The Role of Iron Carbide in the Abyssal Formation of Hydrocarbons in the Upper Mantle
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Iron Carbide as a Possible Donor of Carbon in the Abiogenic Synthesis of Hydrocarbons in the Upper Mantle. The Influence of pT Conditions of the Upper Mantle on the Composition of Hydrocarbon Fluid
4.2. Chemical Mechanisms of the Formation of Hydrocarbons from Iron Carbide
4.3. Oxidation of Iron Compounds in the Abiogenic Synthesis of Hydrocarbons
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolesnikov, A.Y.; Saul, J.M.; Kutcherov, V.G. Chemistry of hydrocarbons under extreme thermobaric conditions. Chem. Sel. 2017, 2, 1336–1352. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Sobolev, N.V. Synthesis of hydrocarbons by CO2 fluid conversion with hydrogen: Experimental modeling at 7.8 GPa and 1350 °C. Dokl. Earth Sci. 2017, 477, 1483–1487. [Google Scholar] [CrossRef]
- Kutcherov, V.G.; Bendeliani, N.A.; Alekseev, V.A.; Kenney, J.F. Synthesis of hydrocarbons from minerals at pressures up to 5 GPa. Dokl. Phys. Chem. 2002, 387, 328–330. [Google Scholar] [CrossRef]
- Kutcherov, V.G.; Kolesnikov, A.; Dyugheva, T.I.; Kulikova, L.F.; Nikolaev, N.N.; Sazanova, O.A.; Braghkin, V.V. Synthesis of complex hydrocarbon systems at temperatures and pressures corresponding to the Earth’s upper mantle conditions. Dokl. Phys. Chem. 2010, 433, 132–135. [Google Scholar] [CrossRef]
- Sokol, A.G.; Tomilenko, A.A.; Bul’bak, T.A.; Sokol, I.A.; Zaikin, P.A.; Palyanova, G.A.; Palyanov, Y.N. Hydrogenation of carbon at 5.5–7.8 GPa and 1100–1400 °C: Implications to formation of hydrocarbons in reduced mantles of terrestrial planets. Phys. Earth Planet. Inter. 2019, 291, 12–23. [Google Scholar] [CrossRef]
