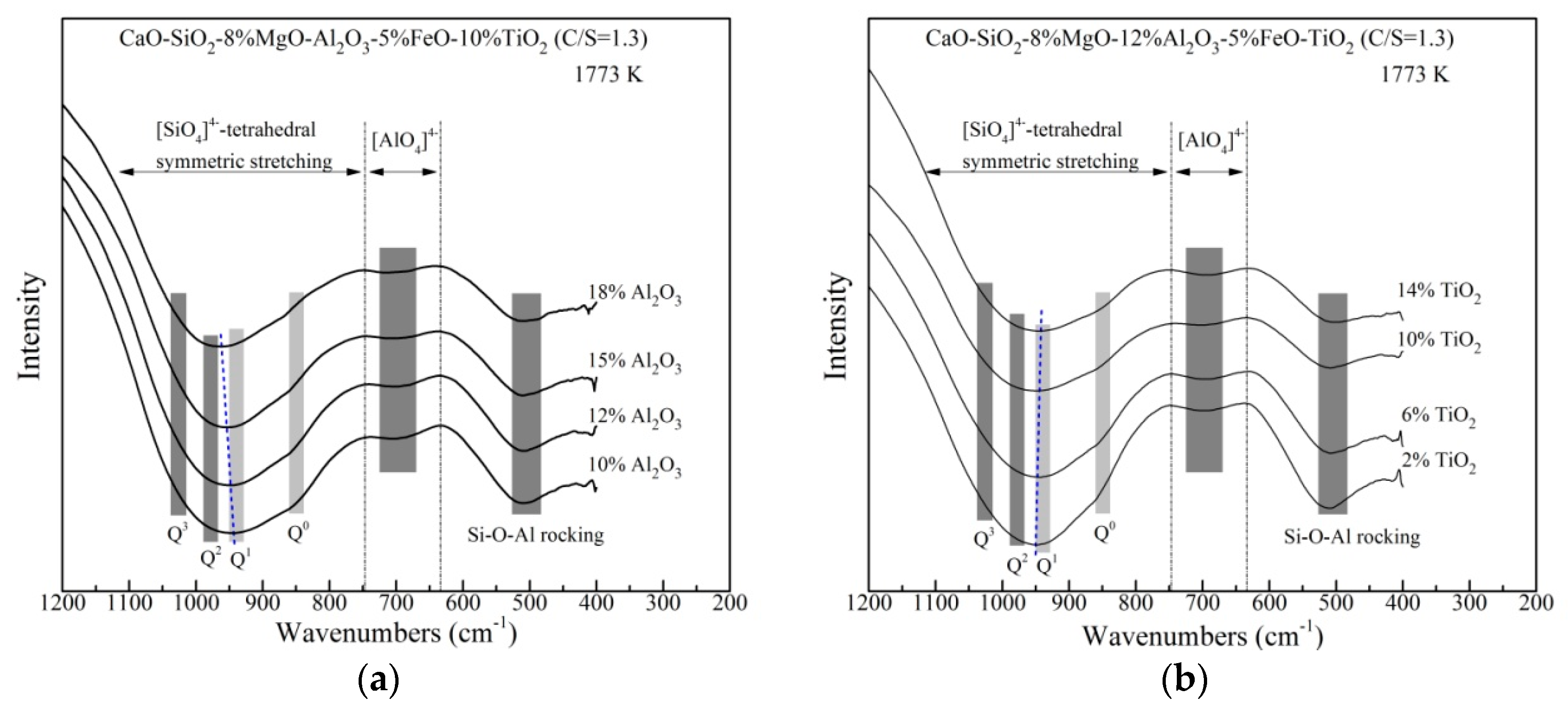
The rapidly quenched slags are performed on FTIR spectroscopy to analyze the structure changes with different Al2O3 and TiO2 content qualitatively. As shown in Figure 7, the bands of 1200–750 cm−1 belongs to the [SiO4]4−–tetrahedral symmetric stretching which includes four kinds of Si–O networks structural units. The bands at ~850, ~940, ~980 and ~1030 cm−1 are assigned to Q0, Q1, Q2 and Q3 (corresponding to non-bridging oxygen per silicon NBO = 4, 3, 2, 1), respectively [31]. In addition, the peaks at 730–630 cm−1, 570–520 cm−1 and ~500 cm−1 are assigned to the asymmetric stretching vibration of [AlO4]5−–tetrahedral (network former), [AlO6]9−–octahedra (network modifier) and the Si–O–Al rocking, respectively [26,32].
