Microstructure of Low-Temperature Gas-Carbonitrided Layers on Austenitic Stainless Steel
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Protocol for Production of the Carbonitrided Diffusion Layers
2.2. Characterization Protocol
3. Results and Discussion
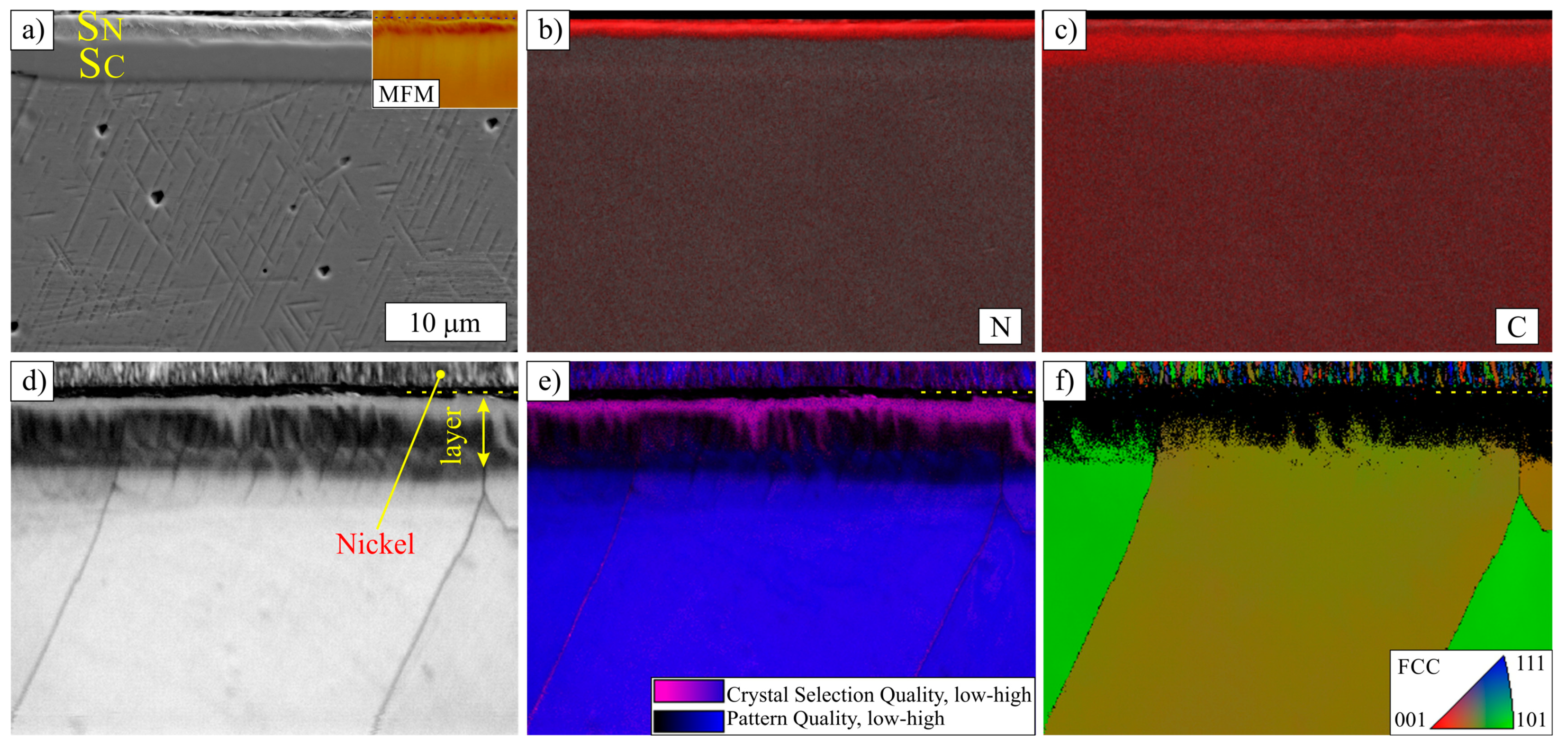
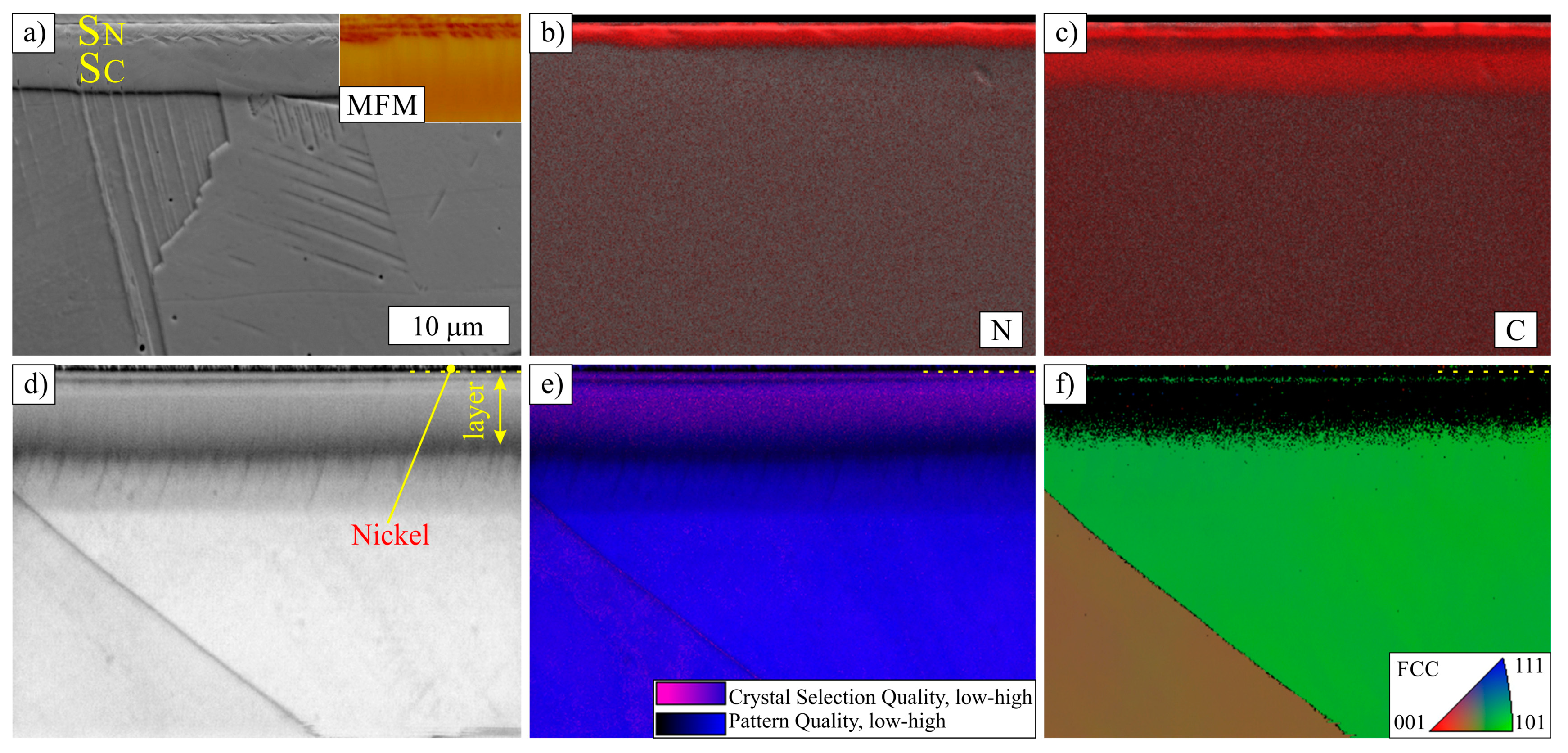
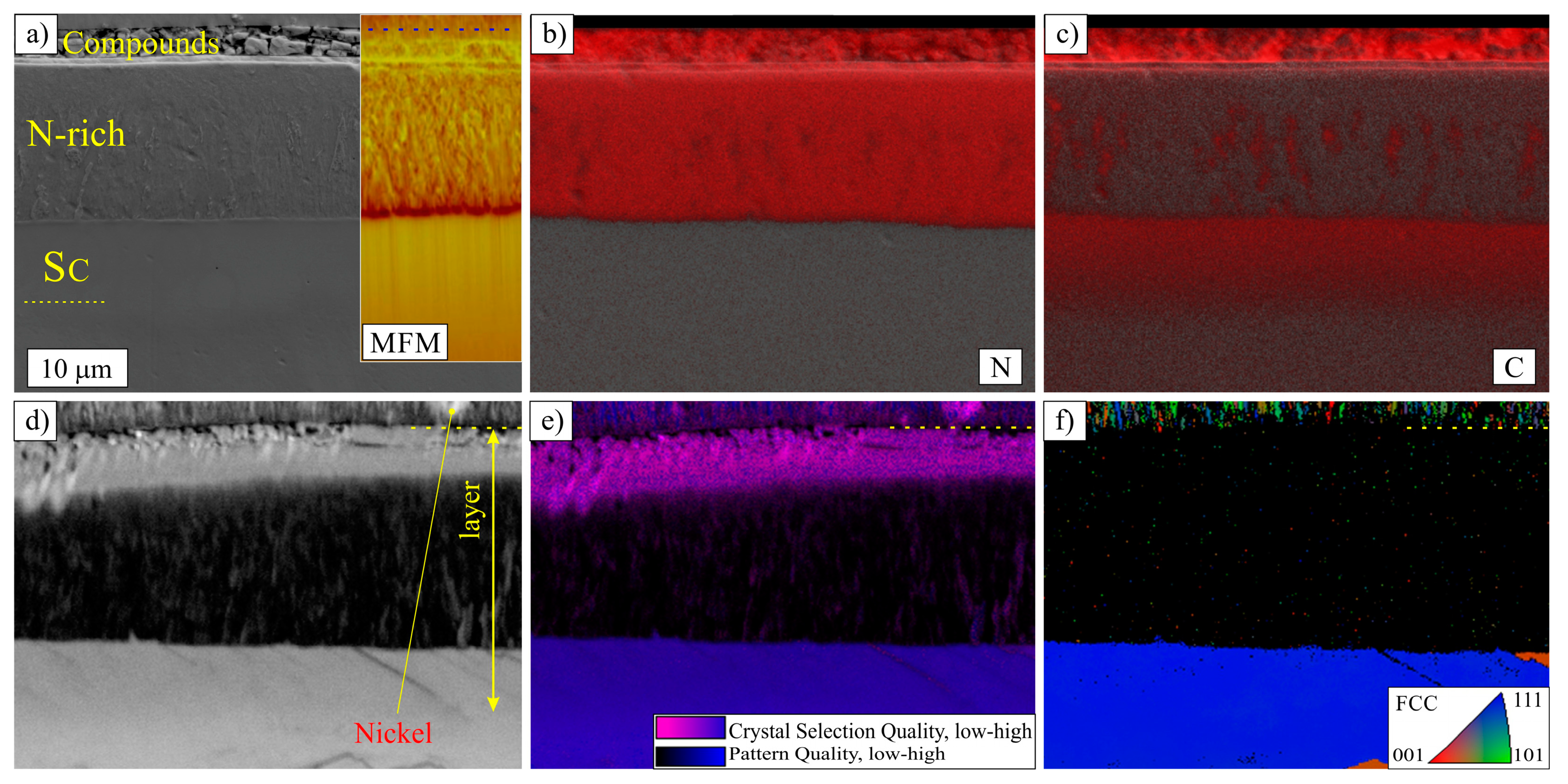
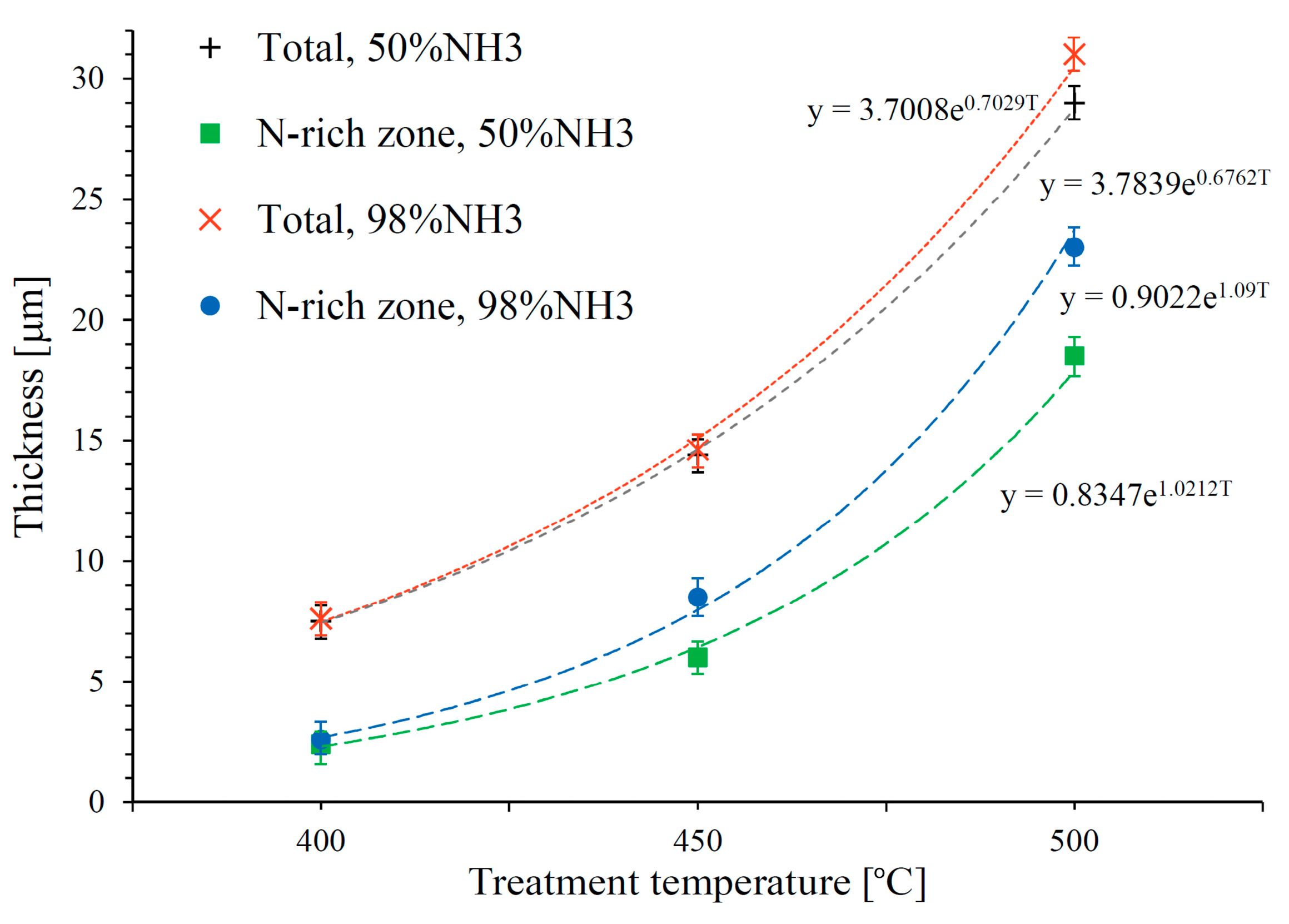
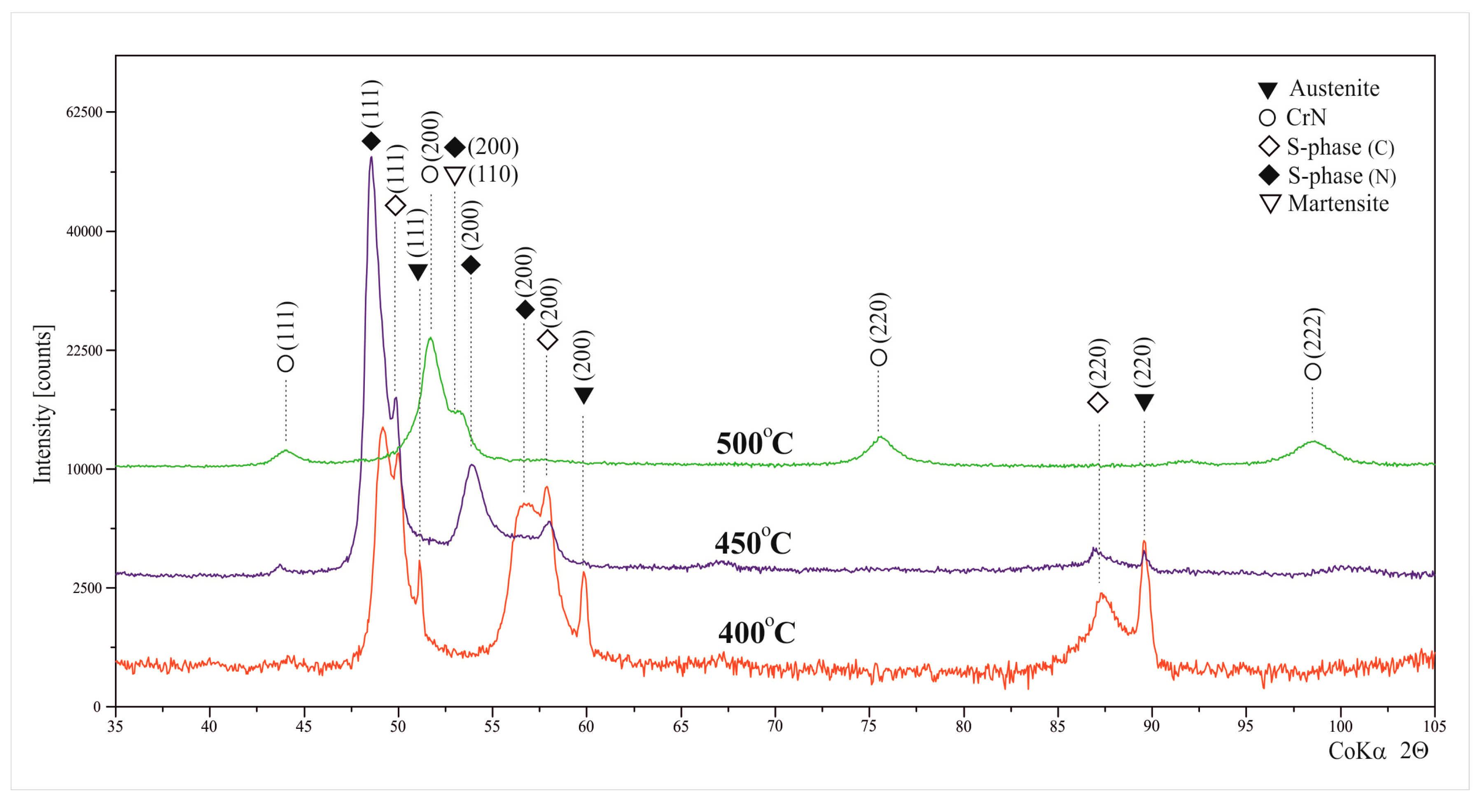
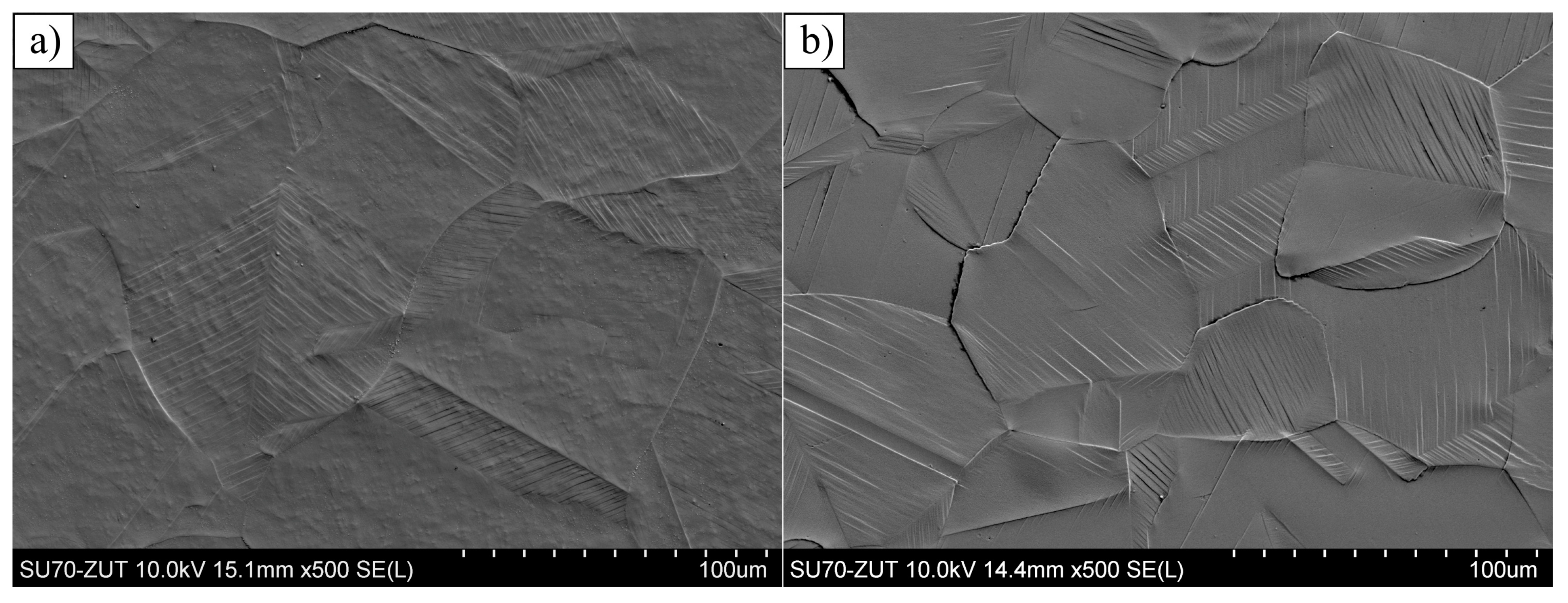
3.1. Layers Treated at 400 and 450 °C
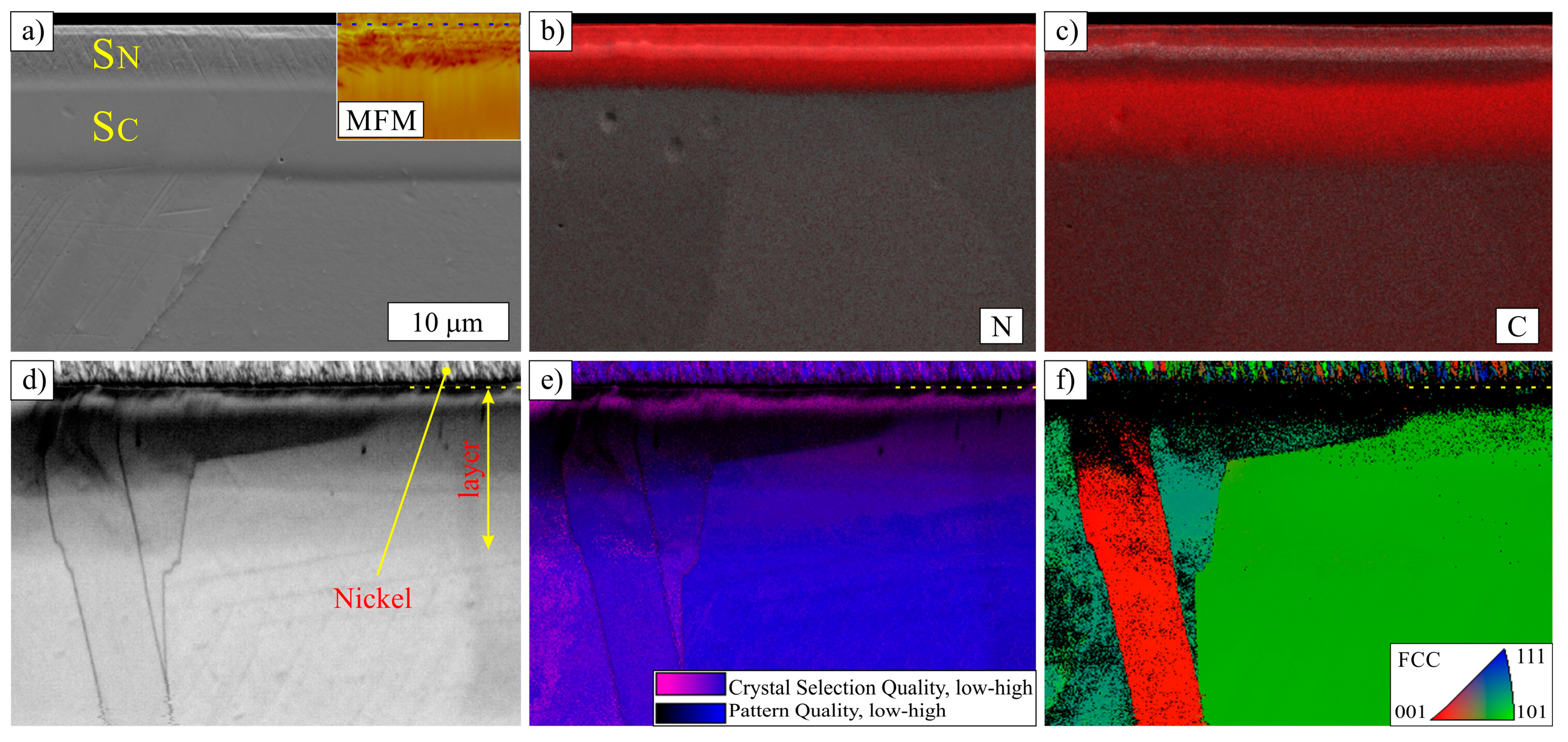
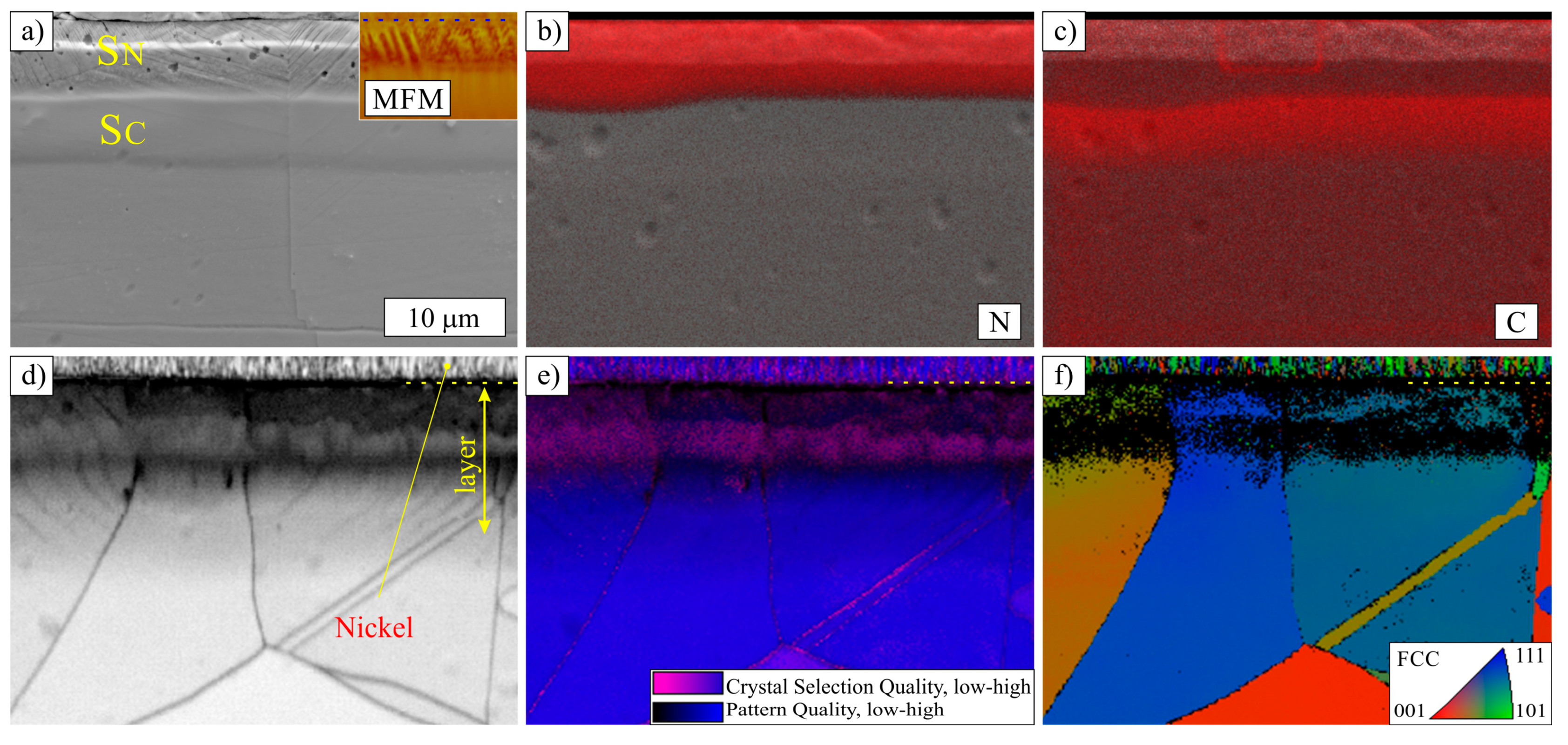
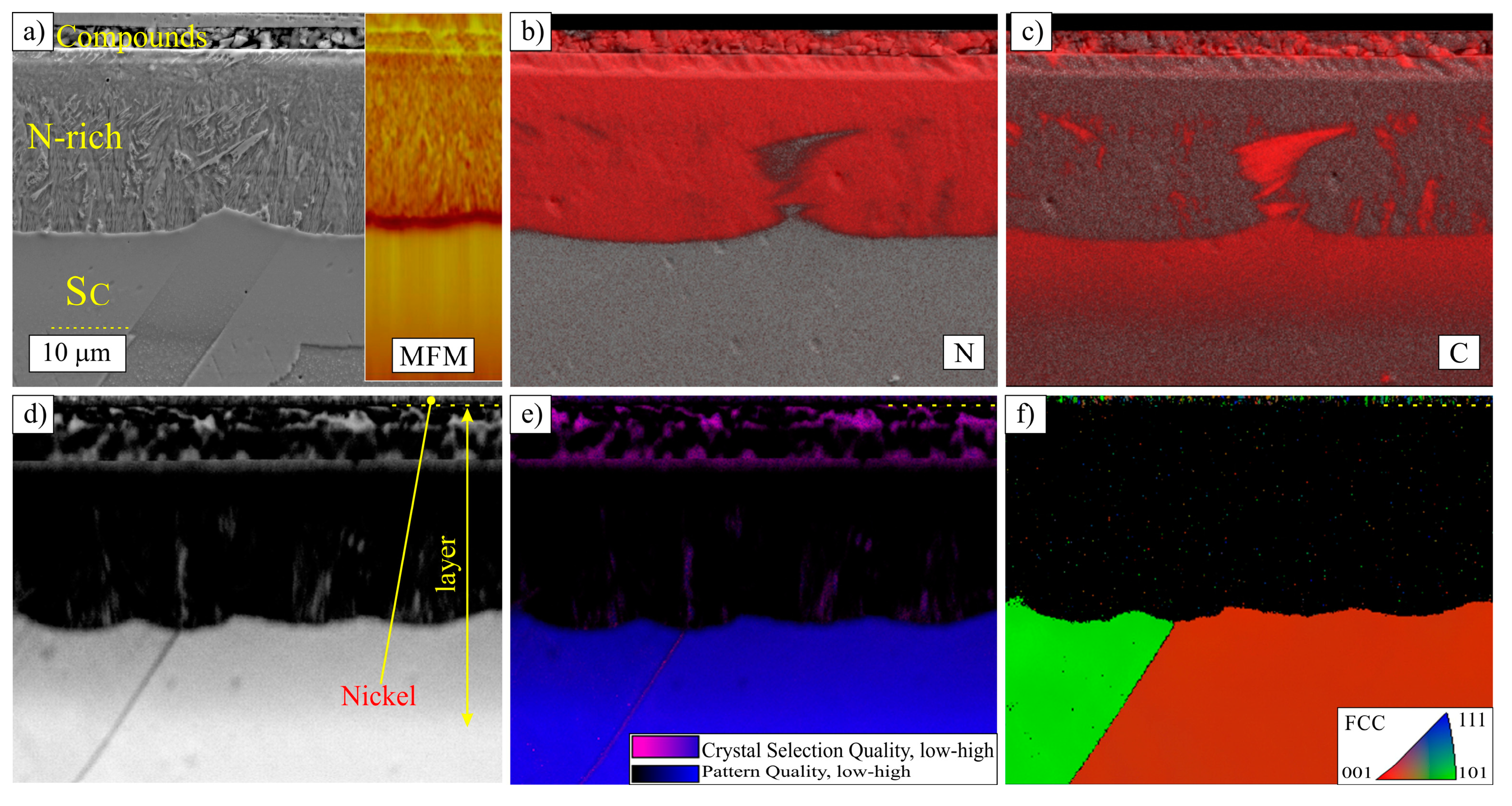
3.2. Layers Treated at 500 °C
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baranowska, J.; Arnold, B. Corrosion resistance of nitrided layers on austenitic steel. Surf. Coat. Technol. 2006, 200, 6623–6628. [Google Scholar] [CrossRef]
- García Molleja, J.; Nosei, L.; Ferrón, J.; Bemporad, E.; Lesage, J.; Chicot, D.; Feugeas, J. Characterization of expanded austenite developed on AISI 316L stainless steel by plasma carburization. Surf. Coat. Technol. 2010, 204, 3750–3759. [Google Scholar] [CrossRef]