- Sokol, A.; Tomilenko, A.; Sokol, I.; Zaikin, P.; Bul’bak, T. Formation of hydrocarbons in the presence of native iron under upper mantle conditions: Experimental constraints. Minerals 2020, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Kutcherov, V.; Ivanov, K.; Mukhina, E.; Serovaiskii, A. Deep hydrocarbon cycle. Carbon Earth Inter. 2020. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.R.; Rossman, G.R. Water in Earth’s Mantle: The role of nominally anhydrous minerals. Science 1992, 255, 1391. [Google Scholar] [CrossRef]
- Ohtani, E. Water in the mantle. Elements 2005, 1, 25–30. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Wirth, R. Iron carbide inclusions in lower-mantle diamond from Juina, Brazil. Can. Mineral. 2011, 49, 555–572. [Google Scholar] [CrossRef] [Green Version]
- Jacob, D.E.; Kronz, A.; Viljoen, K.S. Cohenite, native iron and troilite inclusions in garnets from polycrystalline diamond aggregates. Contrib. Mineral. Petrol. 2004, 146, 566–576. [Google Scholar] [CrossRef]
- Shatsky, V.S.; Ragozin, A.L.; Logvinova, A.M.; Wirth, R.; Kalinina, V.V.; Sobolev, N.V. Diamond-rich placer deposits from iron-saturated mantle beneath the northeastern margin of the Siberian Craton. Lithos 2020, 364–365, 105514. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 2010, 298, 1–13. [Google Scholar] [CrossRef]
