Evaluation of Zeolite as a Potential Reactive Medium in a Permeable Reactive Barrier (PRB): Batch and Column Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
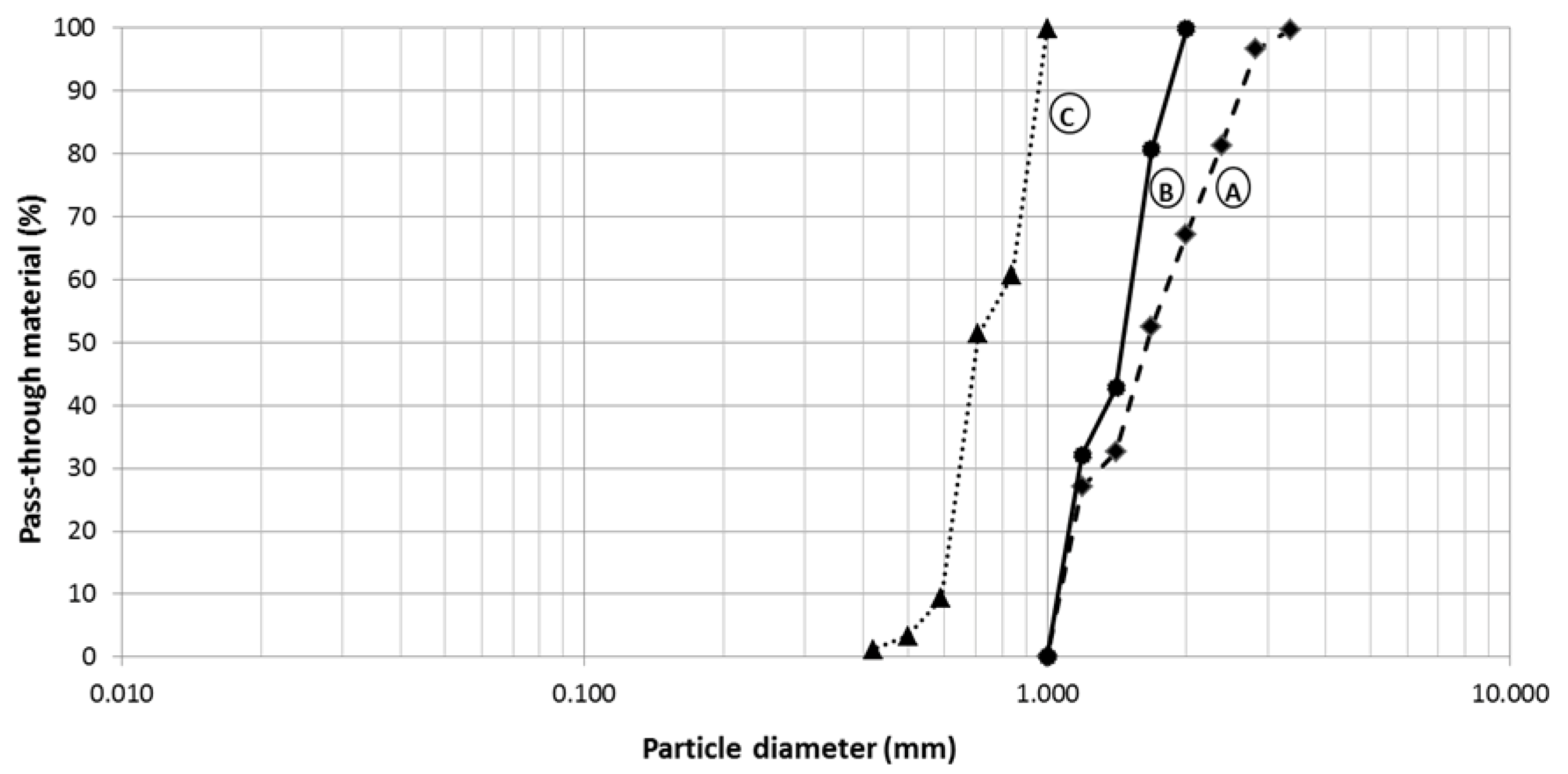
2.2. Characterization of the Zeolite
2.3. Mineralogical Characterization
2.4. Physicochemical and Chemical Characterization
2.5. Batch Studies
2.6. Column Experiments
2.7. Flushing Procedure
3. Results and Discussion
3.1. Basic Characterization of the Zeolite
3.2. Mineralogical and Chemical Characterization
3.3. Batch Studies
3.4. Kinetic Studies
3.5. Column Test Results
3.6. Flushing Results
3.7. Chemical Characterization after Sorption Processes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mizukawa, A.; Filippe, T.C.; Peixoto, L.O.M.; Scipioni, B.; Leonardi, I.R.; De Azevedo, J.C.R. Caffeine as a chemical tracer for contamination of urban rivers. RBRH 2019, 24, 29. [Google Scholar] [CrossRef] [Green Version]
- Sollecito, F.; Vitone, C.; Miccoli, D.; Plötze, M.; Puzrin, A.M.; Cotecchia, F. Marine Sediments from a Contaminated Site: Geotechnical Properties and Chemo-Mechanical Coupling Processes. Geosciences 2019, 9, 333. [Google Scholar] [CrossRef] [Green Version]
- WWAP- World Water Assessment Programme. The United Nations World Water Development Report 2015: Water for a Sustainable World. Paris: United Nations World Water Assessment Programme, UNESCO, 2015. Available online: http://unesdoc.unesco.org/images/0023/002318/231823E.pdf (accessed on 5 September 2019).
