Stiffness and Strength of Stabilized Organic Soils—Part I/II: Experimental Database and Statistical Description for Machine Learning Modelling
Abstract
:1. Introduction
2. Database Experimental Design
3. Base Materials
Soil Types
4. Cementitious Materials
5. Binder Mixes
6. Sample Preparation
7. Testing Procedures
7.1. Unconfined Compression Tests
7.2. Scanning Electron Microscopy
8. Experimental Results
8.1. Descriptive Statistics
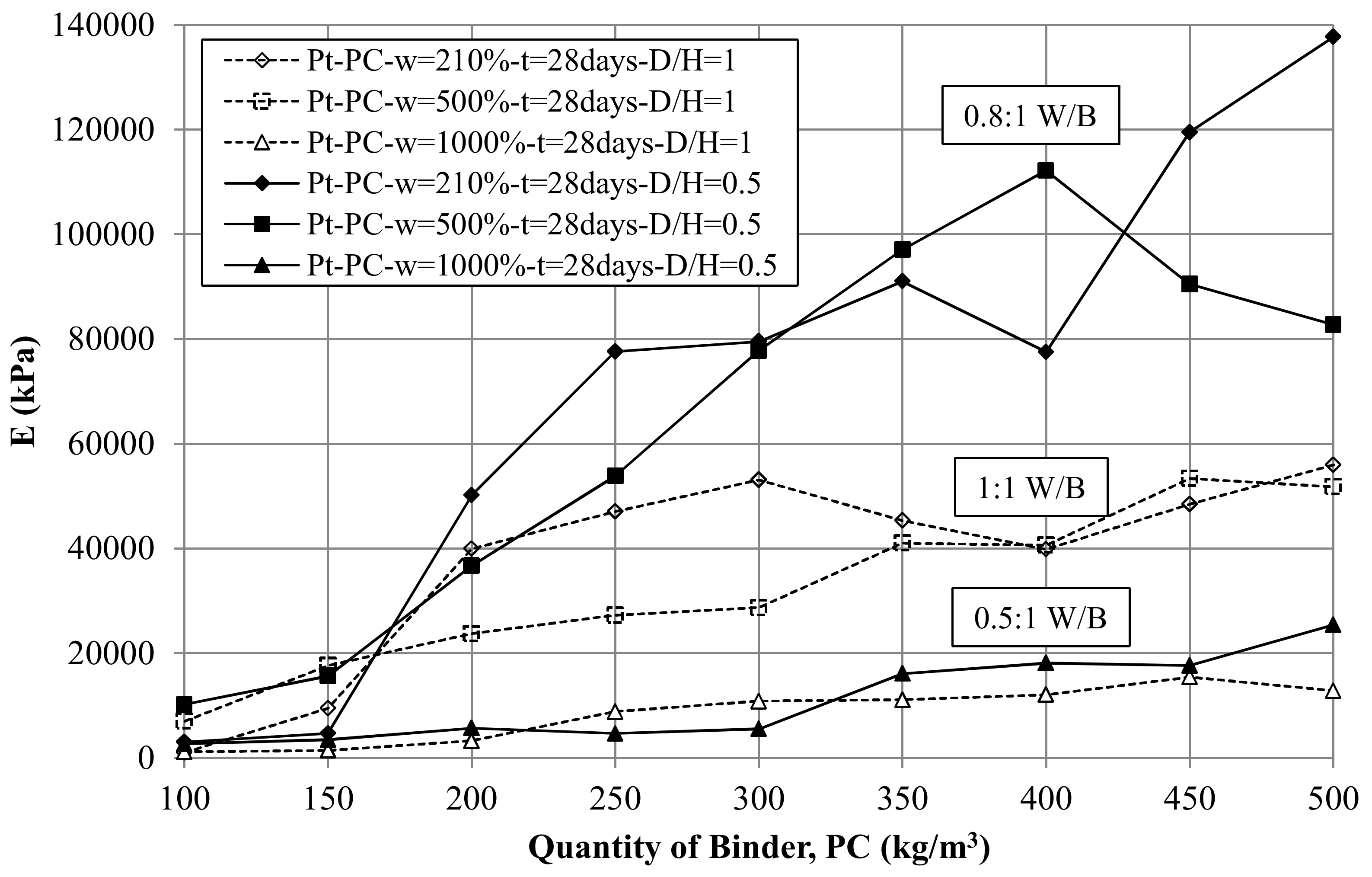
8.2. Effect of Binder Quantity on E and UCS
8.3. Effect of Curing Time on E and UCS
8.4. Effect of Binder Type E and UCS
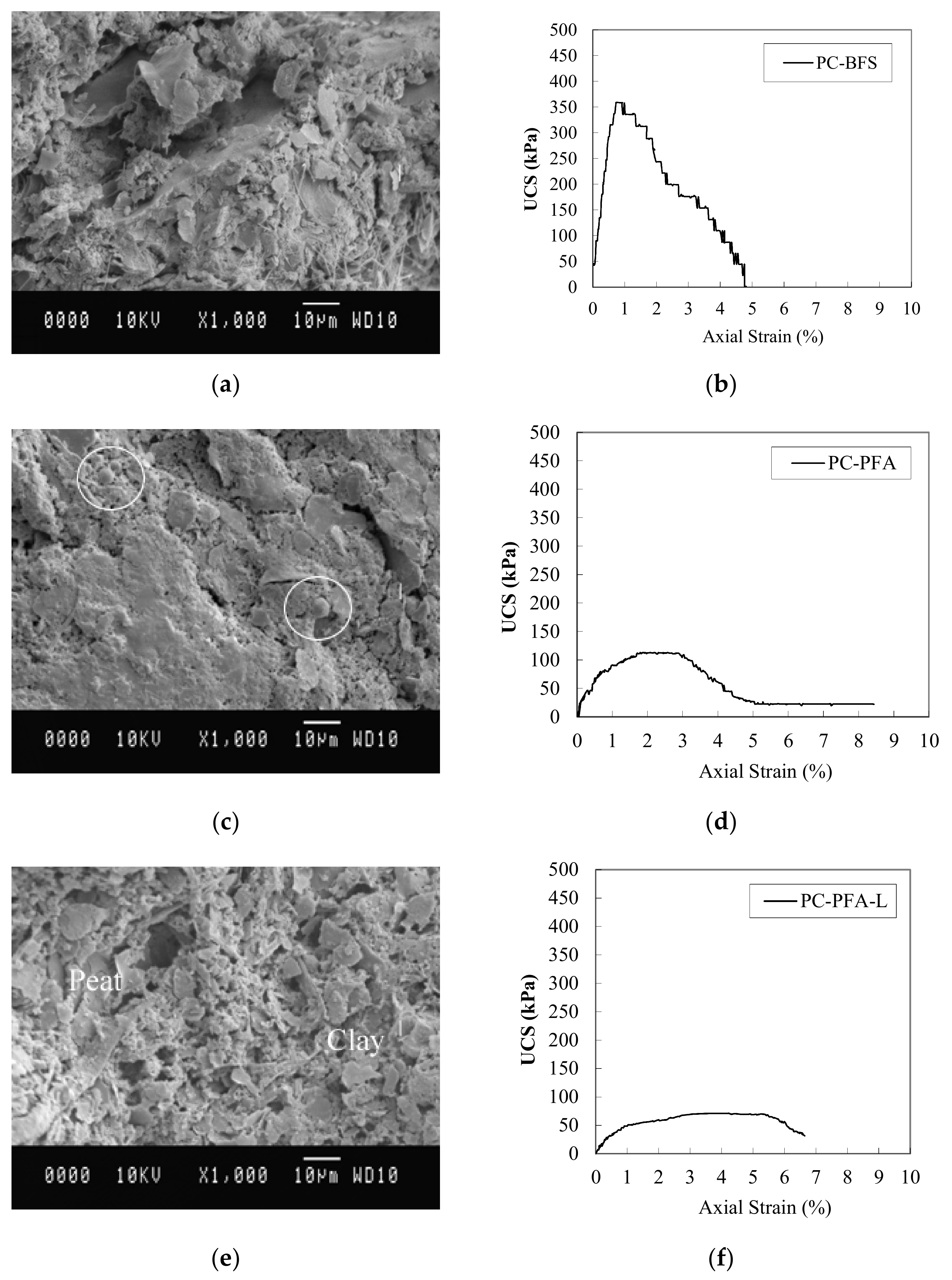
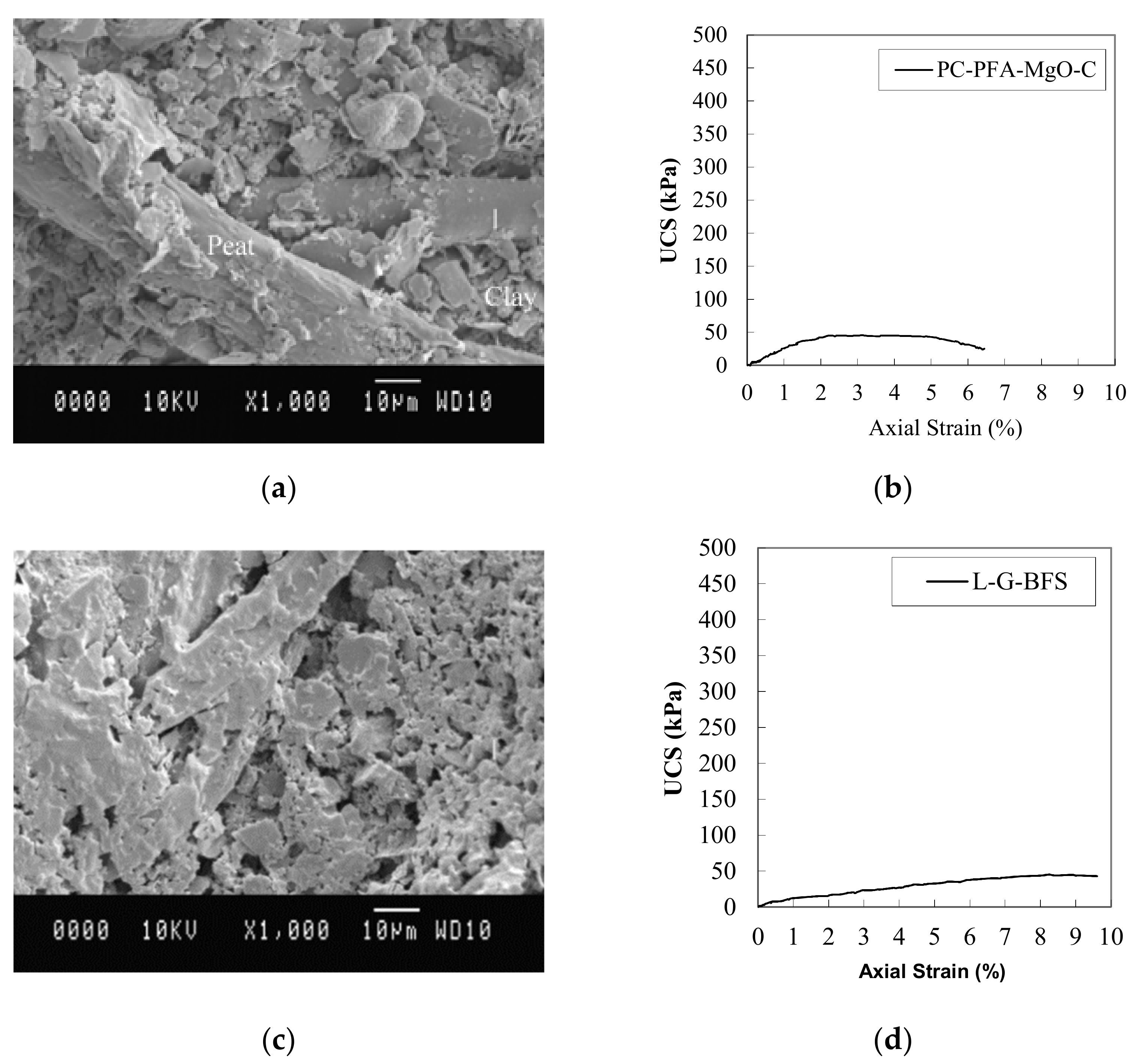
8.5. Scanning Electron Microscopy (SEM)
8.6. Unstabilized Soils
8.7. Stabilized Soils
8.8. Pt-PC Mix
8.9. OH1-Binder Mixes
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahgozar, M.A.; Saberian, M. Geotechnical Properties of peat soil stabilised with shredded waste tyre chips. Mires Peat 2016, 18, 1–13. [Google Scholar] [CrossRef]
- Hernandez-Martinez, F.G. Ground Improvement of Organics Soils using Wet Deep Soil Mixing. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2006. [Google Scholar]
- Hampton, M.B.; Edil, T. Strength Gain of Organic Ground with Cement-Type Binders; Geotechnical Special Publication: Reston, VA, USA, 1998; pp. 135–148. [Google Scholar]
- Åhnberg, H.; Bengtsson, P.E.; Holm, G. Effect of initial loading on the strength of stabilised peat. Proc. Inst. Civil Eng. Improv. 2001, 5, 35–40. [Google Scholar] [CrossRef]
- DGSSS; EuroSoilStab. Design Guide: Soft Soil Stabilisation. Development of Design and Construction Methods to Stabilize Soft Organic Soils; Building Research Establishment: Watford, UK, 2001. [Google Scholar]
- Jelisic, N.; Leppänen, M. Mass Stabilization of Organic Soils and Soft Clay. In Grouting and Ground Treatment; Geotechnical Special Publication: Reston, VA, USA, 2003. [Google Scholar]
- Lambrechts, J.R.; Ganse, M.A.; Layhee, C.A. Soil Mixing to Stabilize Organic Clay for I-95 Widening, Alexandria, VA. In Grouting and Ground Treatment; Geotechnical Special Publication: Reston, VA, USA, 2003. [Google Scholar]
- McGinn, A.J.; O’Rourke, T.D. Performance of Deep Mixing Methods at Fort Point Channel; Bechtel/Parsons Brinckerhoff, Massachussetts Turnpike Authority; Federal Highway Administration, Cornell University: Ithaca, NY, USA, 2003. [Google Scholar]
- Hayashi, H.; Nishimoto, S. Strength characteristics of stabilized peat using different types of binders. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; Volume 1.1, pp. 55–62. [Google Scholar]
- Porbaha, A.; Shibuya, S.; Kishida, T. State of the Art in Deep Mixing Technology. Part III: Geomaterial characterization. Proc. Inst. Civil Eng. -Ground Improv. 2000, 4, 91–110. [Google Scholar] [CrossRef]
- Coastal Development Institute of Technology (CDIT). In Deep Mixing Method, Principle, Design and Construction; Kitazume, M.; Terashi, M. (Eds.) Balkema: Rotterdam, The Netherlands, 2001. [Google Scholar]