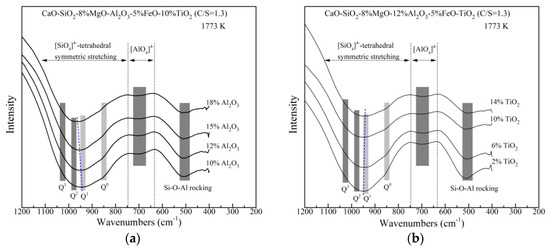
Figure 7.
Spectra of the rapidly quenched slag samples with various (a) Al2O3 content and (b) TiO2 content.
In Figure 7a, by increasing Al2O3 content, the center of the [SiO4]4−–tetrahedral bands shifts to higher wavenumbers from about 945 to 962 cm−1, resulted from the decrease of Q0 and Q1 and the increase of Q2. This indicates that higher Al2O3 contentis likely to polymerize Si–O networks. For Al–O networks, the trough of [AlO4]5−–tetrahedral has little change. No peaks of [AlO6]9−–octahedral are found. This means that Al2O3 is certified only to be a network former in the slags, playing an active role in increasing viscosity. The depth of the Si–O–Al rocking does not change with the addition of Al2O3 content up to 15 mass%, whereas it decreases with higher Al2O3 content. The decrease in the trough of Si–O–Al rocking suggests that the linkage between [SiO4]4− and [AlO4]5−–tetrahedrals becomes weaker, which can be the reason for decreasing viscosity in Figure 4a. As can be noted from Figure 7b, with increasing TiO2 content, the bands of the [SiO4]4−–tetrahedral symmetric stretching vibration becomes shallower and its center shifts to lower wavenumbers slightly. No significant variation of the trough of [AlO4]5−–tetrahedral is found. However, the depth of the Si–O–Al rocking trough decreases continuously. It is confirmed that TiO2 behaves as a basic oxide providing free oxygen ions in these slags. As TiO2 content increases, more O2− ions react with bridged oxygen and depolymerize the silicates, which results in the negative center shift of Si–O stretching bands and the weaker Si–O–Al linkages. This leads to decreasing viscosity as shown in Figure 4b. The FTIR analysis also seems to suggest that TiO2 prefers to modify the Si–O networks rather than Al–O networks, which is similar to the work done by Park [15]. Ti–O networks structure is difficult to be identified in FTIR spectra and it will be clarified by Raman analysis.