- Manikandan, S.; Chidambaram, S.; Prasanna, M.V.; Ganayat, R.R. Assessment of Heavy Metals Pollution and Stable Isotopic Signatures in Hard Rock Aquifers of Krishnagiri District, South India. Geosciences 2019, 9, 200. [Google Scholar] [CrossRef] [Green Version]
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H. Water Pollution from Agriculture: A Global Review; FAO—Food and Agriculture Organization of the United Nations and the IWMI—International Water Management Institute: Colombo, Sri Lanka, 2017. [Google Scholar]
- Mathews, B.W.; Sollenberger, L.E.; Nair, V.D.; Staples, C.R. Impact of Grazing Management on Soil Nitrogen, Phosphorus, Potassium, and Sulfur Distribution. J. Environ. Qual. 1994, 23, 1006–1013. [Google Scholar] [CrossRef]
- Bertol, I.; Engel, F.; Mafra, A.; Bertol, O.; Ritter, S. Phosphorus, potassium and organic carbon concentrations in runoff water and sediments under different soil tillage systems during soybean growth. Soil Tillage Res. 2007, 94, 142–150. [Google Scholar] [CrossRef]
- Wahba, M.M.; Aziz, A.M.; Zaghloul, A.M. Evaluation of Kinetic Approach in Describing Potassium Bioavailability. Am. J. Heterocycl. Chem. 2017, 3, 78. [Google Scholar] [CrossRef]
- Dimirkou, A. Uptake of Zn2+ ions by a fully iron-exchanged clinoptilolite. Case study of heavily contaminated drinking water samples. Water. Res. 2007, 41, 2763–2773. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- He, F.; Gao, J.; Pierce, E.; Strong, P.J.; Wang, H.; Liang, L. In situ remediation technologies for mercury-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 8124–8147. [Google Scholar] [CrossRef]
- Barragán, P.P.; Macedo, M.G.M.; Olguín, M.T. Cadmium sorption by sodium and thiourea-modified zeolite-rich tuffs. J. Environ. Sci. 2017, 52, 39–48. [Google Scholar] [CrossRef]
- Yin, S.; Herath, G.; Heng, S.; Kalpage, S. Using Permeable Reactive Barriers to Remediate Heavy Metal-Contaminated Groundwater through a Laboratory Column Experiment. Am. J. Environ. Sci. 2017, 13, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Huang, G.; Han, Z.; Xu, Y.; Zhu, M.; Zhang, Z. Evaluation of zeolite-supported microscale zero-valent iron as a potential adsorbent for Cd2+ and Pb2+ removal in permeable reactive barriers. Environ. Sci. Pollut. Res. 2017, 24, 13837–13844. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Martinez, A.; Marcaide, A.; Aranzabe, E. Heterogeneous Fenton Catalyst for the Efficient Removal of Azo Dyes in Water. Am. J. Anal. Chem. 2014, 5, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Shabalala, A.N.; Ekolu, S.O.; Diop, S.; Solomon, F. Pervious concrete reactive barrier for removal of heavy metals from acid mine drainage—column study. J. Hazard. Mater. 2017, 323, 641–653. [Google Scholar] [CrossRef]
- Ugrina, M.; Medvidović, N.V.; Perić, J.; Trgo, M. A study of kinetics and successive sorption/desorption of Zn and Cd uptake onto iron-modified zeolite. Clay Miner. 2015, 50, 117–132. [Google Scholar] [CrossRef]
- Sun, Y.; Fang, Q.; Dong, J.; Cheng, X.; Xu, J. Removal of fluoride from drinking water by natural stilbite zeolite modified with Fe(III). Desalination 2011, 277, 121–127. [Google Scholar] [CrossRef]
- Dowson, G.R.M.; Dimitriou, I.; Owen, R.E.; Reed, D.G.; Allen, R.W.K.; Styring, P. Kinetic and economic analysis of reactive capture of dilute carbon dioxide with Grignard reagents. Faraday Discuss. 2015, 183, 47–65. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Mesa, S.J.G.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- Powell, R.M.; Blowes, D.W.; Gillham, R.W.; Schultz, D.; Sivavec, T.; Puls, R.W.; Vogan, J.L.; Powell, P.D.; Landis, R. Permeable Reactive Barrier Technologies for Contaminant Remediation; Report EPA/600//R-98/125; U.S. Environmental Protection Agency: Washington, DC, USA, 1998.