- Serovaiskii, A.Y.; Kolesnikov, A.Y.; Kutcherov, V.G. Formation of iron hydride and iron carbide from hydrocarbon systems at ultra-high thermobaric conditions. Geochem. Int. 2019, 57, 1008–1014. [Google Scholar] [CrossRef]
- Serovaiskii, A.; Mukhina, E.; Dubrovinsky, L.; Chernoutsan, A.; Kudryavtsev, D.; McCammon, C.; Aprilis, G.; Kupenko, I.; Chumakov, A.; Hanfland, M.; et al. Fate of hydrocarbons in iron-bearing mineral environments during subduction. Minerals 2019, 9, 651. [Google Scholar] [CrossRef] [Green Version]
- Martirosyan, N.S.; Litasov, K.D.; Shatskiy, A.; Ohtani, E. The reactions between iron and magnesite at 6 GPa and 1273–1873 K: Implication to reduction of subducted carbonate in the deep mantle. J. Mineral. Petrol. Sci. 2015, 110, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Bayukov, O.A.; Zdrokov, E.V. Iron carbide as a source of carbon for graphite and diamond formation under lithospheric mantle P-T parameters. Lithos 2017, 286–287, 151–161. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Bayukov, O.A.; Sobolev, N.V. Conditions for diamond and graphite formation from iron carbide at the PT parameters of lithospheric mantle. Russ. Geol. Geophys. 2016, 57, 176–189. [Google Scholar] [CrossRef]
- Lord, O.T.; Walter, M.J.; Dasgupta, R.; Walker, D.; Clark, S.M. Melting in the Fe-C system to 70 GPa. Earth Planet. Sci. Lett. 2009, 284, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Rohrbach, A.; Ghosh, S.; Schmidt, M.W.; Wijbrans, C.H.; Klemme, S. The stability of Fe–Ni carbides in the Earth’s mantle: Evidence for a low Fe–Ni–C melt fraction in the deep mantle. Earth Planet. Sci. Lett. 2014, 388, 211–221. [Google Scholar] [CrossRef]