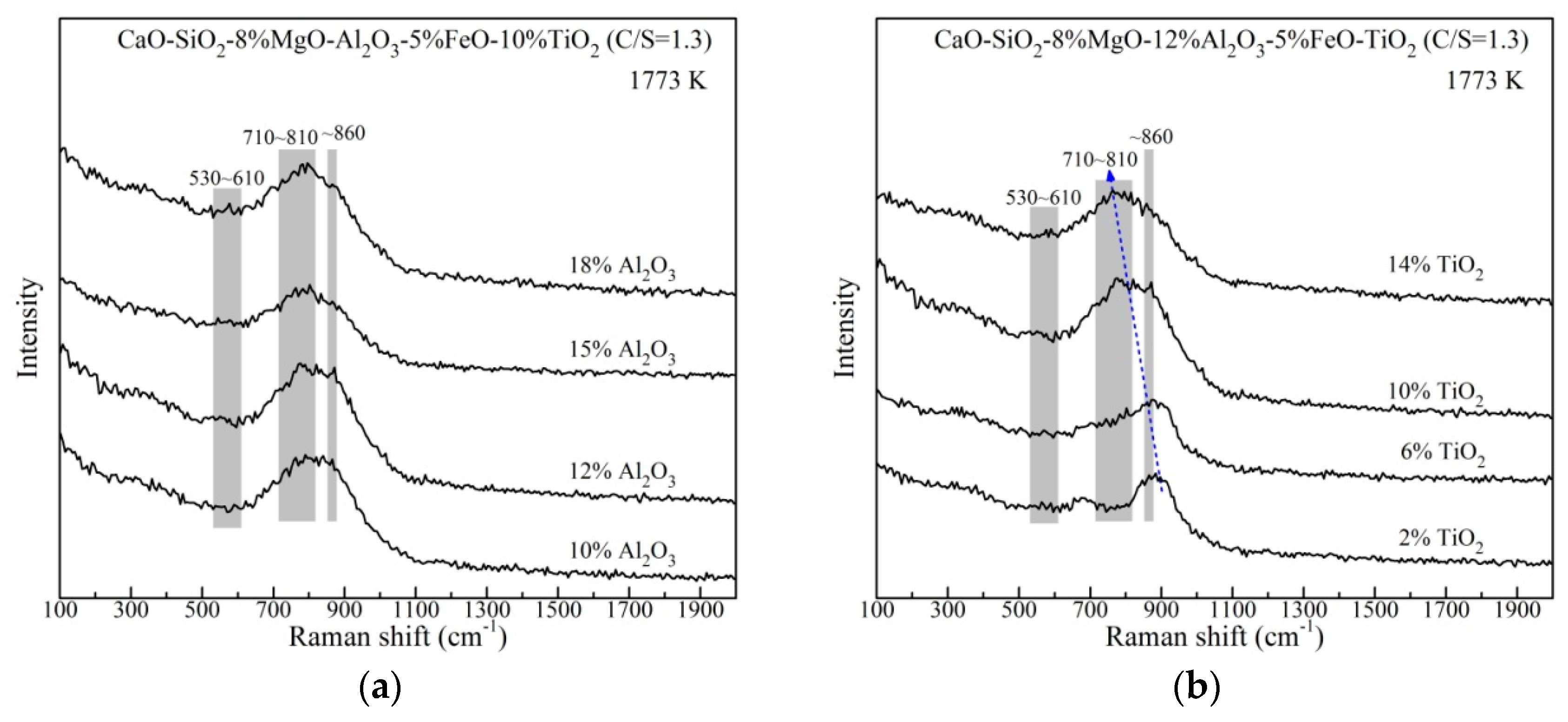
The slag structural units with changing Al2O3 and TiO2 content are further studied by Raman spectroscopy. The original Raman spectra of the rapidly quenched slags at 1773 K are shown in Figure 8. It is obviously discovered that the strong bands locate the frequency region of 600–1100 cm−1 in every spectra curve and change with compositions. The bands of 710~810 cm−1 become more intense relative to the bands at about 860 cm−1. This tendency with changing TiO2 is much more pronounced, which presents as the position of the highest peak shifting to lower wavenumbers. The bands at 790–830 cm−1 were associated with Ti–O stretching vibrations in TiO44− monomer which is a simple and small structural unit [19,33]. Additionally, the bands corresponding to O–(Ti, Si)–O deformation vibrations were proposed at 700–750 cm−1 [33,34], which are suggested to be complex structural units [19,24]. The Raman peaks of Al–O stretching vibrations were reported at 530–610 cm−1 [35,36]. It is found that the bands of 530–610 cm−1 change little with varying both Al2O3 and TiO2 content, which is consistent with the FTIR results. Hence, it is believed that the bands between 710 and 810 cm−1 relate to Ti–O networks, such as O–(Ti, Si)–O and TiO44−. The Si–O networks have been successfully studied on the basis of the Qn concept. As mentioned in FTIR analysis, Qn includes Q0, Q1, Q2 and Q3, which are corresponding to silicate forms of [SiO4]4−, [Si2O7]6−, [SiO3]2− and [Si2O5]2− with the structure of monomers, dimers, chains and sheets, respectively. According to the previous works [32,37,38,39], these silicate units pertain to the Raman peaks at 840~860 cm−1, 900~920 cm−1, 960~1000 cm−1 and 1050~1100 cm−1, respectively. Considering the characteristics of Raman curves of the slags, there are little Q3 units in the present slags.
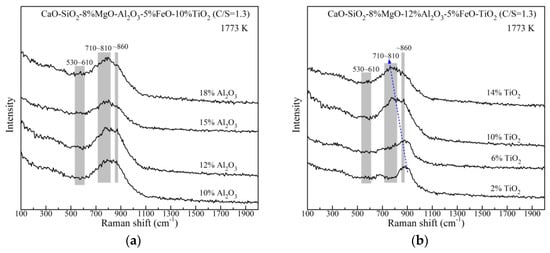
Figure 8.
Original Raman spectroscopy of the rapidly quenched slags with different (a) Al2O3 content and (b) TiO2 content.