- Chen, H.; Park, E.; Hu, C. A design solution of PRB with multispecies transport based on a multi-domain system. Environ. Earth Sci. 2018, 77, 630. [Google Scholar] [CrossRef]
- Mesa, S.J.G.; Malina, G. Screening reactive materials for a permeable barrier to treat TCE-contaminated groundwater: Laboratory studies. Environ. Earth Sci. 2016, 75, 772. [Google Scholar] [CrossRef]
- Luo, X.; Liu, H.; Huang, G.; Li, Y.; Zhao, Y.; Li, X. Remediation of arsenic-contaminated groundwater using media-injected permeable reactive barriers with a modified montmorillonite: Sand tank studies. Environ. Sci. Pollut. Res. 2016, 23, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Gómez, N.; Macedo-Miranda, M.; Olguín, M. Chromium VI adsorption from sodium chromate and potassium dichromate aqueous systems by hexadecyltrimethylammonium-modified zeolite-rich tuff. Appl. Clay Sci. 2014, 95, 197–204. [Google Scholar] [CrossRef]
- Dalal, U.; Reddy, S.N. A novel nano zero-valentironbiomaterial for chromium (Cr6+ to Cr3+) reduction. Environ. Sci. Pollut. Res. 2019, 26, 10631–10640. [Google Scholar] [CrossRef] [PubMed]
- Bibiano-Cruz, L.; Garfias, J.; Salas-García, J.; Martel, R.; Llanos, H. Batch and column test analyses for hardness removal using natural and homoionic clinoptilolite: Breakthrough experiments and modeling. Sustain. Water Resour. Manag. 2016, 2, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Buenaño, X.; Canoira, L.; Sánchez, D.M.; Costafreda, J. Zeolitic tuffs for acid mine drainage (AMD) treatment in Ecuador: Breakthrough curves for Mn2+, Cd2+, Cr3+, Zn2+, and Al3+. Environ. Sci. Pollut. Res. 2017, 24, 6794–6806. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Durand, P.; Roques-Carmes, T.; Eastoe, J.; Pasc, A. Metallo-Solid Lipid Nanoparticles as Colloidal Tools for Meso–Macroporous Supported Catalysts. Langmuir 2015, 31, 1842–1849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pleasant, S.; Jain, P.; Powell, J.; Townsend, T. Calcium carbonate-based permeable reactive barriers for iron and manganese groundwater remediation at landfills. Waste Manag. 2016, 53, 128–135. [Google Scholar] [CrossRef]
- Lee, S.M.; Lalhmunsianma; Tiwari, D. Sericite in the remediation of Cd(II)− and Mn(II)− contaminated waters: Batch and column studies. Environ. Sci. Pollut. Res. 2014, 21, 3686–3696. [Google Scholar] [CrossRef]
- Hong, C.S.; Shackelford, C.D. Long-Term Column Testing of Zeolite-Amended Backfills. I: Testing Methodology and Chemical Compatibility. J. Geotech. Geoenviron. Eng. 2017, 143, 04017050. [Google Scholar] [CrossRef]
- Li, S.; Huang, G.; Kong, X.; Yang, Y.; Liu, F.; Hou, G.; Chen, H. Ammonium removal from groundwater using a zeolite permeable reactive barrier: A pilot-scale demonstration. Water Sci. Technol. 2014, 70, 1540–1547. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Zeolite nanoparticle as a superior adsorbent with high capacity: Synthesis, surface modification and pollutant adsorption ability from wastewater. Microchem. J. 2019, 145, 74–83. [Google Scholar] [CrossRef]
- Nikashina, V.A.; Serova, I.B.; Kats, E.M.; Tokmachev, M.G.; Toropchenova, E.S.; Zhilkina, A.V.; Kuz’Mina, T.G.; Bulenova, K. Permeable reactive barriers based on natural zeolites from Kazakhstan in solving ecological problems: Mathematical model and simulation. Geochem. Int. 2017, 55, 38–46. [Google Scholar] [CrossRef]