- Sun, Y. Production of nitrogen and carbon S phases in austenitic stainless steels by hybrid plasma surface alloying. Surf. Eng. 2009, 26, 114–122. [Google Scholar] [CrossRef]
- Ichii, K.K. Structure of the ion-nitrided layer of 18-8 stainless steel. Technol. Rep. Kansai Univ. 1986, 27, 135. [Google Scholar]
- Bell, T. Current Status of Supersaturated Surface Engineered S-Phase Materials. Key Eng. Mater. 2009, 373–374, 289–295. [Google Scholar]
- Zhang, T.; Tong, X.S. The Effect of Wear and Corrosion Resistance of Austenitic Stainless Steel on Plasma Carburizing. Adv. Mater. Res. 2014, 941–944, 1357–1361. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, H.; Zhao, C. Nitrocarburising of AISI 316 stainless steel at low temperature. Surf. Eng. 2018, 34, 205–210. [Google Scholar] [CrossRef]
- Souza, R.M.; Ignat, M.; Pinedo, C.E.; Tschiptschin, A.P. Structure and properties of low temperature plasma carburized austenitic stainless steels. Surf. Coat. Technol. 2009, 204, 1102–1105. [Google Scholar] [CrossRef]
- Fewell, M.P.; Mitchell, D.R.G.; Priest, J.M.; Short, K.T.; Collins, G.A. The nature of expanded austenite. Surf. Coat. Technol. 2000, 131, 300306. [Google Scholar] [CrossRef]
- Dong, H. S-phase surface engineering of Fe-Cr, Co-Cr and Ni-Cr alloys. Int. Mater. Rev. 2010, 55, 65–98. [Google Scholar] [CrossRef]
- Baranowska, J.; Franklin, S.E.; Pelletier, C.G.N. Tribological behaviour and mechanical properties of low temperature gas nitrided austenitic steel in relation to layer morphology. Wear 2005, 259, 432–438. [Google Scholar] [CrossRef]
- Kochmański, P.; Baranowska, J. Structure and properties of gas nitrided layers on nanoflex stainless steel. Defect Diffus. Forum 2012, 326, 291–296. [Google Scholar] [CrossRef]
- Kochmański, P.; Baranowska, J. Kinetics of low temperature nitriding of precipitation hardened stainless steel. Defect Diffus. Forum 2011, 312, 530–535. [Google Scholar] [CrossRef]
