Removal of Residual Element Tin in the Ferrous Metallurgy Process: A Review
Abstract
:1. Introduction
2. The Sources of Residual Tin in Steel
2.1. Tin-Bearing Iron Ore
2.2. Ferroalloys
2.3. Scrap Steel
3. The Existing Forms of Residual Tin in Steel
- (1)
- Solidification segregation: As tin is a low melting point metal element (with melting point 232 °C), its distribution is not uniform during the solidification process. At the same time, the solidification segregation coefficient of the residual tin in iron is 0.5 [28] and, so, tin generally does not cause severe macro-segregation under normal solidification conditions. Due to the difference in solubility between solid and liquid phases and selective crystallization, the content of residual tin in the latter dendrites is higher than that in the dendrites formed at the initial stage of crystallization; this will cause a heterogeneous concentration distribution in the internal grain.
- (2)
- Grain boundary segregation: Residual tin may cause grain boundary segregation during the solid phase transformation process or heating process. Compared with solidification segregation, as the residual elements can only diffuse in a short range, the generation of grain boundary segregation generally requires a specific temperature and time, and the position is usually located at the crystal defects; for example, the interfaces between pro-eutectoid ferrite and austenite, and the prior austenite grain boundaries [29,30,31,32]. The segregation amount of tin at the grain boundary changes with the cooling rate during the continuous cooling process and the segregation process is dynamic and consistent with non-equilibrium grain boundary segregation [33,34].
- (3)
- Surface segregation: In addition to segregation at the grain boundaries, the residual tin is also enriched at the interface between the steel substrate and the oxide layer. The oxidation potential of tin is lower than that of iron and the melting point of tin is lower than iron, and so the preferential oxidation rate of iron is significantly higher than the diffusion rate of residual tin into the substrate during the high-temperature cooling and secondary heating of the slab [35,36]. As a result of selective oxidation, low-melting point tin-concentrating phases inevitably form [37,38].
4. Tin Removal Processes in the Ferrous Metallurgy Process
4.1. Tin-Bearing Iron Ore Roasting Treatment Process
4.1.1. Sulfurization Volatilization Process
4.1.2. Chlorination Volatilization Process
4.1.3. Reduction Volatilization Process
4.2. Tin-Bearing Scrap Steel Pre-Treatment Technology
4.2.1. Alkaline Electrolysis Process
4.2.2. Alkaline Solution Leaching Process
4.2.3. Chlorination Process
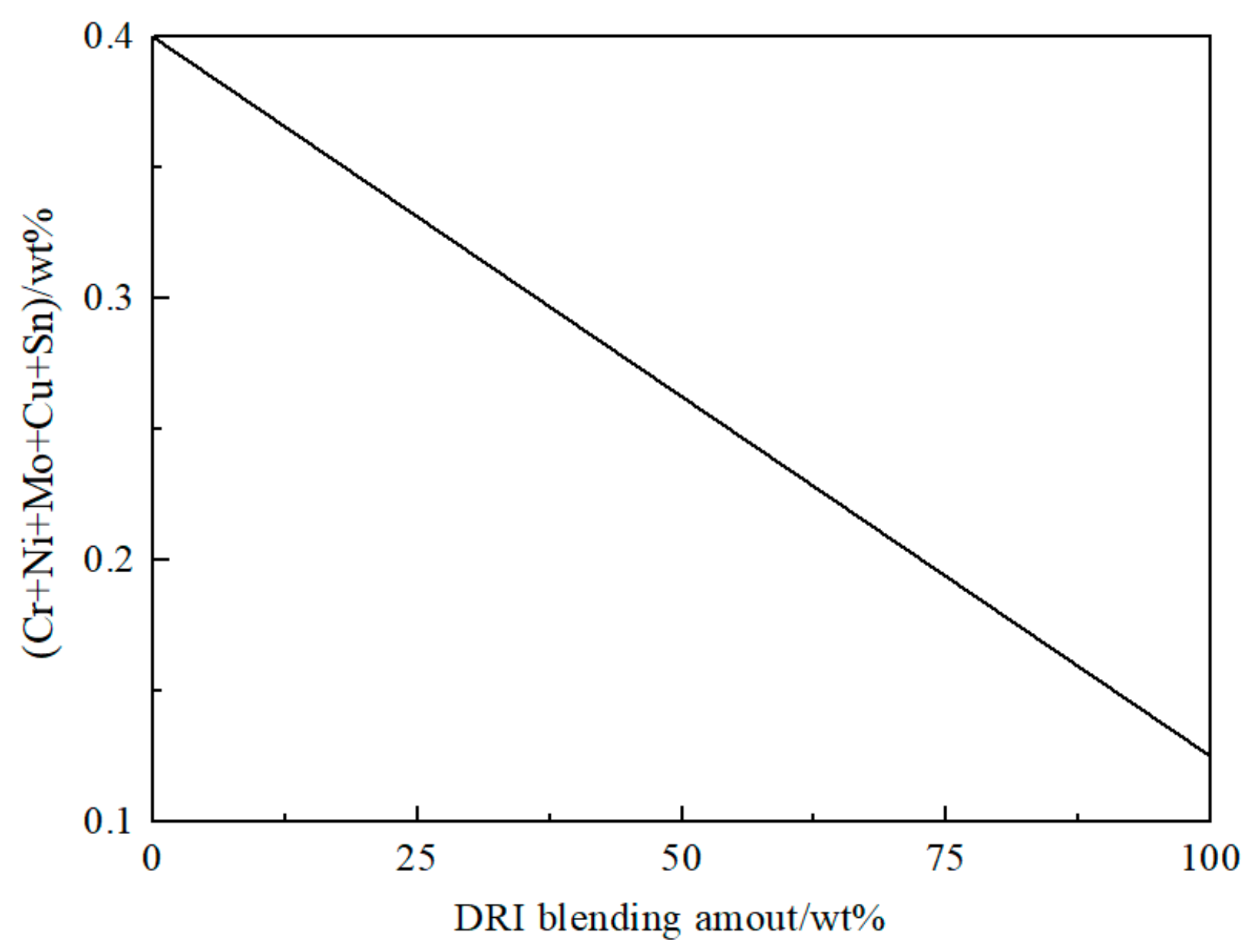
4.3. Ingredient Dilution Process
4.4. Vapor Pressure Process
4.5. Calcium Reaction Process