- Simsek, E.B.; Tuna, A.O.A.; Beker, U. A statistical approach for arsenic adsorption onto Turkey clinoptilolite. Environ. Sci. Pollut. Res. 2015, 22, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Statham, T.M.; Stark, S.C.; Snape, I.; Stevens, G.W.; Mumford, K.A. A permeable reactive barrier (PRB) media sequence for the remediation of heavy metal and hydrocarbon contaminated water: A field assessment at Casey Station, Antarctica. Chemosphere 2016, 147, 368–375. [Google Scholar] [CrossRef]
- Jovanovic, M.; Rajic, N.; Obradovic, B. Novel kinetic model of the removal of divalent heavy metal ions from aqueous solutions by natural clinoptilolite. J. Hazard. Mater. 2012, 233, 57–64. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; De Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; De Mello, J.M.M.; Fiori, M.A.; Oliveira, J.; Magro, J.D. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process. Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Kinoshita, N. Applicability of Natural Zeolite for NH-Forms Removal in Enzyme-Mediated Calcite Precipitation Technique. Geosciences 2017, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Hou, G.; Zheng, P.; Liu, R.; Liu, C. H2S in-situ removal from biogas using a tubular zeolite/TiO2 photocatalytic reactor and the improvement on methane production. Chem. Eng. J. 2016, 294, 105–110. [Google Scholar] [CrossRef]
- Nasonova, A.; Kim, K.-S. Effects of TiO2 coating on zeolite particles for NO and SO2 removal by dielectric barrier discharge process. Catal. Today 2013, 211, 90–95. [Google Scholar] [CrossRef]
- Arimi, M.M. Modified natural zeolite as heterogeneous Fenton catalyst in treatment of recalcitrants in industrial effluent. Prog. Nat. Sci. 2017, 27, 275–282. [Google Scholar] [CrossRef]
- Ambrozova, P.; Kynicky, J.; Urubek, T.; Nguyen, V.D. Synthesis and Modification of Clinoptilolite. Molecules 2017, 22, 1107. [Google Scholar] [CrossRef] [Green Version]
- Koyama, K.; Takéuchi, Y. Clinoptilolite: The distribution of potassium atoms and its role in thermal stability. Zeitschrift für Kristallographie-Crystalline Materials 1977, 145, 216–239. [Google Scholar] [CrossRef]
- Aharoni, C.; Sparks, D.L. Kinetics of Soil Chemical Reactions—A Theoretical Treatment. Rates Soil Chem. Processes 1991, 27, 1–18. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Tanner, C.C. Ammonium removal from wastewaters using natural New Zealand zeolites. N. Z. J. Agric. Res. 1998, 41, 427–446. [Google Scholar] [CrossRef] [Green Version]
- Olah, J.; Papp, J.; Meszaros-Kis, A.; Muscy, G.; Kallo, D. Removal of suspended solids, phosphate and ammonium ions from communal sewage using clinoptilolite derivatives. In Occurrence, Properties and Utilization of Natural Zeolites; Kalló, D., Sherry, H.S., Eds.; Akademiai Kiado: Budapeste, Hungary, 1988; pp. 511–520. [Google Scholar]
- Rodríguez, G.; Brito-Rojas, A.; Countín-Correa, D. Sedimentary Zeolite Deposits in Cuba. In Zeolites; International Zeolite Association (IZA): La Habana, Cuba, 2014; Available online: http://www.iza-online.org/natural/Catalog/Cuba.pdf (accessed on 8 October 2019).
