Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications
Abstract
:1. Introduction
- (1)
- Good bio-functionality. Considering the adaptability of the implant to the bone after implantation, the metal material of the implant should have adequate biocompatibility, which ensures that no rejection reaction occurs after implantation. Moreover, to further improve the biological functionality of the implant, surface modification is an effective and common way to enable the implant with desirable osseointegration properties.
- (2)
- Suitable mechanical properties. In order to avoid the phenomenon of “stress shielding” [5], the implant requires mechanical properties such as Young’s modulus and strength compatible with the bone. For solid metals, the mechanical properties are higher than the mechanical parameters of the bone, resulting in the mismatch in modulus, thereby causing the “stress shielding”, which can be easily solved by using a porous metal material, the topological configuration and relative density of which can be adjusted to meet the mechanical and biological functional requirements of the implant [6,7,8,9].
- (3)
- Appropriate morphological structure and structural processability. It is also a requisite that the shape and outer contour of the implant match the defect portion of the bone [10] to ensure a stable support function. Moreover, because of the limitations of manufacturing equipment and the influence of processing parameters, when designing customized metallic implants, the structure should be modified based on the process to ensure the machinability of the implant.
2. Additive Manufacturing Metallic Implants
Biocompatible Metallic Materials
- (1)
- Common metals
- (2)
- Expensive metals
- (3)
- Degradable metals
3. Design Customized Implants to Match the Bone
3.1. Topology Structure Design
3.1.1. Lattice Structure Design
- (1)
- Lattice structure based on the polyhedral structure
- (2)
- Lattice structure based on the implicit function surface
- (3)
- Lattice structure based on topology optimization design
3.1.2. Gradient Structure Design
- (1)
- Unit cell changes in rod diameter [123]. For a polyhedral structure, it can be viewed as a myriad of rods connecting each other. Thus, altering the rod diameter can achieve a density gradient of the overall structure. When modeling, the structure is sliced into multiple layers. The rod diameters of each layer vary, thereby changing the overall density of the lattice structure.
- (2)
- Unit cell changes in size [124]. The implant is composed of a plurality of unit cells. By adjusting the cell size in different regions, the gradient of the implant structure can be realized while the rod diameter and the pore diameter are ensured.
- (3)
- Unit cell changes in type [125]. Due to the change of bone structure, the unit cells in different regions will theoretically have certain variability. The implants containing multiple isomers can realize the gradient of the structure via different unit cells.
- (4)
- Material composition changes [126]. The change of the material composition can also achieve the structural gradient. The most common method is to change the content of the other materials within one matrix material to realize the functional gradient of the structure.
3.2. Surface Modification
- (1)
- Chemically roughening the surface
- (2)
- Directly filling the coating
- (3)
- Using mechanical methods
4. Discussion and Future Development of AM Metallic Implants
4.1. Discussion
4.2. Future Development
- (1)
- New metallic biomaterials can improve the implant performance
- (2)
- Biomimetic gradient structure can restore the characteristics of natural bone
- (3)
- The surface modification further enhances the biocompatibility of the implant
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Das, M.; Balla, V.K.; Kumar, T.S.; Manna, I. Fabrication of biomedical implants using laser engineered net shaping (LENS™). Trans. Indian Ceram. Soc. 2013, 72, 169–174. [Google Scholar] [CrossRef]
- Larosa, M.A.; Jardini, A.L.; Zavaglia, C.A.D.C.; Kharmandayan, P.; Calderoni, D.R.; Maciel Filho, R. Microstructural and mechanical characterization of a custom-built implant manufactured in titanium alloy by direct metal laser sintering. Adv. Mech. Eng. 2014, 6, 945819. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F. 3D Printing and Additive Manufacturing: Principles and Applications (with Companion Media Pack) of Rapid Prototyping, 4th ed.; World Scientific Publishing Company: Singapur, Singapore, 2014. [Google Scholar]
- Gibson, I. Advanced Manufacturing Technology for Medical Applications: Reverse Engineering, Software Conversion and Rapid Prototyping; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Huiskes, R.; Weinans, H.; Van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Rel. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- Wieding, J.; Wolf, A.; Bader, R. Numerical optimization of open-porous bone scaffold structures to match the elastic properties of human cortical bone. J. Mech. Behav. Biomed. Mater. 2014, 37, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Yavari, S.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A. Additively manufactured open-cell porous biomaterials made from six different space-filling unit cells: The mechanical and morphological properties. Materials 2015, 8, 1871–1896. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, F.S.L.; Lietaert, K.; Eftekhari, A.A.; Pouran, B.; Ahmadi, S.M.; Weinans, H.; Zadpoor, A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017, 53, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Onal, E.; Frith, J.E.; Jurg, M.; Wu, X.; Molotnikov, A. Mechanical Properties and in Vitro Behavior of Additively Manufactured and Functionally Graded Ti6Al4V Porous Scaffolds. Metals 2018, 8, 200. [Google Scholar] [CrossRef]