- Terashi, M. Theme lecture: Deep mixing method—Brief state of the art. In Proceedings of the 14th International Confernece on Soil Mechanics and Foundation Engineering, Hamburg, Germany, 6–12 September 1997; pp. 2475–2478. [Google Scholar]
- Broms, B.B.; Boman, P. Lime stabilized column. In Proceedings of the 5th Asian Regional Conference on Soil Mechanics and Foundation Engineering, Southeast Asian Geotechnical Society, Asian Institute of Technology and Canadian International Development Agency, Kuala, Malaysia, 7–10 April 1975; Volume 1, pp. 227–234. [Google Scholar]
- Rathmayer, H. Deep mixing method for soft subsoil improvement in the nordic countries, Grouting and deep mixing. In Proceedings of the 2nd International Conference on Ground Improvement Geosystems, Tokyo, Japan, 14–17 May 1996; Volume 2, pp. 869–877. [Google Scholar]
- Al-Tabbaa, A. State of practice report–Stabilisation/solidification of contaminated materials with wet deep soil mixing. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; Volume 2, pp. 697–731. [Google Scholar]
- Bruce, D.A.; Bruce, M.E.C.; DiMillio, A.E. Dry mix methods: A brief overview of international practice. In Dry Mixing Methods for Deep Soil Stabilisation; Brendenberg, H., Holm, G., Broms, B., Eds.; Balkema: Rotterdam, The Netherlands, 1999; pp. 15–25. [Google Scholar]
- EuroSoilStab. Development of design and construction methods to stabilise soft organic soils. In Design Guide Soft Soil Stabilisation; BRE: Wadford, UK, 2001. [Google Scholar]
- Holm, G. Keynote lecture: Applications of dry mix methods for deep soil stabilization. In Dry Mixing Methods for Deep Soil Stabilisation; Brendenberg, H., Holm, G., Broms, B., Eds.; Balkema: Rotterdam, The Netherlands, 1999; pp. 3–13. [Google Scholar]
- Kitazume, M. State of Practice Report—Field and laboratory investigations, properties of binders and stabilized soil. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; Volume 2, pp. 660–684. [Google Scholar]
- Larsson, S. State of practice report—Execution, monitoring and quality control. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; Volume 2, pp. 732–785. [Google Scholar]
- Porbaha, A. Special report: Research and advancement of deep mixing technology in the USA. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; Volume 2, pp. 796–809. [Google Scholar]
- Porbaha, A. State of the art in deep mixing technology, part I: Basic concepts and overview. Ground Improv. 1998, 2, 81–92. [Google Scholar] [CrossRef]
- Porbaha, A.; Tanaka, H.; Kobayashi, M. State of the art in deep mixing technology, part II: Applications. Ground Improv. 1998, 3, 125–139. [Google Scholar] [CrossRef]
- Hansson, T. Dry deep mixing; A working procedure for improvement of organic soil. In Deep Mixing Workshop 2002; Port and Airport Research Institute: Tokyo, Japan; Coastal Development Institute of Technology: Tokyo, Japan, 2002. [Google Scholar]