- Stagno, V.; Frost, D.J. Carbon speciation in the asthenosphere: Experimental measurements of the redox conditions at which carbonate-bearing melts coexist with graphite or diamond in peridotite assemblages. Earth Planet. Sci. Lett. 2010, 300, 72–84. [Google Scholar] [CrossRef]
- Akiyama, T.; Miyazaki, A.; Nakanishi, H.; Hisa, M.; Tsutsumi, A. Thermal and gas analyses of the reaction between iron carbide and steam with hydrogen generation at 573K. Int. J. Hydrogen Energy 2004, 29, 721–724. [Google Scholar] [CrossRef]
- Hisa, M.; Tsutsumi, A.; Akiyama, T. Materialographic investigation on the mechanism of hydrogen production through the reaction between iron carbide and steam at a temperature of 673 K. Mater. Trans. 2004, 45, 1911–1914. [Google Scholar] [CrossRef] [Green Version]
- Arabczyk, W.; Ekiert, E.; Jȩdrzejewski, R. Kinetics of the oxidation of iron carbide dispersed in a carbon matrix with water vapor. J. Phys. Chem. A 2009, 113, 4947–4953. [Google Scholar] [CrossRef] [PubMed]
- Sonin, V.M.; Bul’bak, T.A.; Zhimulev, E.I.; Tomilenko, A.A.; Chepurov, A.I.; Pokhilenko, N.P. Synthesis of heavy hydrocarbons under P-T conditions of the Earth’s upper mantle. Dokl. Earth Sci. 2014, 454, 32–36. [Google Scholar] [CrossRef]
- Serovaiskii, A.; Kutcherov, V. Formation of complex hydrocarbon systems from methane at the upper mantle thermobaric conditions. Sci. Rep. 2020, 10, 4559. [Google Scholar] [CrossRef]
- Khurshid, H.; Li, W.; Chandra, S.; Phan, M.-H.; Hadjipanayis, G.C.; Mukherjee, P.; Srikanth, H. Mechanism and controlled growth of shape and size variant core/shell FeO/Fe3O4 nanoparticles. Nanoscale 2013, 5, 7942–7952. [Google Scholar] [CrossRef]