- Brazilian Association of Technical Standards. NBR 7181: Soil—Particle Size Analysis; The Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 1984. [Google Scholar]
- Brazilian Association of Technical Standards. NBR 6508: Soil Grains Passing through the Sieve 4.8 mm: Determination of Specific Mass: Test Method; The Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 1984. [Google Scholar]
- Brazilian Association of Technical Standards. NBR 12004: Solo—Determination of the Maximum Void Index of Non-Cohesive Soils; The Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 1990. [Google Scholar]
- Brazilian Association of Technical Standards. NBR 12051: Solo—Determination of the Minimum Void Index of Non-Cohesive Soils; The Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 1991. [Google Scholar]
- Chapman, H.D. Cation Exchange Capacity. In Methods of Soil Analysis; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 891–901. [Google Scholar]
- Choo, K.Y.; Bai, K. The effect of the mineralogical composition of various bentonites on CEC values determined by three different analytical methods. Appl. Clay Sci. 2016, 126, 153–159. [Google Scholar] [CrossRef]
- Zhou, L.; Boyd, C.E. Total ammonia nitrogen removal from aqueous solutions by the natural zeolite, mordenite: A laboratory test and experimental study. Aquaculture 2014, 432, 252–257. [Google Scholar] [CrossRef]
- Pejon, O.J. Regional Geotechnical Mapping of the Leaf of Piracicaba-SP (Scale 1: 100.000) Study of Methodological Aspects of Characterization and Presentation of Attributes. Ph.D. Thesis, São Carlos School of Engineering, University of São Paulo, São Carlos, Brazil, 1992. [Google Scholar]
- Roy, W.; Krapac, I.; Chou, S.; Griffin, R. Batch—Type Procedures for Estimating Soil Adsorption of Chemicals; United States Environmental Protection Agency: Washington, DC, USA, 1992; EPA/530/SW-87/006F.
- Shackelford, C.D. Critical Concepts for Column Testing. J. Geotech. Eng. 1994, 120, 1804–1828. [Google Scholar] [CrossRef]
- Shackelford, C.D. Cumulative Mass Approach for Column Testing. J. Geotech. Eng. 1995, 121, 696–703. [Google Scholar] [CrossRef]
- Ming, D.W.; Mumpton, F.A. Zeolites in Soils. In Minerals in Soil Environments; Soil Science Society of America: Madison, WI, USA, 1989; pp. 873–907. [Google Scholar]
- Rodriguez-Fuentes, G.; Ruiz-Salvador, A.R.; Mir, M.; Picazo, O.; Quintana, G.; Delgado, M. Thermal and cation influence on ir vibrations of modified natural clinoptilolite. Microporous Mesoporous Mater. 1998, 20, 269–281. [Google Scholar] [CrossRef]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended nomenclature for zeolite minerals: Report of the subcommittee on zeolites of the International Mineralogical Association, Comission on New Minerals and Mineral Names. Mineral. Mag. 1997, 35, 1571–1606. [Google Scholar]
- Wasielewski, S.; Rott, E.; Minke, R.; Steinmetz, H. Evaluation of Different Clinoptilolite Zeolites as Adsorbent for Ammonium Removal from Highly Concentrated Synthetic Wastewater. Water 2018, 10, 584. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, S.A. Study of Heavy Metal Adsorption in Zeolites for use in Reactive Barrier. Master’s Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil, 2011; p. 220f. [Google Scholar]
- Barros, M.A.S.D. Removal of Cr3+ of Industrial and Synthetic Effluents by Naturally Action of Clinoptilolite. Master’s Thesis, Maringá State University, Maringá, Brazil, 1996; p. 140f. [Google Scholar]