- Timoney, M.J.; McCabe, B.A.; Bell, A.L. Experiences of dry soil mixing in highly organic soils. Proc. Inst. Civ. Eng. Ground Improv. 2012, 165, 3–14. [Google Scholar] [CrossRef]
- Bredenberg, H. Keynote Lecture: Equipment for Deep Soil Mixing with the Dry Jet Mix Method. In Dry Mixing Methods for Deep Soil Stabilisation; Brendenberg, H., Holm, G., Broms, B., Eds.; Balkema: Rotterdam, The Netherlands, 1999; pp. 323–331. [Google Scholar]
- Taki, O.; Bell, R.A. Soil-cement pile/column—A systems of deep mixing. In Proceedings of the Geo-Congress 98, Boston, MA, USA, 18–21 October 1998; pp. 59–71. [Google Scholar]
- Usui, H. Quality control of cement deep mixing method (wet mixing method) in Japan. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; Volume 1.2, pp. 635–638. [Google Scholar]
- Larsson, S.; Nilsson, L. Findings of the work on influencing factors on the installation process for lime-cement columns. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; pp. 561–569. [Google Scholar]
- Larsson, S.; Dahlstrom, M.; Nilsson, B. A complementary field study on the uniformity of lime-cement columns for deep mixing. Ground Improv. 2005, 9, 67–77. [Google Scholar] [CrossRef]
- Yang, D.S.; Coutu, C.J.; Scheibel, L.L. Quality control of cement deep soil mixing work for the Port of Oakland Projects. In Proceedings of the 5th International Conference on Case Histories in Geotechnical Engineering, New York, NY, USA, 13–17 April 2004. [Google Scholar]
- Anagnostopoulus, C.A.; Chatziangelou, M. Compressive Strength of Cement Stabilized Soils. A New Statistical Model. Electron. J. Geotech. Eng. 2009, 13, 1–10. [Google Scholar]
- Yousefpour, N.; Medina-Cetina, Z.; Hernandez-Martinez, F.G.; Al-Tabbaa, A. Stiffness and Strength of Stabilized Organic Soils—Part II/II: Para-metric Analysis and Modeling with Machine Learning. Geosciences 2021, 11, 218. [Google Scholar] [CrossRef]
- Hernández Martínez, F.G.; Osman, A.A.-M.; Al-Tabbaa, A. Wet soil improvement of soft clays and organic soils: Laboratory investigation. In Proceedings of the XIV European Conference on Soils Mechanics and Geotechnical Engineering, Madrid, Spain, 24–27 September 2007; Cuellar, V., Dapena, E., Alonso, E., Gens, A., Justo, J.L., Oteo, C., Rodriguez-Ortiz, J.M., Sagasera, C., Sola, P., Soriano, A., Eds.; Millpress: Madrid, Spain, 2007; Volume 3, pp. 1329–1334. ISBN 978-90-5966-055-7. [Google Scholar]
- Hernández Martínez, F.G.; Al-Tabbaa, A. Laboratory strength correlations for cement-treated peat. In GEO-TRANS 2004, Geotechnical Engineering for Transportation Projects; Yegian, M., Kavazanjian, E., Eds.; Geotechnical Special Publication No. 126; ASCE: Los Angeles, CA, USA, 2004; Volume 2, pp. 1403–1411. ISBN 0-7844-0744-4. [Google Scholar]
- Hernández Martínez, F.G.; Al-Tabbaa, A. Strength Properties of Stabilised Peat. In Proceedings of the International Conference of Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; Volume 1.1, pp. 69–78. [Google Scholar]