- Song, C.-H. Study on Digital Design and Direct Manufacturing of Customized Implant Based on Selective Laser Melting. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2014. [Google Scholar]
- Stoffelen, D.V.; Eraly, K.; Debeer, P. The use of 3D printing technology in reconstruction of a severe glenoid defect: A case report with 2.5 years of follow-up. J. Shoulder Elbow Surg. 2015, 24, e218–e222. [Google Scholar] [CrossRef]
- Spetzger, U.; Frasca, M.; König, S.A. Surgical planning, manufacturing and implantation of an individualized cervical fusion titanium cage using patient-specific data. Eur. Spine J. 2016, 25, 2239–2246. [Google Scholar] [CrossRef]
- Fan, H.; Fu, J.; Li, X.; Pei, Y.; Li, X.; Pei, G.; Guo, Z. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: A case series and review of the literature. World J. Surg. Oncol. 2015, 13, 308. [Google Scholar] [CrossRef]
- Utela, B.; Storti, D.; Anderson, R.; Ganter, M. A review of process development steps for new material systems in three dimensional printing (3DP). J. Manuf. Process. 2008, 10, 96–104. [Google Scholar] [CrossRef]
- Guo, C.; Ge, W.; Lin, F. Effects of scanning parameters on material deposition during Electron Beam Selective Melting of Ti-6Al-4V powder. J. Manuf. Process. Technol. 2015, 217, 148–157. [Google Scholar] [CrossRef]
- Basalah, A.; Esmaeili, S.; Toyserkani, E. On the influence of sintering protocols and layer thickness on the physical and mechanical properties of additive manufactured titanium porous bio-structures. J. Mater. Process. Technol. 2016, 238, 341–351. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, J.-B.; Luo, Z.-Y.; Wang, D. Accuracy and density optimization in directly fabricating customized orthodontic production by selective laser melting. Rapid Prototyp. J. 2012, 18, 482–489. [Google Scholar] [CrossRef]
- Berretta, S.; Evans, K.; Ghita, O. Additive manufacture of PEEK cranial implants: Manufacturing considerations versus accuracy and mechanical performance. Mater. Des. 2018, 139, 141–152. [Google Scholar] [CrossRef]
- Jande, Y.A.; Erdal, M.; Dag, S. Production of graded porous polyamide structures and polyamide-epoxy composites via selective laser sintering. J. Reinf. Plast. Compos. 2014, 33, 1017–1036. [Google Scholar] [CrossRef]
- Miron-Borzan, C.S.; Dudescu, M.C.; Berce, P. Bending and compression tests for PA 2200 parts obtained using Selective Laser Sintering method. In Proceedings of the 4th International Conference on Computing and Solutions in Manufacturing Engineering 2016–CoSME’16, Braşov, Romania, 3–4 November 2016; Oancea, G., Dragoi, M.V., Eds.; EDP Sciences: Les Ulis, France, 2017; Volume 94, p. 03010. [Google Scholar]
- Vanderesse, N.; Ky, I.; González, F.Q.; Nuño, N.; Bocher, P. Image analysis characterization of periodic porous materials produced by additive manufacturing. Mater. Des. 2016, 92, 767–778. [Google Scholar] [CrossRef]
- Weißmann, V.; Bader, R.; Hansmann, H.; Laufer, N. Influence of the structural orientation on the mechanical properties of selective laser melted Ti6Al4V open-porous scaffolds. Mater. Des. 2016, 95, 188–197. [Google Scholar] [CrossRef]
- Yavari, S.A.; van der Stok, J.; Ahmadi, S.; Wauthlé, R.; Schrooten, J.; Weinans, H.; Zadpoor, A. Mechanical analysis of a rodent segmental bone defect model: The effects of internal fixation and implant stiffness on load transfer. J. Biomech. 2014, 47, 2700–2708. [Google Scholar] [CrossRef]
- Van Hooreweder, B.; Lietaert, K.; Neirinck, B.; Lippiatt, N.; Wevers, M. CoCr F75 scaffolds produced by additive manufacturing: Influence of chemical etching on powder removal and mechanical performance. J. Mech. Behav. Biomed. Mater. 2017, 70, 60–67. [Google Scholar] [CrossRef]
- Ahn, Y.-K.; Kim, H.-G.; Park, H.-K.; Kim, G.-H.; Jung, K.-H.; Lee, C.-W.; Kim, W.-Y.; Lim, S.-H.; Lee, B.-S. Mechanical and microstructural characteristics of commercial purity titanium implants fabricated by electron-beam additive manufacturing. Mater. Lett. 2017, 187, 64–67. [Google Scholar] [CrossRef]
- Mohammadhosseini, A.; Masood, S.; Fraser, D.; Jahedi, M. Dynamic compressive behaviour of Ti-6Al-4V alloy processed by electron beam melting under high strain rate loading. Adv. Manuf. 2015, 3, 232–243. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Krishna, B.V.; Xue, W.; Bose, S. Application of laser engineered net shaping (LENS) to manufacture porous and functionally graded structures for load bearing implants. J. Mater. Sci. Mater. Med. 2009, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Bertol, L.S.; Júnior, W.K.; Silva, F.P.d.; Aumund-Kopp, C. Medical design: Direct metal laser sintering of Ti–6Al–4V. Mater. Des. 2010, 31, 3982–3988. [Google Scholar] [CrossRef]
- George, N.; Nair, A.B. Porous tantalum: A new biomaterial in orthopedic surgery. In Fundamental Biomaterials: Metals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 243–268. [Google Scholar]
- Baltzer, N.; Copponnex, T. Properties and processing of precious metal alloys for biomedical applications. In Precious Metals for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–36. [Google Scholar]