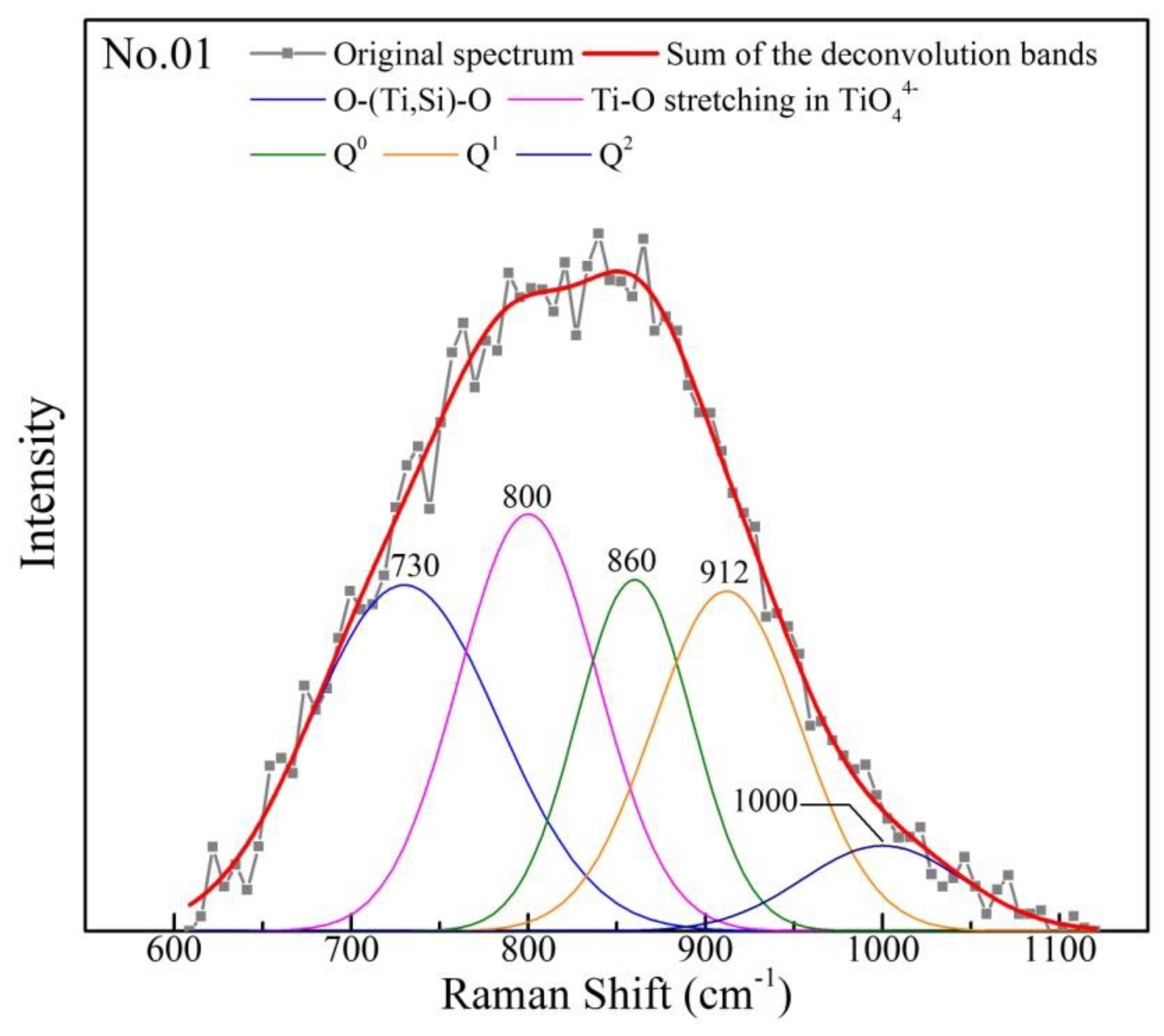
It is necessary to deconvolute the Raman spectra ranged from 600–1100 cm−1 for obtaining detailed information. First, the backgrounds of Raman spectra is determined and subtracted. Then, based on the proposed Raman peaks of each units, the Raman spectra is deconvoluted by the Gaussian–Deconvolution method with correction coefficient more than 0.99. In current study, ~730 cm−1, ~800 cm−1, ~860 cm−1, ~910 cm−1 and ~1000 cm−1 are assigned to O–(Ti, Si)–O deformation, stretching vibrations of TiO44−, Q0, Q1 and Q2, respectively. The typical deconvolution of the Raman spectra is shown in Figure 9. The area of Ti–O networks structural units and Qn can be obtained. The amount of Qn is calculated by Formula 1. Raman scattering coefficient (θn) has been summarized in several studies [40,41]. The average number of non-bridging oxygen per silicon (NBO/Si) is employed to represent the polymerization degree of Si–O networks and calculated by the mole fraction and the number of non-bridge oxygen of Qn. The calculation of NBO is expressed as Formula 2. Lower value of NBO/Si, higher polymerization degree of Si–O networks.
where Xn, An and θn is the mole fraction, Raman band area and Raman scattering coefficient of Qn (n = 0~3).
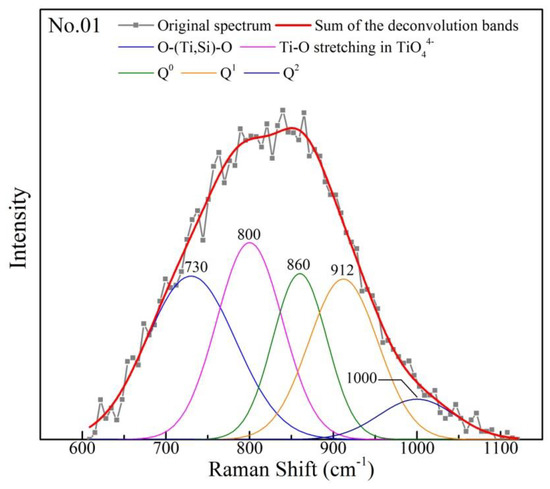
Figure 9.