- Hernández Martínez, F.G.; Al-Tabbaa, A. Mechanical properties of stabilised peat based on laboratory testing. In Proceedings of the 16th International Conference on Soil Mechanics and Geotechnical Engineering, 16 ICSMGE, Osaka, Japan, 12–16 September 2005; Millpress: Bethlehem, PA, USA, 2005; Volume 3, pp. 1193–1198. [Google Scholar]
- Hernández-Martínez, F.G.; Al-Tabbaa, A. Effectiveness of different binders in the stabilisation of organic soils. In Proceedings of the International Symposium on Deep Mixing and Admixture Stabilization, Okinawa, Japan, 19–21 May 2009. Paper CT-3. [Google Scholar]
- Hobbs, N.B. Mire morphology and the properties and behaviour of some British and foreign peats. Quat. J. Eng. Geol. 1986, 19, 7–80. [Google Scholar] [CrossRef]
- Hartlén, J.; Wolski, W. (Eds.) Embankment on Organic Soils; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- British Standard Institution (BSI). Soil improvers and growing media: Determination of a quantity. BS EN 12580:2000; BSI Publications: London, UK, 2000. [Google Scholar]
- ASTM International. D2974-14 Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils; West ASTM International: Conshohocken, PA, USA, 2014. [Google Scholar]
- Head, K.H. Manual of Soil Laboratory Testing: Volume 1: Soil Classification and Compaction Tests; Pentech Press: London, UK, 1992. [Google Scholar]
- British Standard Institution (BSI). Cement-part 1: Composition, specifications and conformity criteria for common cements. In BS EN 197-1; BSI: London, UK, 2000. [Google Scholar]
- Al-Tabbaa, A.; Perera, A.S.R. State of practice report UK stabilisation/solidification treatment and remediation: Binder and technologies—Part, I. Basic principles. In Proceedings of the International Conference on Stabilisation/Solidification Treatment and Remediation, Cambridge, UK, 12–13 April 2005; CRC Press: Boca Raton, FL, USA, 2005; pp. 365–386. [Google Scholar]
- Neville, A.M. Properties of Concrete, 4th ed.; Pearson Education Limited: Harlow, UK, 1995. [Google Scholar]
- Neville, A.M.; Brooks, J.J. Concrete Technology, 2nd ed.; Longman Group UK limited: Harlow, UK, 1993. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Dyer, O. A Rock and Hard place, Eco-cement yet to Cover Ground in the Building Industry. The Guardian, 28 May 2003. [Google Scholar]
- Pearce, F. Green foundations. New Sci. 2002, 175, 39–41. [Google Scholar]
- Harrison, A.J.W. The Case for and Ramification of Blending Reactive Magnesia with Portland cement. In Proceedings of the 28th Annual Conference on Our World in Concrete Structures, Singapore, 28–29 August 2003. [Google Scholar]
- Bensted, J.; Barnes, P. (Eds.) Structure and Performance of Cements, 2nd ed.; Spon Press: London, UK, 2002. [Google Scholar]
- Holm, G. Keynote lecture: Towards a sustainable society—Recent advances in deep mixing. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; Volume 1.1, pp. K13–K24. [Google Scholar]