- Kumari, R.; Krishnia, L.; Kumar, V.; Singh, S.; Singh, H.K.; Kotnala, R.K.; Juluri, R.R.; Bhatta, U.M.; Satyam, P.V.; Yadav, B.S. Fe3C-filled carbon nanotubes: Permanent cylindrical nanomagnets possessing exotic magnetic properties. Nanoscale 2016, 8, 4299–4310. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Mouayd, A.A.; Koltsov, A.; Sutter, E.; Tribollet, B. Effect of silicon content in steel and oxidation temperature on scale growth and morphology. Mater. Chem. Phys. 2014, 143, 996–1004. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.M.; Righter, K. Melting of clinopyroxene+ magnesite in iron-bearing planetary mantles and implications for the Earth and Mars. Contrib. Mineral. Petrol. 2013, 166, 1067–1098. [Google Scholar] [CrossRef]
- Ishizaka, K.; Lewis, S.R.; Hammond, D.; Lewis, R. Chemistry of black leaf films synthesised using rail steels and their influence on the low friction mechanism. RSC Adv. 2018, 8, 32506–32521. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Clifford, D.; El-Gendy, A.A.; Carpenter, E.E. Solvothermal synthesis of Fe7C3 and Fe3C nanostructures with phase and morphology control. J. Appl. Phys. 2016, 120, 033904. [Google Scholar] [CrossRef]
- Pollack, H.N.; Chapman, D.S. On the regional variation of heat flow, geotherms, and lithospheric thickness. Tectonophysics 1977, 38, 279–296. [Google Scholar] [CrossRef] [Green Version]
- Kutcherov, V.G.; Krayushkin, V.A. Deep-seated abiogenic origin of petroleum: From geological assessment to physical theory. Rev. Geophys. 2010, 48, 1–30. [Google Scholar] [CrossRef]