- Amini, N.; Soleimani, M.; Mirghaffari, N. Photocatalytic removal of SO2 using natural zeolite modified by TiO2 and polyoxypropylene surfactant. Environ. Sci. Pollut. Res. 2018, 26, 16877–16886. [Google Scholar] [CrossRef] [PubMed]
- Jaskunas, A.; Subacius, B.; Slinksiene, R. Adsorption of potassium ions on natural zeolite: Kinetic and equilibrium studies. Chemija 2015, 26, 69–78. [Google Scholar]
- Panayotova, M.; Velikov, B. Kinetics of heavy metal ions removal by use of natural zeolite. J. Environ. Sci. Heal. Part A 2002, 37, 139–147. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3973. [Google Scholar] [CrossRef]
- Shaban, M.; Hassouna, M.E.M.; Nasief, F.M.; Abukhadra, M.R. Adsorption properties of kaolinite-based nanocomposites for Fe and Mn pollutants from aqueous solutions and raw ground water: Kinetics and equilibrium studies. Environ. Sci. Pollut. Res. 2017, 24, 22954–22966. [Google Scholar] [CrossRef]
- Allen, S.J.; Gan, Q.; Matthews, R.; Johnson, P.A. Comparison of optimized isotherm models for basic dye adsorption by kudzu. Bioresour. Technol. 2003, 88, 143–152. [Google Scholar] [CrossRef]
- Bueno, B.Y.M. Removal of Pb, Cr and Cu Removal by Combined Biosorption/Bioflotting Process Using Rhodococcus Opacus Strain. Ph.D. Thesis, Pontifical Catholic University of Rio de Janeiro, Rio de Janeiro, Brazil, 2007; p. 172f. [Google Scholar]
- Ho, Y.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process. Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Correia, T.A.; Campos, M.L.; Almeida, J.A.; Miquelluti, D.J.; Souza, M.C. Characterization of zeolites from Urupema, SC, and their ability to remove Cu2+ from aqueous solutions. Revista de Ciências Agroveterinárias 2010, 9, 29–38. [Google Scholar]
- Kitsopoulos, K.P. Cation-Exchange Capacity (CEC) of Zeolitic Volcaniclastic Materials: Applicability of the Ammonium Acetate Saturation (AMAS) Method. Clays Clay Miner. 1999, 47, 688–696. [Google Scholar] [CrossRef]
- Lim, S.-F.; Lee, A.Y.W. Kinetic study on removal of heavy metal ions from aqueous solution by using soil. Environ. Sci. Pollut. Res. 2015, 22, 10144–10158. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, N.; Kexiang, L.; Zhang, Y. Heavy metal removal from MSWI fly ash by electrokinetic remediation coupled with a permeable activated charcoal reactive barrier. Sci. Rep. 2015, 5, 15412. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mou, H.; Chen, L.; Mirza, Z.A.; Liu, L. Cr(VI)-contaminated groundwater remediation with simulated permeable reactive barrier (PRB) filled with natural pyrite as reactive material: Environmental factors and effectiveness. J. Hazard. Mater. 2015, 298, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ames, L.L. The cation sieve properties of clinoptilolite. Am Miner. 1960, 45, 689–700. [Google Scholar]
- Karadag, D.; Koç, Y.; Turan, M.; Armagan, B.; Armaǧan, B. Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite. J. Hazard. Mater. 2006, 136, 604–609. [Google Scholar] [CrossRef]










| Oxides | Mass/Mass (%) | Standard Deviation (%) |
|---|---|---|
| MgO | 0.715 | 0.081 |
| Al2O3 | 10.107 | 0.025 |
| SiO2 | 79.280 | 0.185 |
| K2O | 2.083 | 0.014 |
| CaO | 4.564 | 0.052 |
| TiO2 | 0.418 | 0.008 |
| Fe2O3 | 2.676 | 0.052 |
| ZnO | 0.010 | 0.001 |
| BaO | 0.147 | 0.002 |
| Si/Al | 6.8 | 0.126 |
| Freündlich | Langmuir | |||||
|---|---|---|---|---|---|---|
| KF (l/g) | NF | r2 | A (mg−1) | B (mg/g) | r2 | RL |