- Zhang, X.; Li, X.-W.; Li, J.-G.; Sun, X.-D. Preparation and mechanical property of a novel 3D porous magnesium scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.G.; Monguzzi, L.; Previtali, B. Selective laser melting of pure Zn with high density for biodegradable implant manufacturing. Addit. Manuf. 2017, 15, 20–28. [Google Scholar] [CrossRef]
- Branemark, R.; Branemark, P.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J. Rehabil. Res. Dev. 2001, 38, 175–182. [Google Scholar]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, Y.; Wu, S.; Lin, H.; Yang, Y.; Fan, S.; Gu, C.; Wang, J.; Song, C. Customized a Ti6Al4V bone plate for complex pelvic fracture by selective laser melting. Materials 2017, 10, 35. [Google Scholar] [CrossRef]
- Patel, N.R.; Gohil, P.P. A review on biomaterials: Scope, applications & human anatomy significance. Int. J. Emerg. Technol. Adv. Eng. 2012, 2, 91–101. [Google Scholar]
- Sumitomo, N.; Noritake, K.; Hattori, T.; Morikawa, K.; Niwa, S.; Sato, K.; Niinomi, M. Experiment study on fracture fixation with low rigidity titanium alloy. J. Mater. Sci. Mater. Med. 2008, 19, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Oldani, C.; Dominguez, A. Titanium as a Biomaterial for Implants. Recent Adv. Arthroplast. 2012, 218, 149–162. [Google Scholar]
- Kobayashi, E.; Ando, M.; Tsutsumi, Y.; Doi, H.; Yoneyama, T.; Kobayashi, M.; Hanawa, T. Inhibition effect of zirconium coating on calcium phosphate precipitation of titanium to avoid assimilation with bone. Mater. Trans. 2007, 48, 301–306. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Gjunter, V.; Sysoliatin, P.; Temerkhamor, T. Superelastic Shape Memory Implants in Maxillofacial Surgery, Traumatology, Orthopaedics and Neurosurgery; Tomsk University Publishing House: Tomsk, Russia, 1995; Volume 15. [Google Scholar]
- Rahmanian, R.; Moghaddam, N.S.; Haberland, C.; Dean, D.; Miller, M.; Elahinia, M. Load Bearing and Stiffness Tailored NiTi Implants Produced by Additive Manufacturing: A Simulation Study; SPIE: Bellingham, WA, USA, 2014; Volume 9058. [Google Scholar]
- Pawlak, A.; Szymczyk, P.; Ziolkowski, G.; Chlebus, E.; Dybala, B. Fabrication of microscaffolds from Ti-6Al-7Nb alloy by SLM. Rapid Prototyp. J. 2015, 21, 393–401. [Google Scholar] [CrossRef]
- Szymczyk, P.; Ziółkowski, G.; Junka, A.; Chlebus, E. Application of Ti6Al7Nb alloy for the manufacture of Biomechanical Functional Structures (BFS) for custom-made bone implants. Materials 2018, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Laheurte, P.; Acquier, P.; Joguet, D.; Peltier, L.; Petithory, T.; Anselme, K.; Mille, P. Synthesis and characterization of Ti-27.5 Nb alloy made by CLAD® additive manufacturing process for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 341–348. [Google Scholar] [CrossRef]
- Schulze, C.; Weinmann, M.; Schweigel, C.; Keßler, O.; Bader, R. Mechanical properties of a newly additive manufactured implant material based on Ti-42Nb. Materials 2018, 11, 124. [Google Scholar] [CrossRef]
- Alvarado, J.; Maldonado, R.; Marxuach, J.; Otero, R. Biomechanics of hip and knee prostheses. In Applications of Engineering Mechanics in Medicine; GED–University of Puerto Rico Mayaguez: Mayaguez, Puerto, 2003; pp. 1–20. [Google Scholar]
- Stenlund, P.; Kurosu, S.; Koizumi, Y.; Suska, F.; Matsumoto, H.; Chiba, A.; Palmquist, A. Osseointegration enhancement by Zr doping of Co-Cr-Mo implants fabricated by electron beam melting. Addit. Manuf. 2015, 6, 6–15. [Google Scholar] [CrossRef]
- Dewidar, M.M.; Khalil, K.A.; Lim, J. Processing and mechanical properties of porous 316L stainless steel for biomedical applications. Trans. Nonferr. Met. Soc. China 2007, 17, 468–473. [Google Scholar] [CrossRef]
- Jandin, G.; Bertin, J.; Dembinski, L.; Coddet, C. Manufacture of stainless steel parts by selective laser melting process. In Proceedings of the 2nd International Conference on Advanced Research in Virtual and Rapid Prototyping, Leiria, Portugal, 28 September–1 October 2005; Taylor & Francis Group: London, UK, 2005; pp. 431–434. [Google Scholar]
- Čapek, J.; Machova, M.; Fousova, M.; Kubásek, J.; Vojtěch, D.; Fojt, J.; Jablonska, E.; Lipov, J.; Ruml, T. Highly porous, low elastic modulus 316L stainless steel scaffold prepared by selective laser melting. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Wehmöller, M.; Warnke, P.; Zilian, C.; Eufinger, H. Implant design and production—A new approach by selective laser melting. In Proceedings of the International Congress Series, Berlin, Germany, 22–25 June 2005; Elsevier: Amsterdam, The Netherlands, 2005; Volume 1281, pp. 690–695. [Google Scholar]
- Perry, C. Biomaterials—A Tantalus experience. Mater. Today 2011, 14, 230. [Google Scholar] [CrossRef]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef] [PubMed]
- Sungail, C.; Abid, A. Spherical tantalum feed powder for metal additive manufacturing. Met. Powder Rep. 2018, 73, 316–318. [Google Scholar] [CrossRef]
- Wauthle, R.; Van Der Stok, J.; Yavari, S.A.; Van Humbeeck, J.; Kruth, J.-P.; Zadpoor, A.A.; Weinans, H.; Mulier, M.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Givan, D.A. Precious metals in dentistry. Dent. Clin. N. Am. 2007, 51, 591–601. [Google Scholar] [CrossRef]