- Axelsson, K.; Johansson, S.E.; Andersson, R. Stabilization of Organic Soils by Cement and Puzzolanic Reactions—Feasibility Study; Report 3; Swedish Deep Stabilization Research CentreL: Linkoping, Sweden, 2002; pp. 1–54. [Google Scholar]
- Åhnberg, H.; Johansson, S.-E.; Pihl, H.; Carlsson, T. Stabilising effects of different binders in some Swedish soils. Ground Improv. 2003, 7, 9–23. [Google Scholar] [CrossRef]
- Hebib, S.; Farrell, E.R. Some experience of stabilising Irish organic soils. In Dry Mixing Methods for Deep Soil Stabilisation; Brendenberg, H., Holm, G., Broms, B., Eds.; Balkema: Rotterdam, The Netherlands, 1999; pp. 81–84. [Google Scholar]
- Lahtinen, P.; Jyrava, H.; Kuusipuro, K. Development of binders for organics soils. In Dry Mixing Methods for Deep Soil Stabilisation; Brendenberg, H., Holm, G., Broms, B., Eds.; Balkema: Rotterdam, The Netherlands, 1999; pp. 109–114. [Google Scholar]
- Head, K.H. Manual of Soil Laboratory Testing: Volume 2: Permeability; Shear Strength and Compressibility Tests Pentech Press: London, UK, 1982. [Google Scholar]
- Butcher, A.P. The durability of deep wet mixed columns in an organic soil. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; Volume 1.1, pp. 47–54. [Google Scholar]
- Hebib, S.; Farrell, E.R. Some experience of the stabilisation of Irish peats. Can. Geotech. J. 2003, 40, 107–120. [Google Scholar] [CrossRef]
- Skels, P.; Bondars, K.; Korjakins, A. Unconfined compressive strength properties of cement stabilized peat. In Proceedings of the Civil Engineering 4th International Science Conference, Jelgava, Latvia, 16–17 May 2013; Volume 4, pp. 202–206. [Google Scholar]
- Sing, W.L.; Hashim, R.; Ali, F. Unconfined compressive strength characteristics of stabilized peat. Sci. Res. Essays 2011, 6, 1915–1921. [Google Scholar]
- Tomac, I.; Van Impe, W.F.; Verástegui-Flores, R.D.; Mengé, P. Binder-soil interaction in cement deep mixing through SEM analysis. In Proceedings of the 16th International Conference on Soil Mechanics and Geotechnical Engineering, Osaka, Japan, 12–16 September 2005; pp. 1149–1936. [Google Scholar]
- Van Impe, W.F.; Verástegui-Flores, R.D. Deep Mixing in underwater conditions: A laboratory and field investigation. Ground Improv. 2006, 10, 15–22. [Google Scholar] [CrossRef]
- Kolay, P.K.; Pui, M.P. Peat Stabilization using Gypsum and Flay Ash. J. Civ. Eng. Sci. Technol. 2010, 1, 1–5. [Google Scholar]
- Boobathiraja, S.; Balamurugan, P.; Dhansheer, M.; Adhikari, A. Study on strength of peat soil stabilised with cement and other pozzolanic materials. Int. J. Civil Eng. Res. 2014, 5, 431–438. [Google Scholar]
- Lakshmi, S.; Ramya, K. Strength Characteristics of Stabilized Peat Soil using Fly Ash. Grenze Int. J. Eng. Technol. 2015, 1. Available online: https://issuu.com/grenze/docs/gijet_volume1_issue2 (accessed on 1 May 2021).