- Mukhina, E.; Kolesnikov, A.; Kutcherov, V. The lower pT limit of deep hydrocarbon synthesis by CaCO3 aqueous reduction. Sci. Rep. 2017, 7, 5749. [Google Scholar] [CrossRef] [Green Version]
- Kolesnikov, A.; Kutcherov, V.G.; Goncharov, A.F. Methane-derived hydrocarbons produced under upper-mantle conditions. Nat. Geosci. 2009, 2, 566–570. [Google Scholar] [CrossRef]
- Chen, J.Y.; Jin, L.J.; Dong, J.P.; Zheng, H.F.; Liu, G.Y. Methane formation from CaCO3 reduction catalyzed by high pressure. Chin. Chem. Lett. 2008, 19, 475–478. [Google Scholar] [CrossRef]
- Sharma, A.; Cody, G.D.; Hemley, R.J. In situ diamond-anvil cell observations of methanogenesis at high pressures and temperatures. Energy Fuel 2009, 23, 5571–5579. [Google Scholar] [CrossRef]
- Kenney, J.F.; Kutcherov, V.A.; Bendeliani, N.A.; Alekseev, V.A. The evolution of multicomponent systems at high pressures: VI. The thermodynamic stability of the hydrogen-carbon system: The genesis of hydrocarbons and the origin of petroleum. Proc. Natl. Acad. Sci. USA 2002, 99, 10976–10981. [Google Scholar] [CrossRef] [Green Version]
- Dominé, F.; Bounaceur, R.; Scacchi, G.; Marquaire, P.-M.; Dessort, D.; Pradier, B.; Brevart, O. Up to what temperature is petroleum stable? New insights from a 5200 free radical reactions model. Org. Geochem. 2002, 33, 1487–1499. [Google Scholar] [CrossRef] [Green Version]
- Dominé, F. High pressure pyrolysis of n-hexane, 2,4-dimethylpentane and 1-phenylbutane. Is pressure an important geochemical parameter? Org. Geochem. 1991, 17, 619–634. [Google Scholar] [CrossRef]