- Baranowska, J.; Kochmański, P.; Bielawski, J. The influence of chemical composition of stainless steel on the formation of low temperature nitrided layer. Defect Diffus. Forum 2012, 326–328, 297–302. [Google Scholar] [CrossRef]
- Kochmański, P.; Baranowska, J. Gas nitrided layers on precipitation hardened stainless steel. Mater. Eng. (Inżynieria Mateiałowa) 2010, 3, 324–327. [Google Scholar]
- Bottoli, F.; Winther, G.; Christiansen, T.L.; Somers, M.A.J. Influence of Microstructure and Process Conditions on Simultaneous Low-Temperature Surface Hardening and Bulk Precipitation Hardening of Nanoflex®. Metall. Mater. Trans. A Phys. Met. Mater. Sci. 2015, 46, 5201–5216. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Hummelshøj, T.S.; Somers, M.A.J. A Method of Activating an Article of Passive Ferrous or non-Ferrous Metal Prior to Carburizing, Nitriding and/or Nitrocarburizing. U.S. Patent No. 8,845,823, 30 September 2014. [Google Scholar]
- Christiansen, T.L.; Somers, M.A.J. Low-temperature gaseous surface hardening of stainless steel: The current status. Int. J. Mater. Res. 2009, 100, 1361–1377. [Google Scholar] [CrossRef]