- Sherwood, P.T. Soil Stabilization with Cement and Lime: Trl State of the Art Review; HSMO: London, UK, 1993. [Google Scholar]
- Hewlett, P.C. (Ed.) Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier: London, UK, 1998. [Google Scholar]




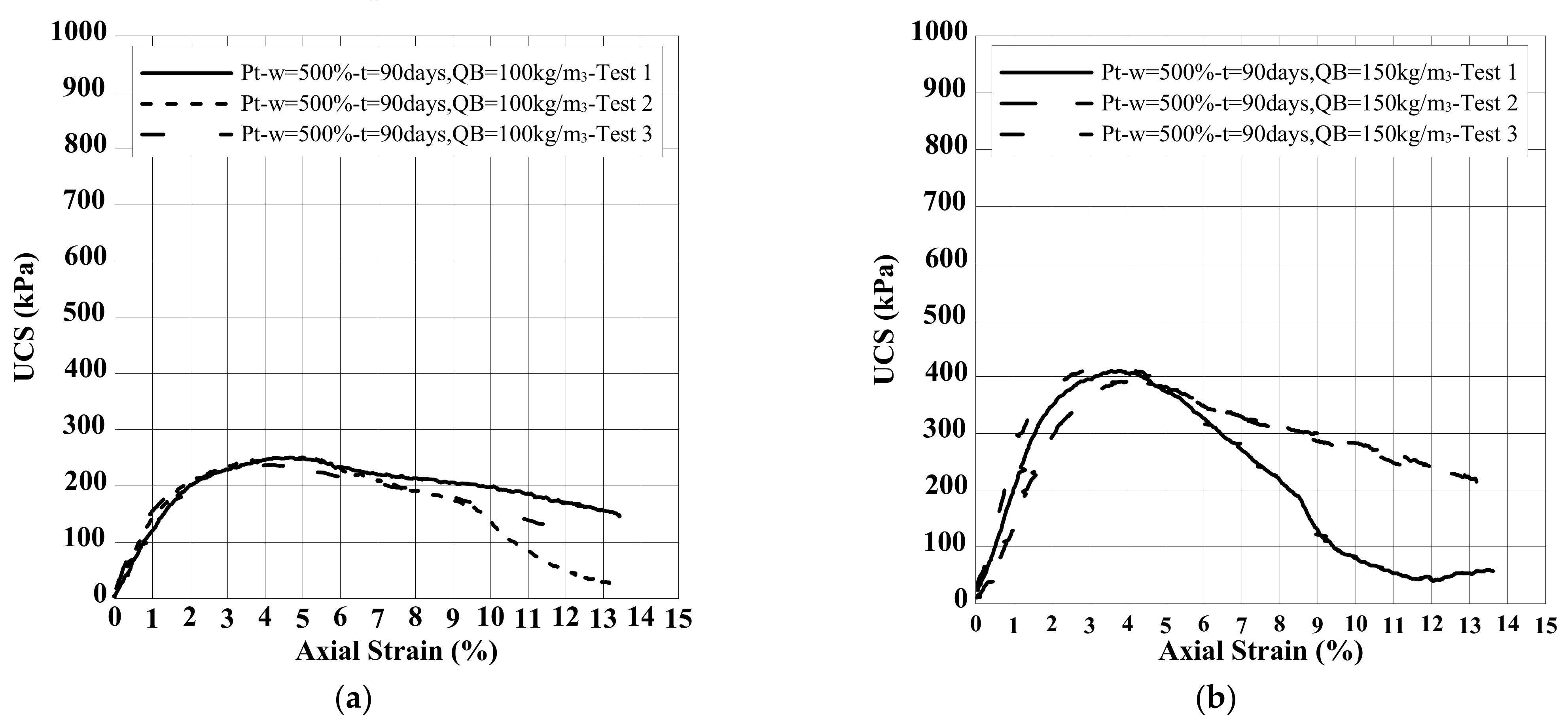