- Wataha, J.C. Alloys for prosthodontic restorations. J. Prosthet. Dent. 2002, 87, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Cart, A.B.; Brantley, W.A. New High-Palladium Casting Alloys: Part 1. Overview and Initial Studies. Int. J. Prosthodont. 1991, 4, 265–275. [Google Scholar]
- Berzins, D.; Kawashima, I.; Graves, R.; Sarkar, N. Heat treatment effects on electrochemical corrosion parameters of high-Pd alloys. J. Mater. Sci. Mater. Med. 2008, 19, 335–341. [Google Scholar] [CrossRef]
- Garau, V.; Masala, M.G.; Cortis, M.C.; Pittau, R. Contact stomatitis due to palladium in dental alloys: A clinical report. J. Prosthet. Dent. 2005, 93, 318–320. [Google Scholar] [CrossRef]
- Muris, J.; Feilzer, A.; Rustemeyer, T.; Kleverlaan, C. Palladium allergy prevalence is underestimated because of an inadequate test allergen. Contact Dermat. 2011, 65, 62. [Google Scholar] [CrossRef]
- Givan, D. Precious metal alloys for dental applications. In Precious Metals for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 109–129. [Google Scholar]
- Kaminski, R.A.; Anusavice, K.; Okabe, T.; Morse, P.; Casteel, P. Castability of silver-base fixed partial denture alloys. J. Prosthet. Dent. 1985, 53, 329–332. [Google Scholar] [CrossRef]
- Revell, P.A.; Damien, E.; Zhang, X.; Evans, P.; Howlett, C.R. The effect of magnesium ions on bone bonding to hydroxyapatite coating on titanium alloy implants. In Key Engineering Materials, Proceedings of the 16th International Symposium on Ceramics in Medicine, Porto, Portugal, 6–9 November 2003; Trans Tech Publications: Zürich, Switzerland, 2004; Volume 254, pp. 447–450. [Google Scholar]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Chung Ng, C.; Savalani, M.; Chung Man, H. Fabrication of magnesium using selective laser melting technique. Rapid Prototyp. J. 2011, 17, 479–490. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tümer, N.; Schröder, K.U.; Mol, J.M.C.; Weinans, H.; et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Huang, Y.; Zhou, L.; Hort, N.; Kainer, K.U. Preparation and properties of high purity Mg–Y biomaterials. Biomaterials 2010, 31, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Hort, N.; Huang, Y.; Fechner, D.; Störmer, M.; Blawert, C.; Witte, F.; Vogt, C.; Drücker, H.; Willumeit, R.; Kainer, K. Magnesium alloys as implant materials—Principles of property design for Mg–RE alloys. Acta Biomater. 2010, 6, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wu, G.; Gao, A.; Feng, H.; Peng, X.; Chu, P.K. Hafnium-implanted WE43 magnesium alloy for enhanced corrosion protection and biocompatibility. Surf. Coat. Technol. 2016, 306, 11–15. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M. Biodegradable Fe-based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.; Leeflang, M.; Pouran, B.; Gonzalez-Garcia, Y.; Weinans, H. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393. [Google Scholar] [CrossRef]
- Francis, A.; Yang, Y.; Virtanen, S.; Boccaccini, A. Iron and iron-based alloys for temporary cardiovascular applications. J. Mater. Sci. Mater. Med. 2015, 26, 138. [Google Scholar] [CrossRef] [PubMed]
- He, J.; He, F.-L.; Li, D.-W.; Liu, Y.-L.; Liu, Y.-Y.; Ye, Y.-J.; Yin, D.-C. Advances in Fe-based biodegradable metallic materials. RSC Adv. 2016, 6, 112819–112838. [Google Scholar] [CrossRef]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, P.; Voshage, M.; Jauer, L.; Chen, Y.; Qin, Y.; Poprawe, R.; Schleifenbaum, J.H. Laser additive manufacturing of Zn metal parts for biodegradable applications: Processing, formation quality and mechanical properties. Mater. Des. 2018, 155, 36–45. [Google Scholar] [CrossRef]
- Wen, P.; Jauer, L.; Voshage, M.; Chen, Y.; Poprawe, R.; Schleifenbaum, J.H. Densification behavior of pure Zn metal parts produced by selective laser melting for manufacturing biodegradable implants. J. Mater. Process. Technol. 2018, 258, 128–137. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.; Previtali, B.; Mantovani, D.; Beanland, R.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, H.; Niu, J.; Zhang, L.; Zhang, H.; Pei, J.; Tan, J.; Yuan, G. Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater. Des. 2017, 117, 84–94. [Google Scholar] [CrossRef]
- Katarivas Levy, G.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants—A review paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]
- Béreš, M.; Silva, C.; Sarvezuk, P.; Wu, L.; Antunes, L.; Jardini, A.; Feitosa, A.; Žilková, J.; de Abreu, H. Mechanical and phase transformation behaviour of biomedical Co-Cr-Mo alloy fabricated by direct metal laser sintering. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2018, 714, 36–42. [Google Scholar] [CrossRef]
- Cowley, A. A healthy future: platinum in medical applications. Platin. Met. Rev. 2011, 55, 98–107. [Google Scholar] [CrossRef]
- Wataha, J.C.; Shor, K. Palladium alloys for biomedical devices. Expert Rev. Med. Devices 2010, 7, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Avedesian, M.M.; Baker, H. ASM Specialty Handbook: Magnesium and Magnesium Alloys; ASM International: Geauga County, OH, USA, 1999. [Google Scholar]
- Chmelik, F.; Trojanova, Z.; Lukáč, P.; Převorovský, Z. Acoustic emission from zinc deformed at room temperature Part I The influence of strain rate on deformation behaviour and acoustic emission in pure zinc. J. Mater. Sci. Lett. 1993, 12, 1086–1087. [Google Scholar] [CrossRef]