- Sun, Y.; Haruman, E. Influence of processing conditions on structural characteristics of hybrid plasma surface alloyed austenitic stainless steel. Surf. Coat. Technol. 2008, 202, 4069–4075. [Google Scholar] [CrossRef]
- Jegou, S.; Christiansen, T.L.; Klaus, M.; Genzel, C.; Somers, M.A.J. Determination of composition, residual stress and stacking fault depth profiles in expanded austenite with energy-dispersive diffraction. Thin Solid Films 2013, 530, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Kahn, H.; Michal, G.M.; Ernst, F.; Heuer, A.H. Ferromagnetism in interstitially hardened austenitic stainless steel induced by low-temperature gas-phase nitriding. Scr. Mater. 2011, 65, 1089–1092. [Google Scholar] [CrossRef]
- Fewell, M.P.; Priest, J.M. High-order diffractometry of expanded austenite using synchrotron radiation. Surf. Coat. Technol. 2008, 202, 1802–1815. [Google Scholar] [CrossRef]
- Hummelshøj, T.S.; Christiansen, T.L.; Somers, M.A.J. Lattice expansion of carbon-stabilized expanded austenite. Scr. Mater. 2010, 63, 761–763. [Google Scholar] [CrossRef]
- Wu, D.; Kahn, H.; Dalton, J.C.; Michal, G.M.; Ernst, F.; Heuer, A.H. Orientation dependence of nitrogen supersaturation in austenitic stainless steel during low-temperature gas-phase nitriding. Acta Mater. 2014, 79, 339–350. [Google Scholar] [CrossRef]
- Buhagiar, J.; Dong, H. Low temperature plasma carbonitriding of ASTM F138 and ASTM F1586 biomedical stainless steels. Surf. Eng. 2009, 26, 256–264. [Google Scholar] [CrossRef]
- Gu, X.; Michal, G.M.; Ernst, F.; Kahn, H.; Heuer, A.H. Numerical simulations of carbon and nitrogen composition-depth profiles in nitrocarburized austenitic stainless steels. Met. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 4268–4279. [Google Scholar] [CrossRef]
- Baranowska, J. Importance of surface activation for nitrided layer formation on austenitic stainless steel. Surf. Eng. 2009, 26, 293–298. [Google Scholar] [CrossRef]
- Baranowska, J.; Szczecinski, K.; Mieczysław, W. Increasing of Gas Nitriding Kinetics; Euromat: Maastricht, The Netherlands, 1997; pp. 279–282. [Google Scholar]