4.6. Rare Earth Treatment
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Global Crude Steel Output Increases by 4.6% in 2018. Available online: http://www.webcitation.org/77nVyqbnp (accessed on 21 April 2019).
- Ramadan, A.; Shash, A.Y.; El-Mahallawi, I.S.; Senk, D.; Mattar, T. Effect of Tempcore Processing on Mitigating Problems of Tramp Elements in Low C Steel Produced from Recycled Material. J. Iron. Steel Res. Int. 2015, 22, 582–589. [Google Scholar] [CrossRef]
- Atkinson, M.; Kavanagh, L.; Mccutcheon, D.; Cox, P.S.; Shultz, R. Steel Industry Technology Roadmap; American Iron and Steel Institute: Washington, DC, USA, 2001; pp. 80–81. [Google Scholar]
- Zhang, L. Influence of Residual Elements in Steel on the Quality of CSP Products. Met. Mater. Metall. Eng. 2009, 37, 12–15. [Google Scholar]
- GB/T 700-2006. Carbon Structural Steel; Standards Press of China: Beijing, China, 2007. [Google Scholar]
- ASTM A36/A36M-14. Standard Specification for Carbon Structural Steel; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Deng, S. The Influence of Residual Elements to Steel Quality and its Controlling. Liu Steel Technol. 2016, 2, 10–15. [Google Scholar]
- Zhang, M. Study on Plasticization Control of Oxide Inclusions in Tire Cord Steel. Steelmaking 2014, 30, 62–65, 78. [Google Scholar]
- Li, L.; Zhu, R.; Guo, H.; Dong, J.; Li, F. Development of Non-leaded Free-Cutting Steel by Adding Tin. Int. J. Min. Met. Mater. 2003, 25, 312–314. [Google Scholar]
- Reynolds, P.; Block, V.; Essel, I.; Klocke, F. Alternatives to Lead as a Machinability Enhancer in Free Cutting Steels. Steel Res. Int. 2007, 78, 908–914. [Google Scholar] [CrossRef]
- Chen, S.C.; Zhu, R.; Xue, L.Q.; Lin, T.C.; Li, J.S.; Lin, Y. Effects of Elemental Sn on the Properties and Inclusions of the Free-cutting Steel. Int. J. Min. Met. Mater. 2015, 22, 141–148. [Google Scholar] [CrossRef]
- Matsuoka, H.; Osawa, K.; Ono, M.; Ohmura, M. Influence of Cu and Sn on Hot Ductility of Steels with Various C Content. ISIJ Int. 1997, 37, 255–262. [Google Scholar] [CrossRef]
- Nagasaki, C.; Kihara, J. Effect of Copper and Tin on Hot Ductility of Ultra-low and 0.2% Carbon Steels. ISIJ Int. 1997, 37, 523–530. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, D.Y. Effect of Residual Elements on Hot-workability of Stainless Steel. Spec. Steel 1990, 1, 24–29. [Google Scholar]
- Wang, H. Harmful Effect of Sn Element on SUS304HC Austenite Stainless Steel. Iron Steel 2004, 39, 64–66. [Google Scholar]
- Zhao, B.; Wang, J.; Du, Y.; Li, J. Effect of As, Sn and Sb on Temper Brittleness of 30CrMnSiA Steel. Trans. Mater. Heat Treat. 1995, 1, 13–17. [Google Scholar]
- Zhou, S.; Guo, Y.; Shen, G. Control Critical Range of P and Sn of Cr-Mo-V Series Gun steel. Ordnance Mater. Sci. Eng. 1998, 1, 13–19. [Google Scholar]