Typical set of Raman spectra deconvolution.
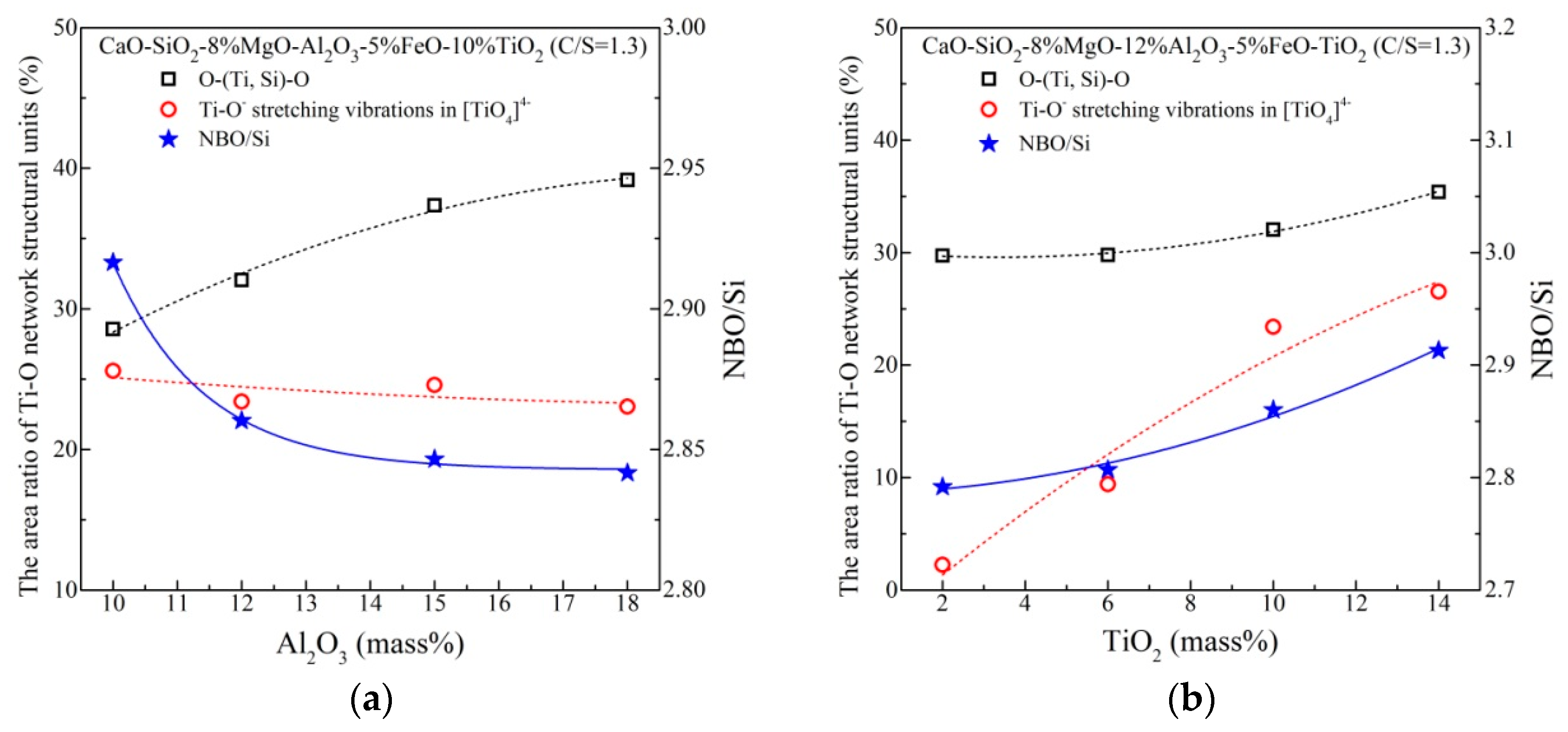
The abundance variations of structural units with increasing Al2O3 and TiO2 content are shown in Figure 10. It can be seen from Figure 10athat as Al2O3 content increases, the percentage of O–(Ti, Si)–O deformation vibrations increases while that of TiO44− stretch vibrations slightly decreases, which is an indication of more complex Ti–O networks. NBO is decreased continuously, suggesting polymerization of Si–O networks. In Figure 10b, with the addition of TiO2 content, both O–(Ti, Si)–O and TiO44− in the slags increase. Notably, the increase of TiO44− is much greater than O–(Ti, Si)–O. Meanwhile, NBO of the slag with more content of TiO2 is higher. The facts indicate that TiO2 acts as a basic oxide and simplifies Si–O and Ti–O networks. There are some consistency between FTIR and Raman analysis, correlating well with the results of viscosity.