- Mittemeijer, E.J.; Somers, M.A.J. (Eds.) Thermochemical Surface Engineering of Steels; Woodhead Publishing: Cambridge, UK, 2015; ISBN 9780857096807. [Google Scholar]
- Tsujikawa, M.; Yamauchi, N.; Ueda, N.; Sone, T.; Hirose, Y. Behavior of carbon in low temperature plasma nitriding layer of austenitic stainless steel. Surf. Coat. Technol. 2005, 193, 309–313. [Google Scholar] [CrossRef]
- Brink, B.K.; Ståhl, K.; Christiansen, T.L.; Frandsen, C.; Hansen, M.F.; Somers, M.A.J. Composition-dependent variation of magnetic properties and interstitial ordering in homogeneous expanded austenite. Acta Mater. 2016, 106, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Prokert, F.; Abd El-Rahman, A.M.; Schell, N.; Negm, N.Z.; Richter, E.; El-Hossary, F.M.; Möller, W. In-situ stability study of nitrocarburized 304 stainless steel during heating. Surf. Coat. Technol. 2005, 200, 602–607. [Google Scholar]
- Li, X.Y.; Sun, Y.; Bell, T. The stability of the nitrogen S-phase in austenitic stainless steel. Z. Met. 1999, 90, 901–907. [Google Scholar]



| Element | C | Cr | Ni | Mo | Mn | Si | Fe |