- Gulyaev, A.P.; Taran, A.V. Effect of Purity on the Temper Brittleness of Improved Structural Steels. Met. Sci. Heat Treat. 1971, 13, 844–848. [Google Scholar] [CrossRef]
- Ding, Z.; Yan, Y.; Wang, S. Investigation on Reversible Temper Embrittlement and the Mechanism of Structional Steel. J. Dalian Railw. Inst. 1998, 19, 68–73. [Google Scholar]
- Liu, F. Surface Enrichment of Residual Elements and Oxidation of the Austenite Grain Boundaries. Acta Metall. Sin. 1978, 14, 310–315. [Google Scholar]
- Stephenson, E.T. Effect of Recycling on Residuals, Processing, and Properties of Carbon and Low-alloy Steels. Metall. Mater. Trans. A 1983, 14, 343–353. [Google Scholar] [CrossRef]
- Su, Z.J. Research on the Behavior of Tin Removal from Tin-Bearing Iron Ores by Reduction Roasting. Master’s Thesis, Central South University, Changsha, China, 2014. [Google Scholar]
- Li, L. Development status and future demand outlook of ferroalloy industry in China. China Steel Focus 2013, 5, 15–24. [Google Scholar]
- Guo, D.; Chu, S. Influence of Manganese Ferroalloys on Iron & Steel Industry and its Purification of China. Ferro Alloy 2012, 43, 9–12. [Google Scholar]
- Savov, L.; Volkova, E.; Janke, D. Copper and Tin in Steel Scrap Recycling. RMZ Mater. Geoenviron. 2003, 50, 627–640. [Google Scholar]
- Noro, K.; Takeuchi, M.; Mizukami, Y. Necessity of Scrap Reclamation Technologies and Present Conditions of Technical Development. ISIJ Int. 1997, 37, 198–206. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Liu, M. Micro-segregation Model Calculation of Residual Tin in Boiler and Pressure Vessel Steel. Philos. Mag. 2019, 99, 1041–1056. [Google Scholar] [CrossRef]
- Seah, M.P.; Hondros, E.D. Grain Boundary Segregation. Proc. R. Soc. Lond. 1973, 335, 191–212. [Google Scholar] [CrossRef]
- Wu, P.; He, X. Grain Boundary Non-equilibrium Segregation: Retrospect/Prospect. Acta Metall. Sin. 1999, 35, 1009–1020. [Google Scholar]
- Nachtrab, W.T.; Chou, Y.T. Grain Boundary Segregation of Copper, Tin and Antimony in C-Mn Steels at 900 °C. J. Mater. Sci. 1984, 19, 2136–2144. [Google Scholar] [CrossRef]
- Wang, M.Q.; Wang, K.; Deng, Q. Non-equilibrium Grain Boundary Co-segregation of Nickel and Tin in 2·6 NiCrMoV Steel. Mater. Sci. Technol. 2009, 25, 1238–1242. [Google Scholar] [CrossRef]
- Sarafianos, N. Influence of the Combined Effect of Grain Size and Tin Grain Boundary Segregation on the Ductility of Carbon Steel During Hot-rolling. J. Mater. Sci. Lett. 1993, 12, 1522–1525. [Google Scholar]
- Yuan, Z.X.; Jia, J.; Guo, A.M.; Shen, D.D.; Song, S.H. Cooling-induced Tin Segregation to Grain Boundaries in a Low-carbon Steel. Scr. Mater. 2003, 48, 203–206. [Google Scholar] [CrossRef]
- Jia, J. Tin Segregation Behavior to Grain-boundary and the Hot Ductility of Low Carbon Steel. Master’ Thesis, Wuhan University of Science and Technology, Wuhan, China, 2003. [Google Scholar]