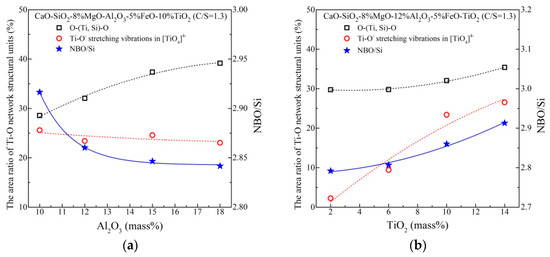
Figure 10.
Aabundance of the structure units with changing (a) Al2O3 content (b) TiO2 content.
4. Conclusions
The evolutions of viscosity and structure of CaO–MgO–Al2O3–SiO2–TiO2–FeO system slag with C/S = 1.3, 8 mass% MgO and 5 mass% FeO, which is relevant Ti–bearing primary slag in blast furnace, are studied by a series of methods. Major findings are concluded as follows:
- (1).
- The change of critical temperatures can be attributed to the variation trend of melting temperature and crystallization capacity, which is able to be explained by the relative amounts of basic phases including perovskite and melilite.
- (2).
- The viscosity of the slag containing 10 mass% TiO2 first increases and then decreases with increasing Al2O3 content from 10 to 18 mass%, exhibiting the maximum value at 15% Al2O3, while an increase in TiO2 content from 2 to 14 mass% causes a decrease in the viscosity of the slag at 12 mass % Al2O3.
- (3).
- FTIR and Raman analysis confirm that Si–O and Ti–O networks are more complex with increasing Al2O3 or decreasing TiO2 content, causing higher viscosity. The dampening of Si–O–Al trough observed in FTIR indicates a decrease in the linkagebetween [SiO4]4− and [AlO4]5− tetrahedrals, which may be the reason for the lower viscosity with excess Al2O3 content.
Author Contributions
Conceptualization, S.S. and Q.W.; methodology, T.L. and C.S.; software, T.L.; validation, C.S.; formal analysis, T.L.; investigation, T.L.; resources, Q.W.; writing—original draft preparation, T.L.; writing—review and editing, C.S. and S.S.; supervision, Q.W.; project administration, Q.W.; funding acquisition, S.S. and Q.W.
Funding
This research was funded by The Rio Tinto–USTL (University of Science and Technology Liaoning) Joint Research Project and Liaoning Province Natural Fund Guidance Plan Project (20180550599).
Acknowledgments
We gratefully express our appreciation to Rio Tinto through The Rio Tinto–USTL (University of Science and Technology Liaoning) Joint Research Project and Liaoning Province Natural Fund Guidance Plan Project (20180550599) for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fan, H.L.; Chen, D.F.; Liu, T.; Duan, H.M.; Huang, Y.W.; Long, M.J.; He, W.J. Crystallization behaviors of anosovite and silicate crystals in high CaO and MgO titanium slag. Metals 2018, 8, 754. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Xue, X.X. Optimized use of MgO flux in the agglomeration of high–chromium vanadium–titanium magnetite. Int. J. Miner. Metall. Mater. 2015, 22, 371–380. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, J.L.; Jiao, K.X. Economical and efficient protection for blast furnace hearth. ISIJ Int. 2018, 58, 1198–1203. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Chu, M.S.; Chen, M.; Zhao, Z.X.; Zhao, B.J.; Liu, Z.G. Titanium distribution between blast furnace slag and iron for blast furnace linings protection. Ironmak. Steelmak. 2018, 45, 1–8. [Google Scholar] [CrossRef]
- Jiao, K.X.; Zhang, J.L.; Liu, Z.J.; Xu, M.; Liu, F. Formation mechanism of the protective layer in a blast furnace hearth. Int. J. Miner. Metall. Mater. 2015, 22, 1017–1024. [Google Scholar] [CrossRef]
- Li, T.L.; Sun, C.Y.; Lan, D.; Song, J.; Song, S.; Wang, Q. Effect of mineral elements migration on softening–melting properties of Ti–bearing high basicity sinter. ISIJ Int. 2019, 59, 245–252. [Google Scholar] [CrossRef]