- Radovan, H.; Jozef, Ž.; Teodor, T.; Jaroslav, M.; Martin, L. Evaluation of custom-made implants using industrial computed tomography. In Proceedings of the 10th International Conference on Digital Technologies, Zilina, Slovakia, 9–11 July 2014; pp. 82–86. [Google Scholar]
- Wubneh, A.; Tsekoura, E.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Campoli, G.; Weinans, H.; Zadpoor, A.A. Computational load estimation of the femur. J. Mech. Behav. Biomed. Mater. 2012, 10, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef]
- Maskery, I.; Aremu, A.O.; Simonelli, M.; Tuck, C.; Wildman, R.D.; Ashcroft, I.A.; Hague, R.J.M. Mechanical Properties of Ti-6Al-4V Selectively Laser Melted Parts with Body-Centred-Cubic Lattices of Varying cell size. Exp. Mech. 2015, 55, 1261–1272. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Montaño, Ó.L.; Cortés-Rodríguez, C.J.; Uva, A.E.; Fiorentino, M.; Gattullo, M.; Monno, G.; Boccaccio, A. Comparison of the mechanobiological performance of bone tissue scaffolds based on different unit cell geometries. J. Mech. Behav. Biomed. Mater. 2018, 83, 28–45. [Google Scholar] [CrossRef]
- Mazur, M.; Leary, M.; Sun, S.; Vcelka, M.; Shidid, D.; Brandt, M. Deformation and failure behaviour of Ti-6Al-4V lattice structures manufactured by selective laser melting (SLM). Int. J. Adv. Manuf. Technol. 2016, 84, 1391–1411. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, D.; Zhou, Y.; Wang, W.; Cao, X. Study on topology optimization design, manufacturability, and performance evaluation of Ti-6Al-4V porous structures fabricated by selective laser melting (SLM). Materials 2017, 10, 1048. [Google Scholar]
- Chantarapanich, N.; Puttawibul, P.; Sucharitpwatskul, S.; Jeamwatthanachai, P.; Inglam, S.; Sitthiseripratip, K. Scaffold library for tissue engineering: A geometric evaluation. Comput. Math. Method Med. 2012, 2012, 14. [Google Scholar] [CrossRef] [PubMed]
- Bucklen, B.S.; Wettergreen, W.A.; Yuksel, E.; Liebschner, M.A.K. Bone-derived CAD library for assembly of scaffolds in computer-aided tissue engineering. Virtual. Phys. Prototy. 2008, 3, 13–23. [Google Scholar] [CrossRef]
- Ushijima, K.; Cantwell, W.J.; Mines, R.A.W.; Tsopanos, S.; Smith, M. An investigation into the compressive properties of stainless steel micro-lattice structures. J. Sandw. Struct. Mater. 2011, 13, 303–329. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Z.; Lu, Z.; Liao, B.; He, X. Effective elastic properties and initial yield surfaces of two 3D lattice structures. Int. J. Mech. Sci. 2018, 138, 146–158. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Campoli, G.; Yavari, S.A.; Sajadi, B.; Wauthle, R.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Mechanical behavior of regular open-cell porous biomaterials made of diamond lattice unit cells. J. Mech. Behav. Biomed. Mater. 2014, 34, 106–115. [Google Scholar] [CrossRef]
- Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. The effect of 3D-printed Ti6Al4V scaffolds with various macropore structures on osteointegration and osteogenesis: A biomechanical evaluation. J. Mech. Behav. Biomed. Mater. 2018, 88, 488–496. [Google Scholar] [CrossRef]
- Choy, S.Y.; Sun, C.-N.; Leong, K.F.; Wei, J. Compressive properties of Ti-6Al-4V lattice structures fabricated by selective laser melting: Design, orientation and density. Addit. Manuf. 2017, 16, 213–224. [Google Scholar] [CrossRef]
- Guo, X.-F.; Ma, L. Periodic topological lattice with different indentation hardness on opposite surfaces. Mater. Des. 2019, 180, 107953. [Google Scholar] [CrossRef]
- Reznikov, N.; Chase, H.; Zvi, Y.B.; Tarle, V.; Singer, M.; Brumfeld, V.; Shahar, R.; Weiner, S. Inter-trabecular angle: A parameter of trabecular bone architecture in the human proximal femur that reveals underlying topological motifs. Acta Biomater. 2016, 44, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jetté, B.; Brailovski, V.; Dumas, M.; Simoneau, C.; Terriault, P. Femoral stem incorporating a diamond cubic lattice structure: Design, manufacture and testing. J. Mech. Behav. Biomed. Mater. 2018, 77, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, D.Z.; Zhang, P.; Zhao, M.; Jafar, S. Mechanical Properties of Optimized Diamond Lattice Structure for Bone Scaffolds Fabricated via Selective Laser Melting. Materials 2018, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Soro, N.; Attar, H.; Wu, X.; Dargusch, M.S. Investigation of the structure and mechanical properties of additively manufactured Ti-6Al-4V biomedical scaffolds designed with a Schwartz primitive unit-cell. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2019, 745, 195–202. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Fraser, D.; Song, G.; Wen, C. Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications. Mater. Des. 2018, 137, 345–354. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Brandt, M.; Wen, C. Ultrahigh-strength titanium gyroid scaffolds manufactured by selective laser melting (SLM) for bone implant applications. Acta Mater. 2018, 158, 354–368. [Google Scholar] [CrossRef]
- Yoo, D.J. Porous scaffold design using the distance field and triply periodic minimal surface models. Biomaterials 2011, 32, 7741–7754. [Google Scholar] [CrossRef] [PubMed]
- Al-Ketan, O.; Abu Al-Rub, R.K.; Rowshan, R. Mechanical Properties of a New Type of Architected Interpenetrating Phase Composite Materials. Adv. Mater. Technol. 2017, 2, 1600235. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Rowshan, R.; Abu Al-Rub, R.K. Topology-mechanical property relationship of 3D printed strut, skeletal, and sheet based periodic metallic cellular materials. Addit. Manuf. 2018, 19, 167–183. [Google Scholar] [CrossRef]