- Scott, H.P.; Hemley, R.J.; Mao, H.-k.; Herschbach, D.R.; Fried, L.E.; Howard, W.M. Generation of methane in the Earth’s mantle: In situ high pressure-temperature measurements of carbonate reduction. PNAS 2004, 101, 14023–14026. [Google Scholar] [CrossRef] [Green Version]
- Vyakhirev, R.I.K.; Yu, P.; Kabanov, N.I. Teoriya i Opyt Dobychi Gaza (Theory and Practice of Gas Mining); Nedra: Moscow, Russia, 1998; p. 479. [Google Scholar]
- Belonoshko, A.B.; Lukinov, T.; Rosengren, A.; Bryk, T.; Litasov, K.D. Synthesis of heavy hydrocarbons at the core-mantle boundary. Sci. Rep. 2015, 5, 18382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobretsov, N.L.; Koulakov, I.Y.; Litasov, Y.D. Migration paths of magma and fluids and lava compositions in Kamchatka. Russ. Geol. Geophys. 2012, 53, 1253–1275. [Google Scholar] [CrossRef] [Green Version]
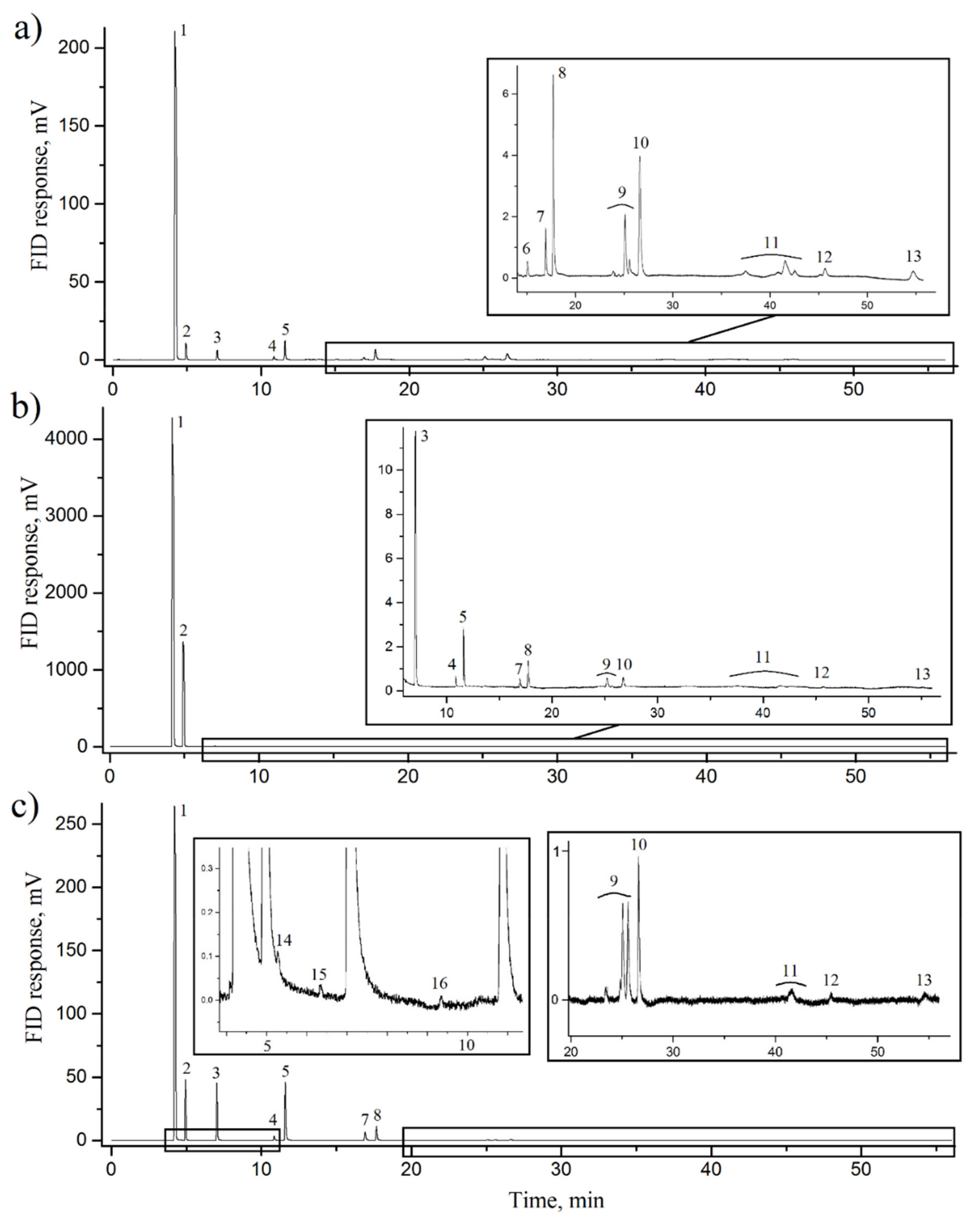

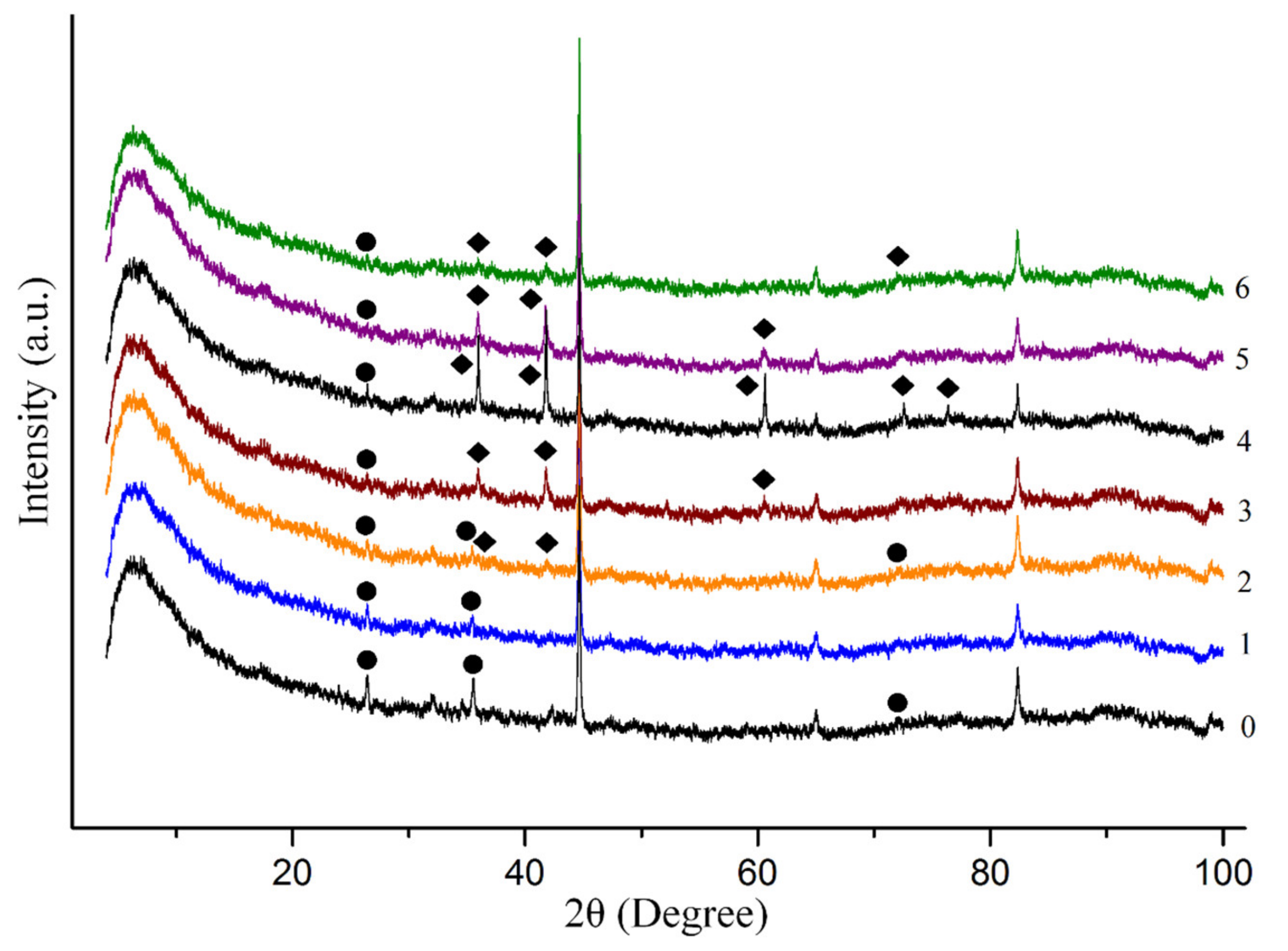
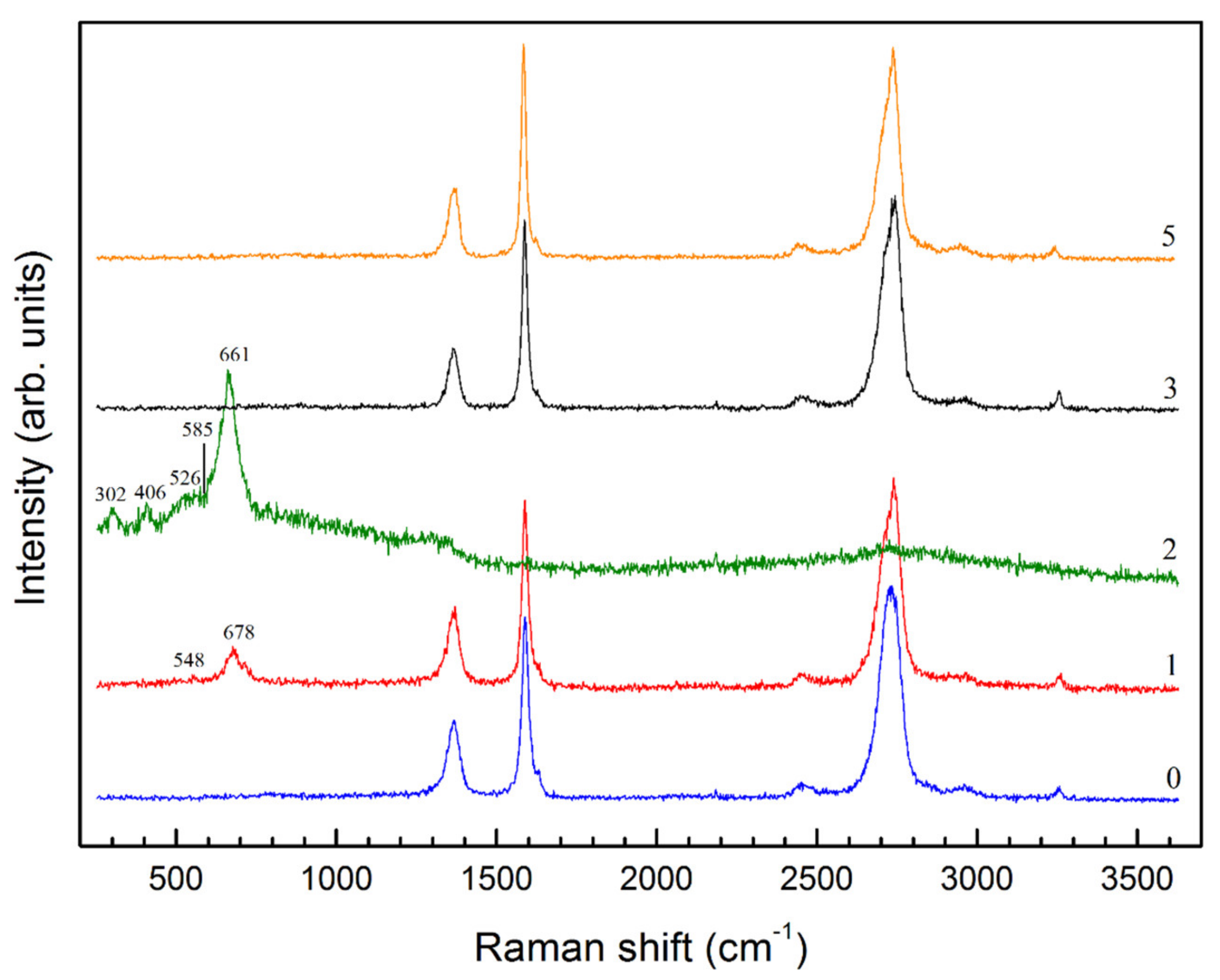
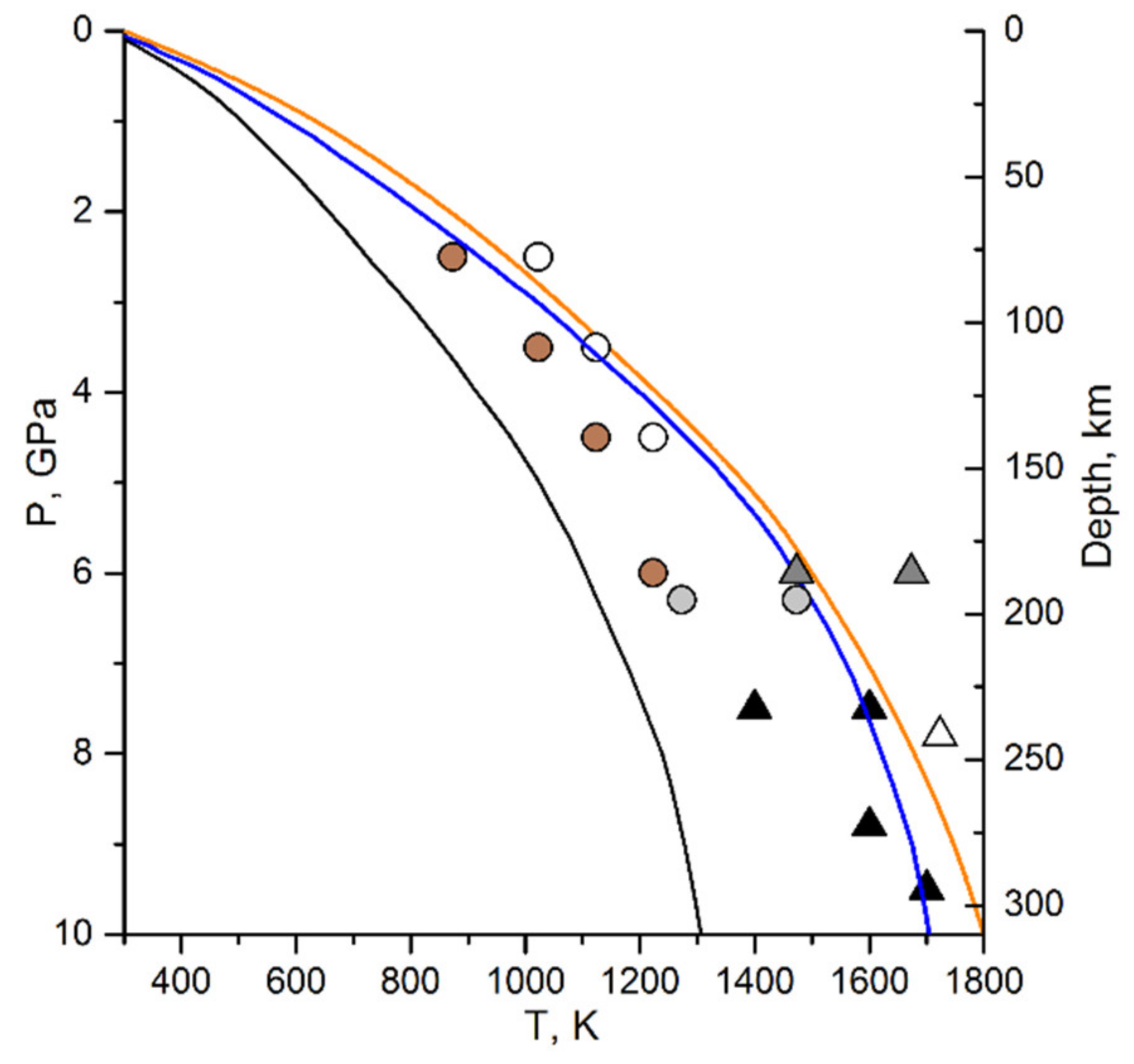
| # | P, GPa (±0.2) | T, K (±25) | CH4, % | C2H6, % | C3H8, % | C4H10, % | C5H12, % | C6H14, % | C7H16, % | C6H6, % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 873 | 81.83 | 3.10 | 1.50 | 3.51 | 3.56 | 4.03 | 0.71 | 0.70 |
| 2 | 2.5 | 1023 | 96.59 | 2.01 | 0.43 | 0.32 | 0.24 | 0.25 | 0.16 | - |
| 3 | 3.5 | 1023 | 87.41 | 2.33 | 0.92 | 0.48 | 2.24 | 3.26 | 2.36 | - |
| 4 | 3.5 | 1123 | 79.30 | 20.38 | 0.14 | 0.04 | 0.03 | 0.03 | 0.04 | 0.01 |
| 5 | 4.5 | 1123 | 84.65 | 13.89 | 0.94 | 0.30 | 0.11 | 0.07 | 0.05 | - |
| 6 | 4.5 | 1223 | 98.60 | 1.32 | 0.03 | 0.03 | 0.01 | - | - | - |