- Zhou, X.L.; Luo, Y.H.; Chen, T.J.; Zhu, D.P. Enhancing the reduction of high-aluminum iron ore by synergistic reducing with high-manganese iron ore. Metals 2019, 9, 15. [Google Scholar] [CrossRef]
- Guo, H.; Shen, F.M.; Zhang, H.Y.; Gao, Q.J.; Jiang, X. High–temperature reduction and melting mechanism of sinter with different MgO content. Metals 2019, 9, 510. [Google Scholar] [CrossRef]
- Pang, Z.D.; Lv, X.W.; Yan, Z.M.; Liang, D.; Dang, J. Transition of blast furnace slag from silicate-based to aluminate-based: Electrical conductivity. Metall. Mater. Trans. B 2019, 50, 385–394. [Google Scholar] [CrossRef]
- Xie, H.E.; Yu, W.Z.; You, Z.X.; Lv, X.W.; Bai, C.G. The effect of titanium carbonitride on the viscosity of high–titanium–type blast furnace slag. Metals 2019, 9, 395. [Google Scholar] [CrossRef]
- Yan, Z.M.; Lv, X.W.; He, W.C.; Xu, J. Effect of TiO2 on the liquid zone and apparent viscosity of SiO2–CaO–8wt%MgO–14wt%Al2O3 system. ISIJ Int. 2017, 57, 31–36. [Google Scholar] [CrossRef]
- Xu, R.Z.; Zhang, J.L.; Ma, R.Y.; Jiao, K.X.; Zhao, Y.A. Influence of TiO2 on the viscosity of a high alumina slag and on carbon brick corrosion. Steel Res. Int. 2017, 89, 1700353. [Google Scholar] [CrossRef]
- Feng, C.; Chu, M.S.; Tang, J.; Qin, J.; Li, F.; Liu, Z.G. Effects of MgO and TiO2 on the viscous behaviors and phase compositions of titanium–bearing slag. Int. J. Miner. Metall. Mater. 2016, 23, 868–880. [Google Scholar] [CrossRef]
- Sohn, I.; Wang, W.L.; Matsuura, H.; Tsukihashi, F.; Min, D.J. Influence of TiO2 on the viscous behavior of calcium silicate melts containing 17 mass% Al2O3 and 10 mass% MgO. ISIJ Int. 2012, 52, 158–160. [Google Scholar] [CrossRef]
- Park, H.; Park, J.Y.; Kim, G.H.; Sohn, I. Effect of TiO2 on the viscosity and slag structure in blast furnace type slags. Steel Res. Int. 2012, 83, 150–156. [Google Scholar] [CrossRef]
- Shankar, A.; Görnerup, M.; Lahiri, A.K.; Seetharaman, S. Experimental investigation of the viscosities in CaO–SiO2–MgO–Al2O3 and CaO–SiO2–MgO–Al2O3–TiO2 slags. Metall. Mater. Trans. B 2007, 38, 911–915. [Google Scholar] [CrossRef]
- Zhang, S.F.; Zhang, X.; Liu, W.; Lv, X.W.; Bai, C.G.; Wang, L. Relationship between structure and viscosity of CaO–SiO2–Al2O3–MgO–TiO2 slag. J. Non-Cryst. Solids 2014, 402, 214–222. [Google Scholar] [CrossRef]
- Liao, J.L.; Li, J.; Wang, X.D.; Zhang, Z.T. Influence of TiO2 and basicity on viscosity of Ti bearing slag. Ironmak. Steelmak. 2013, 39, 133–139. [Google Scholar] [CrossRef]
- Feng, C.; Tang, J.; Gao, L.H.; Liu, Z.G.; Chu, M.S. Effects of CaO/SiO2 on viscous behaviors and structure of CaO–SiO2–11.00wt%MgO–11.00wt%Al2O3–43.00wt%TiO2 slag systems. ISIJ Int. 2019, 59, 31–38. [Google Scholar] [CrossRef]
- Liang, H.L.; Chu, M.S.; Feng, C.; Tang, J.; Liu, Z.G.; Wang, W.P. Optimisation study and affecting mechanism of CaO/SiO2 and MgO on viscous behaviours of titanium–bearing blast furnace slag. Ironmak. Steelmak. 2018, 45. [Google Scholar] [CrossRef]
- Yan, Z.M.; Lv, X.W.; Zhang, J.; Qin, Y.; Bai, C.G. Influence of MgO, Al2O3 and CaO/SiO2 on the viscosity of blast furnace type slag with high Al2O3 and 5 wt–% TiO2. Can. Metall. Q. 2016, 55, 186–194. [Google Scholar] [CrossRef]
- Feng, C.; Chu, M.S.; Tang, J.; Tang, Y.T.; Liu, Z.G. Effect of CaO/SiO2 and Al2O3 on viscous behaviors of the titanium–bearing blast furnace slag. Steel Res. Int. 2016, 87, 1274–1283. [Google Scholar] [CrossRef]
- Bian, L.T.; Gao, Y.H. Influence of Al2O3, CaO/SiO2, and B2O3 on viscous behavior of high alumina and medium titania blast furnace slag. J. Chem. 2017, 2017, 6895928. [Google Scholar] [CrossRef]