- Yang, L.; Yan, C.; Han, C.; Chen, P.; Yang, S.; Shi, Y. Mechanical response of a triply periodic minimal surface cellular structures manufactured by selective laser melting. Int. J. Mech. Sci. 2018, 148, 149–157. [Google Scholar] [CrossRef]
- Tang, Y.; Kurtz, A.; Zhao, Y.F. Bidirectional Evolutionary Structural Optimization (BESO) based design method for lattice structure to be fabricated by additive manufacturing. Comput. Aided Des. 2015, 69, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Zegard, T.; Paulino, G.H. Bridging topology optimization and additive manufacturing. Struct. Multidiscip. Optim. 2016, 53, 175–192. [Google Scholar] [CrossRef]
- Maheshwaraa Namasivayam, U.; Conner Seepersad, C. Topology design and freeform fabrication of deployable structures with lattice skins. Rapid Prototyp. J. 2011, 17, 5–16. [Google Scholar] [CrossRef]
- Huang, X.; Xie, Y. Bidirectional Evolutionary Topology Optimization for Structures with Geometrical and Material Nonlinearities. AIAA J. 2006, 45, 308–313. [Google Scholar] [CrossRef]
- Huang, X.; Radman, A.; Xie, Y. Topological design of microstructures of cellular materials for maximum bulk or shear modulus. Comput. Mater. Sci. 2011, 50, 1861–1870. [Google Scholar]
- Burblies, A.; Busse, M. Computer based porosity design by multi phase topology optimization. In Multiscale and Functionally Graded Materials, Oahu Island, HI, USA, 15–18 October 2006; Paulino, G.H., Pindera, M.J., Dodds, R.H., Rochinha, F.A., Dave, E.V., Chen, L., Eds.; American Institute of Physics: College Park, MD, USA, 2008; Volume 973, pp. 285–290. [Google Scholar]
- Surmeneva, M.A.; Surmenev, R.A.; Chudinova, E.A.; Koptioug, A.; Tkachev, M.S.; Gorodzha, S.N.; Rännar, L.-E. Fabrication of multiple-layered gradient cellular metal scaffold via electron beam melting for segmental bone reconstruction. Mater. Des. 2017, 133, 195–204. [Google Scholar] [CrossRef]
- Choy, S.Y.; Sun, C.-N.; Leong, K.F.; Wei, J. Compressive properties of functionally graded lattice structures manufactured by selective laser melting. Mater. Des. 2017, 131, 112–120. [Google Scholar] [CrossRef]
- Liu, F.; Mao, Z.; Zhang, P.; Zhang, D.Z.; Jiang, J.; Ma, Z. Functionally graded porous scaffolds in multiple patterns: New design method, physical and mechanical properties. Mater. Des. 2018, 160, 849–860. [Google Scholar] [CrossRef]
- Li, S.; Zhao, S.; Hou, W.; Teng, C.; Hao, Y.; Li, Y.; Yang, R.; Misra, R.D.K. Functionally Graded Ti-6Al-4V Meshes with High Strength and Energy Absorption. Adv. Eng. Mater. 2015, 18, 34–38. [Google Scholar] [CrossRef]
- Chung, H.; Das, S. Functionally graded Nylon-11/silica nanocomposites produced by selective laser sintering. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2008, 487, 251–257. [Google Scholar] [CrossRef]
- Dumas, M.; Terriault, P.; Brailovski, V. Modelling and characterization of a porosity graded lattice structure for additively manufactured biomaterials. Mater. Des. 2017, 121, 383–392. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Fang, G.; Xing, L.-L.; Liu, W.; Zhou, J. Effect of porosity variation strategy on the performance of functionally graded Ti-6Al-4V scaffolds for bone tissue engineering. Mater. Des. 2018, 157, 523–538. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Fang, G.; Leeflang, S.; Zadpoor, A.A.; Zhou, J. Topological design, permeability and mechanical behavior of additively manufactured functionally graded porous metallic biomaterials. Acta Biomater. 2019, 84, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Maskery, I.; Aremu, A.O.; Parry, L.; Wildman, R.D.; Tuck, C.J.; Ashcroft, I.A. Effective design and simulation of surface-based lattice structures featuring volume fraction and cell type grading. Mater. Des. 2018, 155, 220–232. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Lei, Z.; Zhang, S.; Fuh, J.Y.H.; Wen, F.L. Triply Periodic Minimal Surfaces Sheet Scaffolds for Tissue Engineering Applications: An Optimization Approach towards Biomimetic Scaffold Design. ACS Appl. Bio Mater. 2018, 1, 259–269. [Google Scholar] [CrossRef]
- Yoo, D.J. Heterogeneous porous scaffold design for tissue engineering using triply periodic minimal surfaces. Int. J. Precis. Eng. Manuf. 2012, 13, 527–537. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Wang, Q.; Wen, S.; Wei, Q.; Yan, C.; Hao, L.; Liu, J.; Shi, Y. Continuous functionally graded porous titanium scaffolds manufactured by selective laser melting for bone implants. J. Mech. Behav. Biomed. Mater. 2018, 80, 119–127. [Google Scholar] [CrossRef]
- Yang, L.; Mertens, R.; Ferrucci, M.; Yan, C.; Shi, Y.; Yang, S. Continuous graded Gyroid cellular structures fabricated by selective laser melting: Design, manufacturing and mechanical properties. Mater. Des. 2019, 162, 394–404. [Google Scholar] [CrossRef]
- Afshar, M.; Anaraki, A.P.; Montazerian, H. Compressive characteristics of radially graded porosity scaffolds architectured with minimal surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 254–267. [Google Scholar] [CrossRef]
- Feng, J.W.; Fu, J.Z.; Shang, C.; Lin, Z.W.; Li, B. Porous scaffold design by solid T-splines and triply periodic minimal surfaces. Comput. Meth. Appl. Mech. Eng. 2018, 336, 333–352. [Google Scholar] [CrossRef]