- Melford, D.A. Surface Hot Shortness in Mild Steel-study of Influence of Residual Elements with Aid of Electron Probe Microanalyser. J. Iron Steel Inst. 1962, 200, 290–299. [Google Scholar]
- Geng, M.; Wang, X.; Zhang, J.; Wang, W.; Xiao, J. Enrichment of Residual Elements in Continuously Cast Slabs and Medium Plates. J. Univ. Sci. Technol. Beijing 2009, 31, 300–305. [Google Scholar]
- Webler, B.A.; Sridhar, S. The Effect of Silicon on the High Temperature Oxidation Behavior of Low-carbon Steels Containing the Residual Elements Copper and Nickel. ISIJ Int. 2007, 45, 1245–1254. [Google Scholar] [CrossRef]
- Suzuki, H.G. Strain Rate Dependence of Cu Embrittlement in Steels. ISIJ Int. 1997, 37, 250–254. [Google Scholar] [CrossRef]
- Han, G.; Zhang, Y.; Jiang, T.; Huang, Y.; Li, G. Study on Preparation of Pellets for Iron-making from Tin, Zinc-bearing Iron Concentrates by Grate-kiln Process. Iron Steel 2009, 44, 8–13. [Google Scholar]
- Zhang, Y.B.; Jiang, T.; Li, G.H.; Huang, Z.C.; Guo, Y.F. Tin and Zinc Separation from Tin, Zinc Bearing Complex Iron Ores by Selective Reduction Process. Ironmak. Steelmak. 2013, 38, 613–619. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Li, G.H.; Jiang, T.; Guo, Y.F.; Huang, Z.C. Reduction Behavior of Tin-bearing Iron Concentrate Pellets using Diverse Coals as Reducers. Int. J. Miner. Process. 2012, 110, 109–116. [Google Scholar] [CrossRef]
- Fu, Z. Volatile Process of Tin in Reduction Roasting. Nonferr. Met. (Extr. Metall.) 1980, 1, 25–29. [Google Scholar]
- Wang, Z.W.; Wang, C.Y.; Lu, H.M. Investigation on Removal of Tin from Sn-bearing Iron Concentrates by Reduction Roasting. Min. Metall. 2005, 14, 63–66. [Google Scholar]
- Yunnan Tin Company; Tin Metallurgy Editing Group of Kunming University of Science and Technology. Tin Metallurgy; Metallurgical Industry Press: Beijing, China, 1977; p. 11. [Google Scholar]
- Kékesi, T.; Török, T.I.; Kabelik, G. Extraction of Tin from Scrap by Chemical and Electrochemical Methods in Alkaline Media. Hydrometallurgy 2000, 55, 213–222. [Google Scholar] [CrossRef]
- Wang, H.B. DRI as a Substitute of Scrap Used for Steelmaking in Electric Arc Furnaces. Res. Iron Steel 2004, 32, 53–57, 61. [Google Scholar]
- Wang, P. Popularization and Application of Hot Briquetted Iron (HBI). Iron Steel Scrap China 2005, 11, 11–18. [Google Scholar]
- Zheng, H.Y.; Jiang, M.F.; Wang, G.H.; Hui, Y.A. Fe3C-a New Raw Material for Steelmaking. J. Iron Steel Res. 1996, 11, 60–63. [Google Scholar]
- Gu, W.B.; Liu, X.; Yang, B.C. Clean Steel Making in the EAF at Baosteel. Iron Steel 2000, 35, 16–18, 38. [Google Scholar]
- Li, S.Q.; Li, S.Q.; Wang, Y.N. Analysis and Study of Controlling Harmful Residuals in Molten Steel. Iron Steel 2001, 36, 70–72, 76. [Google Scholar]
- Sun, G.L. Fundamentals on Precipitation Behavior of Residual Elements Tin and Antimony in Steel. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2016. [Google Scholar]