|---|---|---|---|---|---|---|---|
| Mass % | 0.1 | 18.67 | 7.54 | 0.23 | 2.03 | 0.33 | balance |
| Sample | Temperature (°C) | Atmosphere Composition (vol. %) | Duration (h) | ||
|---|---|---|---|---|---|
| NH3 | Dissociated NH3 | C2H2 | |||
| 1 | 400 | 49 | balance | 2 | 5 |
| 2 | 400 | 98 | - | ||
| 3 | 450 | 49 | balance | ||
| 4 | 450 | 98 | - | ||
| 5 | 500 | 49 | balance | ||
| 6 | 500 | 98 | - | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochmański, P.; Baranowska, J.; Fryska, S. Microstructure of Low-Temperature Gas-Carbonitrided Layers on Austenitic Stainless Steel. Metals 2019, 9, 817. https://doi.org/10.3390/met9080817
Kochmański P, Baranowska J, Fryska S. Microstructure of Low-Temperature Gas-Carbonitrided Layers on Austenitic Stainless Steel. Metals. 2019; 9(8):817. https://doi.org/10.3390/met9080817
Chicago/Turabian StyleKochmański, Paweł, Jolanta Baranowska, and Sebastian Fryska. 2019. "Microstructure of Low-Temperature Gas-Carbonitrided Layers on Austenitic Stainless Steel" Metals 9, no. 8: 817. https://doi.org/10.3390/met9080817