- Sudarmadji, N.; Tan, J.Y.; Leong, K.F.; Chua, C.K.; Loh, Y.T. Investigation of the mechanical properties and porosity relationships in selective laser-sintered polyhedral for functionally graded scaffolds. Acta Biomater. 2011, 7, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.; Trueba, P.; Pavon, J.J.; Chicardi, E.; Kamm, P.; Garcia-Moreno, F.; Rodriguez-Ortiz, J.A. Design, processing and characterization of titanium with radial graded porosity for bone implants. Mater. Des. 2016, 110, 179–187. [Google Scholar] [CrossRef]
- Svehla, M.; Morberg, P.; Zicat, B.; Bruce, W.; Sonnabend, D.; Walsh, W.R. Morphometric and mechanical evaluation of titanium implant integration: Comparison of five surface structures. J. Biomed. Mater. Res. Part B 2000, 51, 15–22. [Google Scholar] [CrossRef]
- Heinl, P.; Müller, L.; Körner, C.; Singer, R.F.; Müller, F.A. Cellular Ti–6Al–4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Korobkova, A.; Kazakbiev, A.; Zhukova, Y.; Sheremetyev, V.; Dubinskiy, S.; Filonov, M. Surface treatment of bulk and porous materials based on superelastic titanium alloys for medical implants. Mater. Today Proc. 2017, 4, 4664–4669. [Google Scholar] [CrossRef]
- Yavari, S.A.; van der Stok, J.; Chai, Y.C.; Wauthle, R.; Birgani, Z.T.; Habibovic, P.; Mulier, M.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Bone regeneration performance of surface-treated porous titanium. Biomaterials 2014, 35, 6172–6181. [Google Scholar] [CrossRef] [PubMed]
- Larsson Wexell, C.; Thomsen, P.; Aronsson, B.-O.; Tengvall, P.; Rodahl, M.; Lausmaa, J.; Kasemo, B.; Ericson, L. Bone response to surface-modified titanium implants: Studies on the early tissue response to implants with different surface characteristics. Int. J. Biomater. 1996, 17, 605–616. [Google Scholar] [CrossRef]
- Li, G.; Cao, H.; Zhang, W.; Ding, X.; Yang, G.; Qiao, Y.; Liu, X.; Jiang, X. Enhanced osseointegration of hierarchical micro/nanotopographic titanium fabricated by microarc oxidation and electrochemical treatment. ACS Appl. Mater. Interfaces 2016, 8, 3840–3852. [Google Scholar] [CrossRef]
- Shi, X.; Nakagawa, M.; Kawachi, G.; Xu, L.; Ishikawa, K. Surface modification of titanium by hydrothermal treatment in Mg-containing solution and early osteoblast responses. J. Mater. Sci. Mater. Med. 2012, 23, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, C.; Xia, W.; Engqvist, H.; Snis, A.; Lausmaa, J.; Palmquist, A. Biomimetic calcium phosphate coating of additively manufactured porous CoCr implants. Appl. Surf. Sci. 2015, 353, 40–47. [Google Scholar] [CrossRef]
- Stuebinger, S.; Nuss, K.; Buerki, A.; Mosch, I.; le Sidler, M.; Meikle, S.T.; von Rechenberg, B.; Santin, M. Osseointegration of titanium implants functionalised with phosphoserine-tethered poly(epsilon-lysine) dendrons: A comparative study with traditional surface treatments in sheep. J. Mater. Sci. Mater. Med. 2015, 26, 87. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Hempel, U.; Zydek, J.; Vladescu, A.; Pietryga, K.; Kaeswurm, J.A.H.; Buchweitz, M.; Surmenev, R.A.; Surmeneva, M.A.; Cotrut, C.M.; et al. Pectin coatings on titanium alloy scaffolds produced by additive manufacturing: Promotion of human bone marrow stromal cell proliferation. Mater. Lett. 2018, 227, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Trujillo, C.; Ternero, F.; Antonio Rodriguez-Ortiz, J.; Jose Pavon, J.; Montealegre-Melendez, I.; Arevalo, C.; Garcia-Moreno, F.; Torres, Y. Improvement of the balance between a reduced stress shielding and bone rit ingrowth by bioactive coatings onto porous titanium substrates. Surf. Coat. Technol. 2018, 338, 32–37. [Google Scholar] [CrossRef]
- Rifai, A.; Nhiem, T.; Lau, D.W.; Elbourne, A.; Zhan, H.; Stacey, A.D.; Mayes, E.L.H.; Sarker, A.; Ivanova, E.P.; Crawford, R.J.; et al. Polycrystalline Diamond Coating of Additively Manufactured Titanium for Biomedical Applications. ACS Appl. Mater. Interfaces 2018, 10, 8474–8484. [Google Scholar] [CrossRef] [PubMed]
- Zebrowski, R.; Walczak, M.; Klepka, T.; Pasierbiewicz, K. Effect of the shot peening on surface properties of ti-6al-4v alloy produced by means of DMLS technology. Eksploat. Niezawodn. 2019, 21, 46–53. [Google Scholar] [CrossRef]
- Bagherifard, S.; Hickey, D.J.; de Luca, A.C.; Malheiro, V.N.; Markaki, A.E.; Guagliano, M.; Webster, T.J. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials 2015, 73, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Andani, M.T.; Qin, H.; Moghaddam, N.S.; Ibrahim, H.; Jahadakbar, A.; Amerinatanzi, A.; Ren, Z.; Zhang, H.; Doll, G.L.; et al. Improving surface finish and wear resistance of additive manufactured nickel-titanium by ultrasonic nano-crystal surface modification. J. Mater. Process. Technol. 2017, 249, 433–440. [Google Scholar] [CrossRef]
- Hou, X.; Qin, H.; Gao, H.; Mankoci, S.; Zhang, R.; Zhou, X.; Ren, Z.; Doll, G.L.; Martini, A.; Sahai, N. A systematic study of mechanical properties, corrosion behavior and biocompatibility of AZ31B Mg alloy after ultrasonic nanocrystal surface modification. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1061–1071. [Google Scholar] [CrossRef]
- Robotti, F.; Bottan, S.; Fraschetti, F.; Mallone, A.; Pellegrini, G.; Lindenblatt, N.; Starck, C.; Falk, V.; Poulikakos, D.; Ferrari, A. A micron-scale surface topography design reducing cell adhesion to implanted materials. Sci. Rep. 2018, 8, 10887. [Google Scholar] [CrossRef]
- Han, E.D.; Kim, B.H.; Seo, Y.H. Anti-cell adhesion characteristics of nanotextured surface for implantable biomedical device. Int. J. Precis. Eng. Manuf. 2017, 18, 239–244. [Google Scholar] [CrossRef]