- Zhen, Y.L.; Zhang, G.H.; Chou, K.C. Influence of Al2O3/TiO2 ratio on viscosities and structure of CaO–MgO–Al2O3–SiO2–TiO2 melts. ISIJ Int. 2014, 54, 985–989. [Google Scholar] [CrossRef]
- Jiao, K.X.; Zhang, J.L.; Wang, Z.Y.; Liu, Y.X.; Xu, R.Z. Melting features and viscosity of TiO2–containing primary slag in a blast furnace. High Temp. Mater. Proc. 2018, 37, 149–156. [Google Scholar] [CrossRef]
- Jiao, K.X.; Zhang, J.L.; Wang, Z.Y.; Chen, C.L.; Liu, Y.X. Effect of TiO2 and FeO on the viscosity and structure of blast furnace primary Slags. Steel Res. Int. 2017, 88, 1600296. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. Amphoteric behavior of alumina in viscous flow and structure of CaO–SiO2(–MgO)–Al2O3 slags. Metall. Mater. Trans. B 2004, 35, 269–275. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, Z.T.; Liu, L.L.; Wang, X.D. Investigation of the viscosity and structural properties of CaO–SiO2–TiO2 slags. Metall. Mater. Trans. B 2014, 45, 1389–1397. [Google Scholar] [CrossRef]
- Qi, J.; Liu, C.J.; Jiang, M.F. Role of Li2O on the structure and viscosity in CaO–Al2O3–Li2O–Ce2O3 melts. J. Non-Cryst. Solids 2017, 475, 101–107. [Google Scholar] [CrossRef]
- Gao, Y.M.; Wang, S.B.; Hong, C.; Ma, X.J.; Yang, F. Effects of basicity and MgO content on the viscosity of the SiO2–CaO–MgO–9wt%Al2O3 slag system. Int. J. Miner. Metall. Mater. 2014, 21, 353–362. [Google Scholar] [CrossRef]
- Kim, J.R.; Lee, Y.S.; Min, D.J.; Jung, S.M.; Yi, S.H. Influence of MgO and Al2O3 contents on viscosity of blast furnace type slags containing FeO. ISIJ Int. 2004, 44, 1291–1297. [Google Scholar] [CrossRef]
- Shen, X.; Chen, M.; Wang, N.; Wang, D. Viscosity property and melt structure of CaO–MgO–SiO2–Al2O3–FeO Slag System. ISIJ Int. 2019, 59, 9–15. [Google Scholar] [CrossRef]
- Mysen, B.O.; Ryerson, F.J.; Virgo, D. The influence of TiO2 on the structure and derivative properties of silicate melts. Am. Mineral. 1980, 65, 1150–1165. [Google Scholar]
- Bihuniak, P.P.; Condrate, R.A. Structures, spectra and related properties of group IVB–doped vitreous silica. J. Non-Cryst. Solids 1981, 44, 331–343. [Google Scholar] [CrossRef]
- McMillan, P.; Piriou, B. Raman spectroscopy of calcium aluminate glasses and crystals. J. Non-Cryst. Solids 1983, 55, 221–242. [Google Scholar] [CrossRef]
- McMillan, P.F.; Poe, B.T.; Gillet, P.H.; Reynard, B. A study of SiO2 glass and supercooled liquid to 1950 K via high–temperature Raman spectroscopy. Geochim. Cosmochim. Ac. 1994, 58, 3653–3664. [Google Scholar] [CrossRef]
- Deng, L.B.; Zhang, X.F.; Zhang, M.X.; Jia, X.L. Effect of CaF2 on viscosity, structure and properties of CaO–Al2O3–MgO–SiO2 slag glass ceramics. J. Non-Cryst. Solids 2018, 500, 310–316. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D.; Scarfe, C.M. Relations between the anionic structure and viscosity of silicate melts–A Raman spectroscopic study. Am. Mineral. 1980, 74, 690–710. [Google Scholar]
- Mysen, B.O.; Finger, L.W.; Virgo, D.; Seifert, F.A. Curve–fitting of Raman spectra of silicate glasses. Am. Mineral. 1982, 67, 686–695. [Google Scholar]
- Sun, Y.Q.; Wang, H.; Zhang, Z.T. Understanding the relationship between structure and thermophysical properties of CaO–SiO2–MgO–Al2O3 molten slags. Metall. Mater. Trans. B 2018, 49, 677–687. [Google Scholar] [CrossRef]
- Frantza, J.D.; Mysen, B.O. Raman spectra and structure of BaO–SiO2, SrO–SiO2 and CaO–SiO2 melts to 1600 °C. Chem. Geol. 1995, 121, 155–176. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).