| 1.685 | 3.31 | 0.77 | 0.0053 | 19.50 | 0.99 | 0.54 |
| qe Experimental (mg/g) | Pseudo-1st- Order | Pseudo-2nd-Order | Elovich | ||||
|---|---|---|---|---|---|---|---|
| K+ 500 mg/L | 6.9 | qe (mg/g) | 2.446 | qe (mg/g) | 6.882 | β (g/mg) | 1.026 |
| k1(s−1) | 0.003 | k2 (s−1) | 0.009 | α (mg/g min) | 1.720 | ||
| r2 | 0.789 | r2 | 0.999 | r2 | 0.937 | ||
| K+ 200 mg/L | 0.9 | qe (mg/g) | 1.296 | qe (mg/g) | 0.949 | β (g/mg) | 6.350 |
| k1(s−1) | 0.003 | k2 (s−1) | 0.008 | α (mg/g min) | 0.872 | ||
| r2 | 0.970 | r2 | 0.998 | r2 | 0.942 | ||
| Zn2+ 200 mg/L | 0.87 | qe (mg/g) | 1.696 | qe (mg/g) | 0.929 | β (g/mg) | 6.361 |
| k1(s−1) | 0.001 | k2 (s−1) | 0.004 | α (mg/g·min) | 1.924 | ||
| r2 | 0.899 | r2 | 0.993 | r2 | 0.992 | ||
| Cycle | Contaminated Solution Percolation | Flushing Process | Final Condition for Cycle | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Pore Volume Percolated | Total Percolated Volume (cm3) | Total Potassium Adsorbed (mg) | Adsorbed Potassium Rate (mg/g) | Total CEC Occupied at the End of a Cycle (%) | Total Void Volume Percolated | Total Percolated Volume (cm3) | Total Potassium Desorption (mg) | Desorption Potassium Rate (mg/g) | CEC Recovered by Flushing (%) | Flushing Efficiency (%) | Potassium Adsorbed (mg) | Adsorbed Potassium Remained after Flushing (mg/g) | Total CEC Occupied after Flushing (%) | |
| 1 | 129 | 81,779.5 | 22,251.65 | 19.05 | 27.14 | 28 | 17,750.6 | 2282.22 | 1.95 | 2.79 | 11.43 | 19,969.43 | 17.09 | 24.35 |
| 2 | 72 | 45,644.4 | 9804.04 | 8.40 | 36.31 | 29 | 18,384.5 | 2567.50 | 2.20 | 3.13 | 9.44 | 27,205.96 | 23.29 | 33.18 |
| 3 | 64 | 40,572.8 | 8082.86 | 6.92 | 43.04 | 200 | 126,790.0 | 3518.42 | 3.01 | 4.29 | 11.07 | 31,770.40 | 27.20 | 38.75 |
| 4 | 125 | 79,243.8 | 18,733.22 | 16.07 | 61.59 | 100 | 63,395.0 | 3518.42 | 3.01 | 4.29 | 7.49 | 46,985.20 | 40.23 | 57.30 |
| 5 | 110 | 69,734.5 | 16,707.75 | 14.31 | 77.68 | 30 | 19,018.5 | 3518.42 | 3.01 | 4.29 | 5.85 | 60,174.53 | 51.52 | 73.39 |
| 6 | 70 | 44,376.5 | 7797.59 | 6.68 | 82.90 | 30 | 19,018.5 | 3328.24 | 2.85 | 4.06 | 5.15 | 64,643.88 | 55.35 | 78.84 |
| 7 | 55 | 34,867.3 | 4374.26 | 3.75 | 84.18 | 35 | 22,188.3 | 2852.78 | 2.44 | 3.48 | 4.31 | 66,165.36 | 56.65 | 80.70 |
| 8 | 40 | 25,358.0 | 8177.96 | 7.00 | 90.67 | 30 | 19,018.5 | 2852.78 | 2.44 | 3.48 | 3.99 | 71,490.54 | 61.21 | 87.19 |
| Elements | Initial | Final |
|---|---|---|
| 100% Mass/Mass | ||
| Mg | 0.716 | 0.423 |
| Al | 9.735 | 8.433 |
| Si | 72.47 | 70.066 |
| K | 4.331 | 8.113 |
| Ca | 8.489 | 6.423 |
| Ti | 0.384 | 0.380 |
| Fe | 3.488 | 3.338 |
| Zn | 0.023 | 0.021 |
| Ba | 0.364 | 0.341 |
| Cl | - | 2.462 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, L.C.C.; Zuquette, L.V. Evaluation of Zeolite as a Potential Reactive Medium in a Permeable Reactive Barrier (PRB): Batch and Column Studies. Geosciences 2020, 10, 59. https://doi.org/10.3390/geosciences10020059
Rocha LCC, Zuquette LV. Evaluation of Zeolite as a Potential Reactive Medium in a Permeable Reactive Barrier (PRB): Batch and Column Studies. Geosciences. 2020; 10(2):59. https://doi.org/10.3390/geosciences10020059
Chicago/Turabian StyleRocha, Liana Carolina Carvalho, and Lazaro Valentin Zuquette. 2020. "Evaluation of Zeolite as a Potential Reactive Medium in a Permeable Reactive Barrier (PRB): Batch and Column Studies" Geosciences 10, no. 2: 59. https://doi.org/10.3390/geosciences10020059