- Weißmann, V.; Boss, C.; Bader, R.; Hansmann, H. A novel approach to determine primary stability of acetabular press-fit cups. J. Mech. Behav. Biomed. Mater. 2018, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]






| Materials | Mechanical Property | Physicochemical Property | Material Degradability | Main Application | ||
|---|---|---|---|---|---|---|
| Young Modulus (GPa) | Yield Strength (MPa) | Tensile Strength (MPa) | ||||
| Pure Ti [34] | 105 | 695 | 785 | Superior biocompatibility, good corrosion resistance, and relatively inert | Non-degradable |
|
| Ti–6Al–4V alloy [34] | 110 | 850–900 | 960–970 | Good biocompatibility, good corrosion resistance, and relatively inert | Non-degradable |
|
| Co–Cr–Mo alloy [47,82] | 230 | 200–823 | 430–1028 | Little biological toxicity, excellent corrosion and wear resistance | Non-degradable |
|
| 316L stainless steel [47,49] | 193 | 290 | 579 | Acceptable biocompatibility and good corrosion resistance, bio-inert materials | Non-degradable |
|
| Tantalum metal [54,56] | 186 | 138–345 | 207–517 | Non-toxic, excellent biocompatibility, relatively inert, stable characteristic | Non-degradable |
|
| Gold [30,58] | 80 | 25 | 130 | Excellent biocompatibility, superior corrosion resistance, and completely unreactive | Non-degradable |
|
| Platinum metal [30,83] | 147 | 150 | 240 | Excellent biocompatibility, superior corrosion resistance, and strong chemic inertia | Non-degradable |
|
| Palladium metal [30,84] | 112 | 50 | 190 | Excellent biocompatibility, superior corrosion resistance, and strong chemic inertia | Non-degradable |
|
| Silver [30,63] | 76 | 28 | 150 | Good biocompatibility, good corrosion resistance, and relative chemic inertia | Non-degradable |
|
| Magnesium metal [71,85] | 44.2 | 162 | 250 | Outstanding biocompatibility, promoting bone tissue regeneration. Active nature, corrosive in a weak alkaline environment, and the corrosion product is non-toxic | Degradable, fast degradation rate |
|
| Iron metal [73] | 200 | 50 | 540 | Appropriate biocompatibility, less active properties, and slower corrosion dissolution | Degradable, slow degradation rate |
|
| Zinc metal [77,86] | 96.5 | 30 | 37 | Good biocompatibility, active nature, and corrosive dissolved substances in the body are not poisonous | Degradable, moderate degradation rate |
|
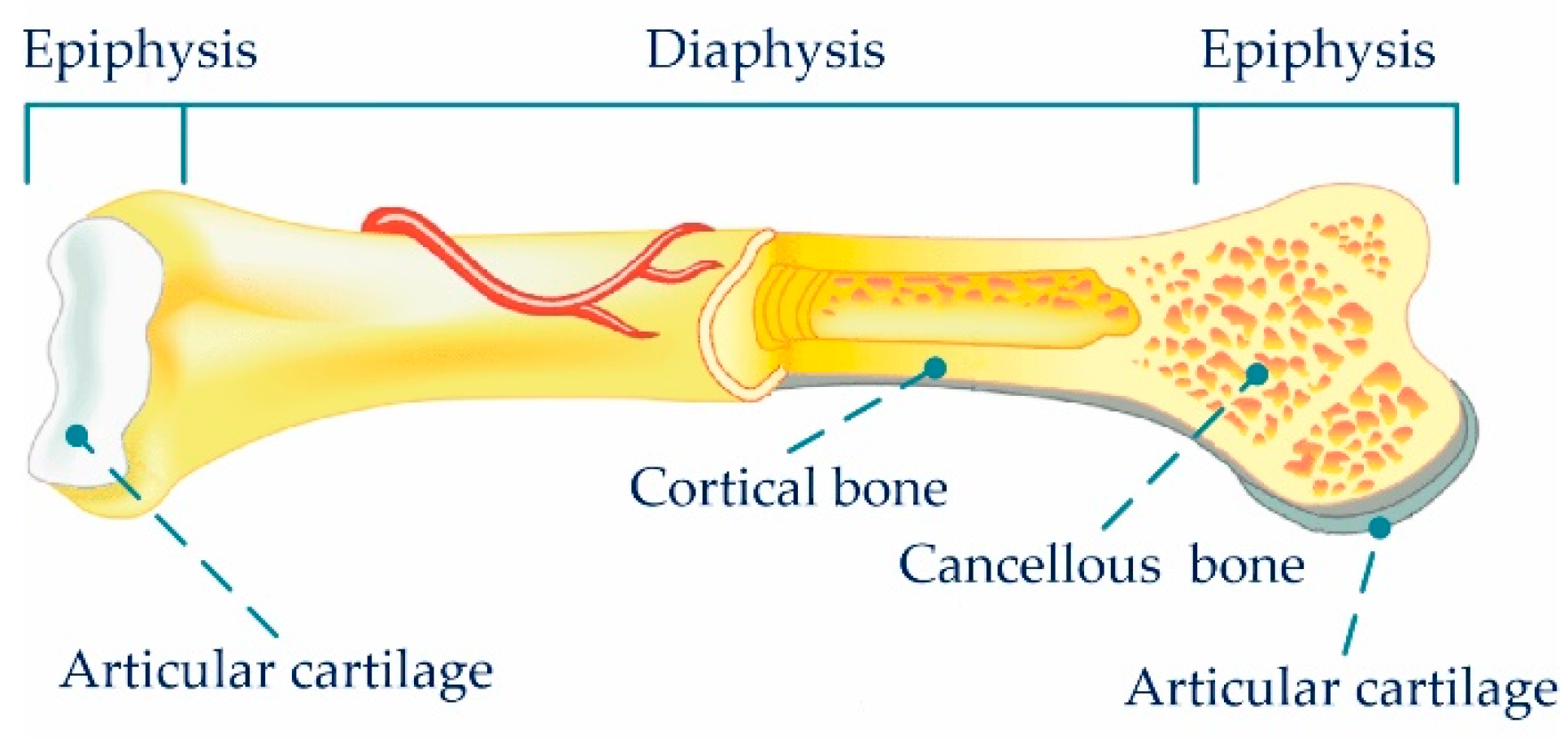
| Cortical bone [36] | 15–30 | 30–70 | 70–150 | null | ||
| Structure Types | Structure Characteristics | Materials/Methods | Process Attributes | |
|---|---|---|---|---|
| Lattice structure | Polyhedron | Based on polyhedral geometry, CAD modeling, simple structure, and predictable mechanical properties, most widely used. | Ti-6Al-7Nb/SLM [44] Ti-6Al-4V/SLM [103,104,107,108] 316L SS/SLM [100] | High resolution; good implant porosity; high strength, low ductility; good osteointegration. |
| Minimal surface | Based on implicit function, complex structure, high specific surface area, and high permeability, similar to trabecular bone. | Ti-6Al-4V/SLM [109,115] Ti-6Al-4V/EBM [110] Pure Ti/SLM [111] Ti-6Al-4V/SLS [114] | Fine molding quality; high porosity; good biocompatibility; excellent mechanical properties. | |
| Topology optimization design | Based on boundary conditions and optimization algorithms, smooth surface, and high strength, similar to natural bone structure. | Ti-6Al-4V/SLM [116] Pure Ti/SLM [121] | Fine molding quality; lightweight design; stable structure. | |
| Gradient structure | Vary in rod diameter | Varied the rod diameter, the transition of the longitudinal gradient is smooth, but the radial transition is abrupt. Relative density varies widely. | Ti-6Al-4V/SLM [127,128,129] | Obvious anisotropy; excellent energy absorption; appropriate modulus; good permeability. |
| Vary in cell size | Varied in cell size; non-stationary transitions between different layers. | Ti-6Al-4V/EBM [123] Ti-6Al-4V/SLM [124] | Fine molding quality; appropriate modulus; high energy absorption. | |
| Vary in cell types [130,131,137] | Varied in unit cell type, different areas have different topologies; non-stationary transitions between different layers. | Ti-6Al-4V/EBM [125] | High energy absorption; appropriate modulus; biomimetic bone structure. | |
| Vary in materials components [126,138] | Varied material components, different areas only show material changes, relatively stable structure, and significant compatibility between different layers. | PA 11/SLS [126] | Biomimetic graded porosity; appropriate modulus; stable microstructure. | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications. Metals 2019, 9, 1004. https://doi.org/10.3390/met9091004
Bai L, Gong C, Chen X, Sun Y, Zhang J, Cai L, Zhu S, Xie SQ. Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications. Metals. 2019; 9(9):1004. https://doi.org/10.3390/met9091004
Chicago/Turabian StyleBai, Long, Cheng Gong, Xiaohong Chen, Yuanxi Sun, Junfang Zhang, Lecai Cai, Shengyan Zhu, and Sheng Quan Xie. 2019. "Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications" Metals 9, no. 9: 1004. https://doi.org/10.3390/met9091004
APA StyleBai, L., Gong, C., Chen, X., Sun, Y., Zhang, J., Cai, L., Zhu, S., & Xie, S. Q. (2019). Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications. Metals, 9(9), 1004. https://doi.org/10.3390/met9091004