| 7 | 6.0 | 1223 | 68.56 | 8.96 | 7.66 | 9.27 | 4.32 | 0.98 | 0.16 | 0.09 |
| 8 | Severo-Stavropolskoe gas field [4], (given for comparison) | 98.90 | 0.29 | 0.16 | 0.05 | - | - | - | - | |
| 9 | Vuktinskoe gas field [4], (given for comparison) | 73.80 | 8.70 | 3.90 | 1.80 | 6.40 | - | - | - | |
| # | P, GPa (±0.2) | T, K (±25) | X-ray Diffraction | Raman Spectroscopy |
|---|---|---|---|---|
| 1 | 2.5 | 873 | Remained Fe3C, no new compounds | Fe3O4 |
| 2 | 2.5 | 1023 | Remained Fe3C, FeO | Fe3O4, FeO |
| 3 | 3.5 | 1023 | Remained Fe3C, FeO | No new compounds |
| 4 | 3.5 | 1123 | Remained Fe3C, FeO | - |
| 5 | 4.5 | 1123 | Remained Fe3C, FeO | No new compounds |
| 6 | 4.5 | 1223 | Remained Fe3C, FeO | - |
| 7 | 6.0 | 1223 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serovaiskii, A.; Kutcherov, V. The Role of Iron Carbide in the Abyssal Formation of Hydrocarbons in the Upper Mantle. Geosciences 2021, 11, 163. https://doi.org/10.3390/geosciences11040163
Serovaiskii A, Kutcherov V. The Role of Iron Carbide in the Abyssal Formation of Hydrocarbons in the Upper Mantle. Geosciences. 2021; 11(4):163. https://doi.org/10.3390/geosciences11040163
Chicago/Turabian StyleSerovaiskii, Aleksandr, and Vladimir Kutcherov. 2021. "The Role of Iron Carbide in the Abyssal Formation of Hydrocarbons in the Upper Mantle" Geosciences 11, no. 4: 163. https://doi.org/10.3390/geosciences11040163
APA StyleSerovaiskii, A., & Kutcherov, V. (2021). The Role of Iron Carbide in the Abyssal Formation of Hydrocarbons in the Upper Mantle. Geosciences, 11(4), 163. https://doi.org/10.3390/geosciences11040163