- Xu, K.D.; Jiang, G.C.; Hong, X.; Zheng, S.B.; Xu, J.L. Discussion on New Process Making Clean-steel from Scrap. Acta Metall. Sin. 2001, 37, 395–399. [Google Scholar]
- Xiong, Y.L.; Yang, B.; Xiong, H.; Xu, B.Q.; Liu, D.C.; Dai, Y.N. Separation of Sn-Fe Alloys by Vacuum Distillation. Chin. J. Vac. Sci. Technol. 2012, 32, 820–824. [Google Scholar]
- Lipart, J.; Łabaj, J.; Słowikowski, M.; Jama, D. Effects of Pressure on the Rate of Tin Evaporation from Liquid Iron. Arch. Metall. Mater. 2014, 59, 825–828. [Google Scholar] [CrossRef]
- Savov, L.; Janke, D. Evaporation of Cu and Sn from Induction-stirred Iron-based Melts Treated at Reduced Pressure. ISIJ Int. 2000, 40, 95–104. [Google Scholar] [CrossRef]
- Savoa, L.; Tu, S.; Janke, D. Methods of Increasing the Rate of Tin Evaporation From Iron-based Melts. ISIJ Int. 2000, 40, 654–663. [Google Scholar] [CrossRef]
- Matsuo, T.; Maya, K.; Nishi, T.; Shinme, K.; Ueno, A.; Anezaki, S. Removal of Copper and Tin in Molten Iron with Decarburization under Reduced Pressure. ISIJ Int. 1996, 36, s62–s65. [Google Scholar] [CrossRef]
- Nishi, T.; Fukagawa, S.; Shinme, K.; Mastuo, T. Removal of Copper and Tin in Molten Iron with Combination of Plasma Heating and Powder Blowing Decarburization under Reduced Pressure. ISIJ Int. 1999, 39, 905–912. [Google Scholar] [CrossRef]
- Sasaki, N.; Uchida, Y.I.; Miki, Y.J.; Matsuno, H. Fundamental Study of Sn Removal from Hot Metal by NH3 Gas Blowing. ISIJ Int. 2014, 54, 1807–1812. [Google Scholar] [CrossRef]
- Matsuo, T. Removal of Copper and Tin in the Molten Steel with Plasma Flame. Tetsu Hagané 1989, 75, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Zaitsev, A.I.; Zaitseva, N.E.; Shakhpazov, E.K.; Mogutnov, B.M. Potentialities of Simultaneous Removal of Tin and Copper from Molten Iron through Evaporation. ISIJ Int. 2004, 44, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Tsukihashi, F. Physical Chemistry of Removal of Tramp Elements from Steel. In Proceedings of the 9th China-Japan Symposium on Science and Technology of Iron and Steel, Xian, China, 8–9 November 2001; pp. 59–66. [Google Scholar]
- Ochifuji, Y.; Tsukihashi, F.; Sano, N. The Activity of Calcium in Calcium-metal-fluoride Fluxes. Metall. Mater. Trans. B 1995, 26, 789–794. [Google Scholar] [CrossRef]
- Ghosh, D. Removal of Tin and Copper from Liquid Iron by Al2O3-Saturated Ca-CaCl2 Slags at 1448 to 1648 K. Metall. Mater. Trans. B 2009, 40, 508. [Google Scholar] [CrossRef]
- Kitamura, K.; Takenouchi, T.; Iwanami, Y. Removal of Impurities from Molten Steel by CaC2. Tetsu Hagané 1985, 71, 220–227. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Gu, L.J. Rare Earth-iron Binary Phase Diagrams. Chin. Rare Earths 1981, 1, 72–85. [Google Scholar]
- Zhao, Y.B.; Wang, F.M.; Li, C.R.; Xiao, J.G. Effect of Lanthanum on GCr15 Bearing Steel with Tin and Antimony. J. Chin. Rare Earth Soc. 2007, 25, 229–233. [Google Scholar]
- Wilson, W.G.; Kay, D.A.R.; Vahed, A. The Use of Thermodynamics and Phase Equilibria to Predict the Behavior of the Rare Earth Elements in Steel. JOM 1974, 26, 14–23. [Google Scholar] [CrossRef]
- Yan, C.L.; Wang, F.M.; Wei, L.J.; Fu, Q.; Wu, C.J. Improvement of Lanthanum on Impact Toughness of 34CrNi3Mo Steel Containing Sn, Sb Residual Elements. J. Univ. Sci. Technol. Beijing 2004, 26, 277–281. [Google Scholar]
- Wei, L.J.; Wang, F.M.; Xiang, C.X.; Zhang, X.; Liu, K.M.; Wu, C.J. Improvement of Lanthanum on Hot Ductility of 34CrNi3Mo Steel Containing Residuals. J. Chin. Rare Earth Soc. 2003, 21, 311–314. [Google Scholar]
- Sha, A.X.; Wang, F.M.; Wu, C.J.; Dong, L.R.; Li, S.Y.; Chen, Y.X.; Zhu, L.X. Fixation of Tramp Elements in Steel by Lanthanum. Chin. J. Rare Met. 2000, 24, 287–291. [Google Scholar]
- Yang, Z.L. Research on the Interaction Rule between the Rare Earth Cerium and the Low Melting Point Metallic Tin in the Steel. Master’s Thesis, Guizhou University, Guiyang, China, 2006. [Google Scholar]
- Yu, J.S.; Yu, Z.S.; Zhang, F.Z. Handbook for Rare Earth Treated Steels; Metallurgical Industry Press: Beijing, China, 1993; p. 22. [Google Scholar]





| Low Carbon Plain Steel Plate | Sn | Cu | As | Cu + 8Sn + 5As |
|---|---|---|---|---|
| American Riverdale | 0.003 | 0.04 | 0.018 | 0.154 |
| Japanese Kawasaki | 0.002 | 0.03 | 0.015 | 0.125 |
| Lianyuan Steel | 0.012 | 0.07 | 0.043 | 0.381 |
| Vf Zhujiang Steel | 0.014 | 0.10 | 0.040 | 0.412 |
| Metal Chloride | SnCl2 | SnCl4 | FeCl2 | FeCl3 | PbCl2 | AsCl3 | ZnCl2 | CaCl2 | MgCl2 |
|---|---|---|---|---|---|---|---|---|---|
| Melting point/°C | 246 | −33 | 674 | 306 | 501 | −18 | 283–293 | 782 | 714 |
| Boiling point/°C | 623 | 114 | 1023 | 315 | 951 | 130 | 732 | 1600 | 1412 |
| Temperature (°C) | 1400 | 1450 | 1500 | 1550 | 1600 | 1650 | 1700 |
|---|---|---|---|---|---|---|---|
| Fe | 0.3327 | 0.7311 | 1.507 | 2.742 | 5.176 | 9.311 | 16.41 |
| Sn | 12.16 | 22.59 | 40.55 | 70.47 | 118.9 | 194.98 | 311.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ma, G.; Liu, M.; Li, Z. Removal of Residual Element Tin in the Ferrous Metallurgy Process: A Review. Metals 2019, 9, 834. https://doi.org/10.3390/met9080834
Zhang X, Ma G, Liu M, Li Z. Removal of Residual Element Tin in the Ferrous Metallurgy Process: A Review. Metals. 2019; 9(8):834. https://doi.org/10.3390/met9080834
Chicago/Turabian StyleZhang, Xiang, Guojun Ma, Mengke Liu, and Zhi Li. 2019. "Removal of Residual Element Tin in the Ferrous Metallurgy Process: A Review" Metals 9, no. 8: 834. https://doi.org/10.3390/met9080834
APA StyleZhang, X., Ma, G., Liu, M., & Li, Z. (2019). Removal of Residual Element Tin in the Ferrous Metallurgy Process: A Review. Metals, 9(8), 834. https://doi.org/10.3390/met9080834