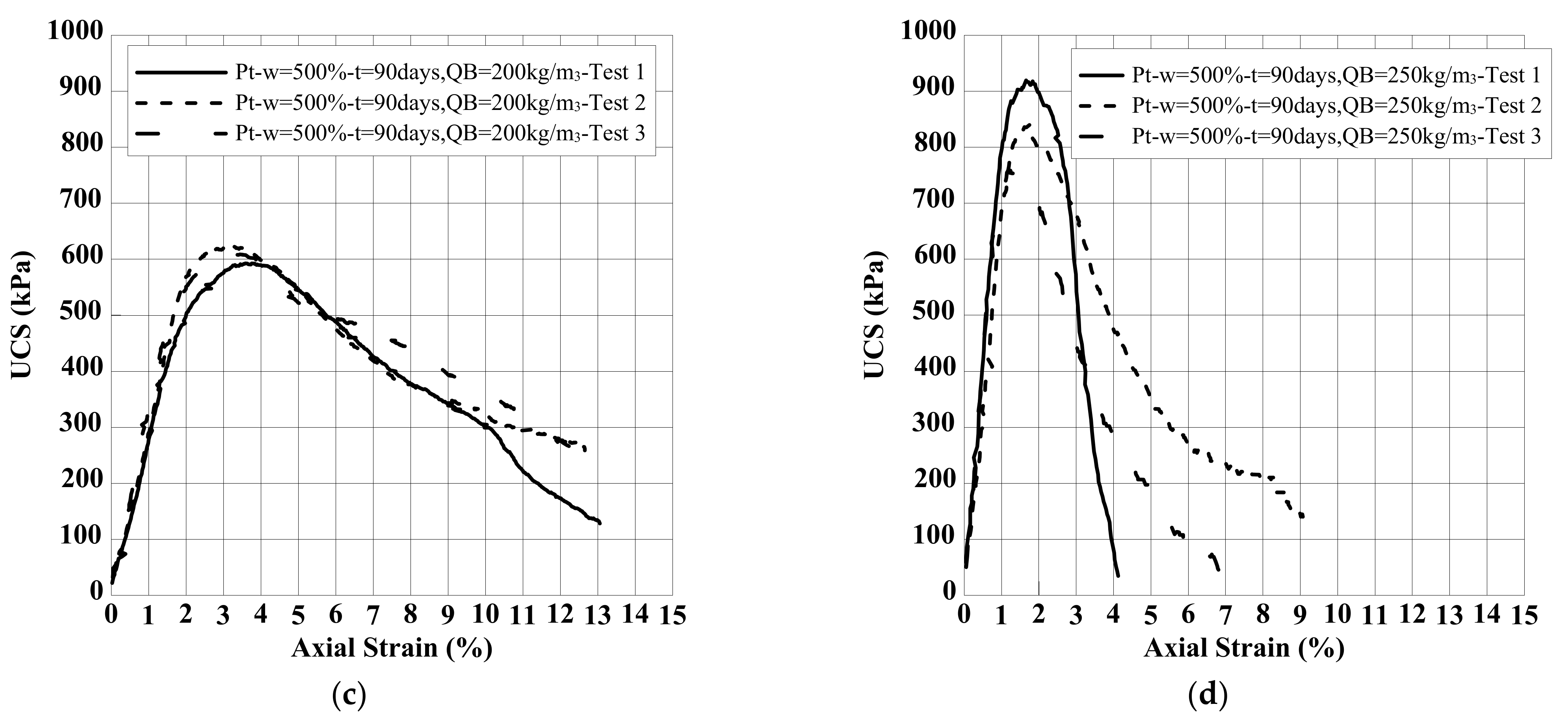


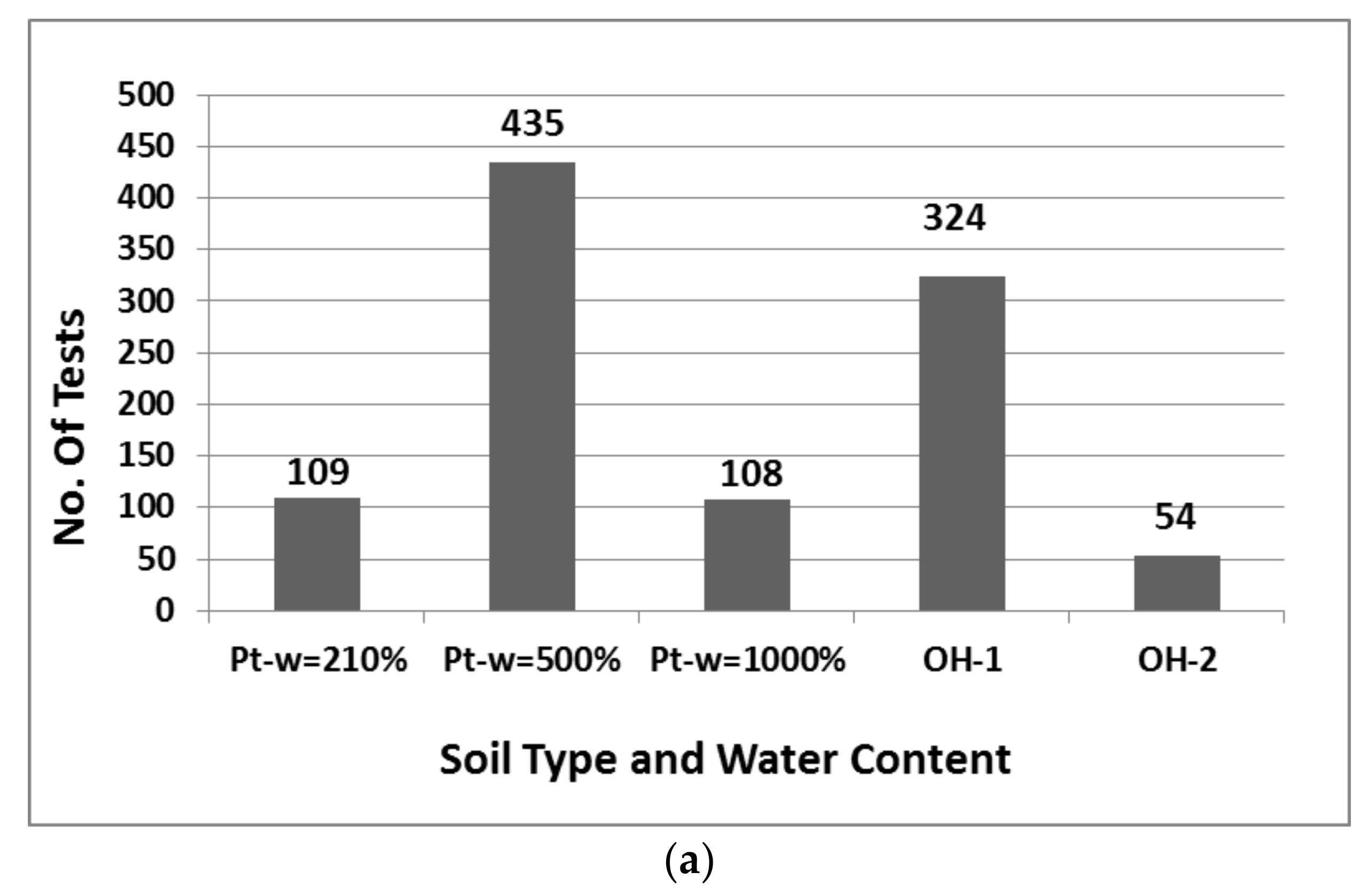
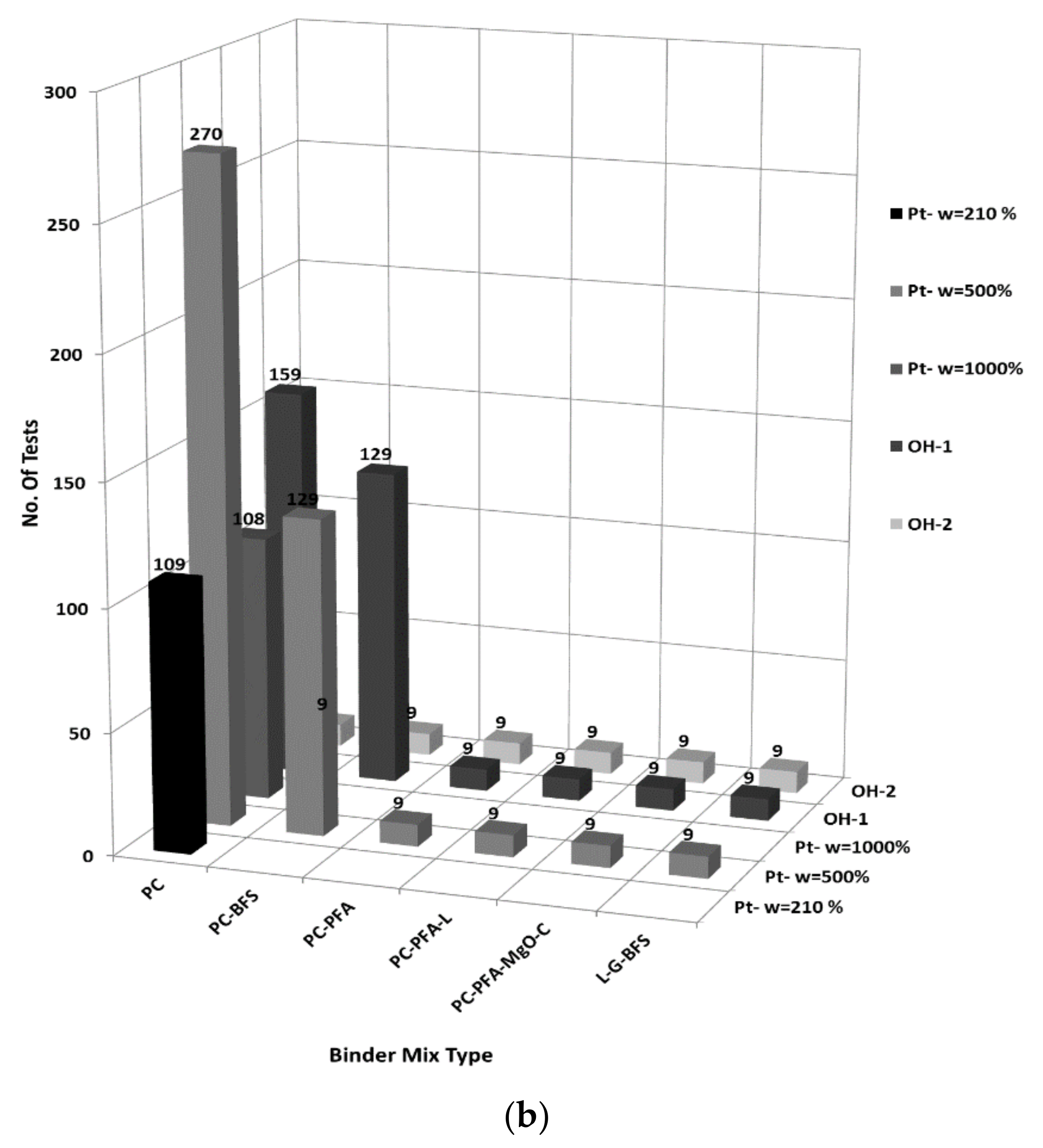


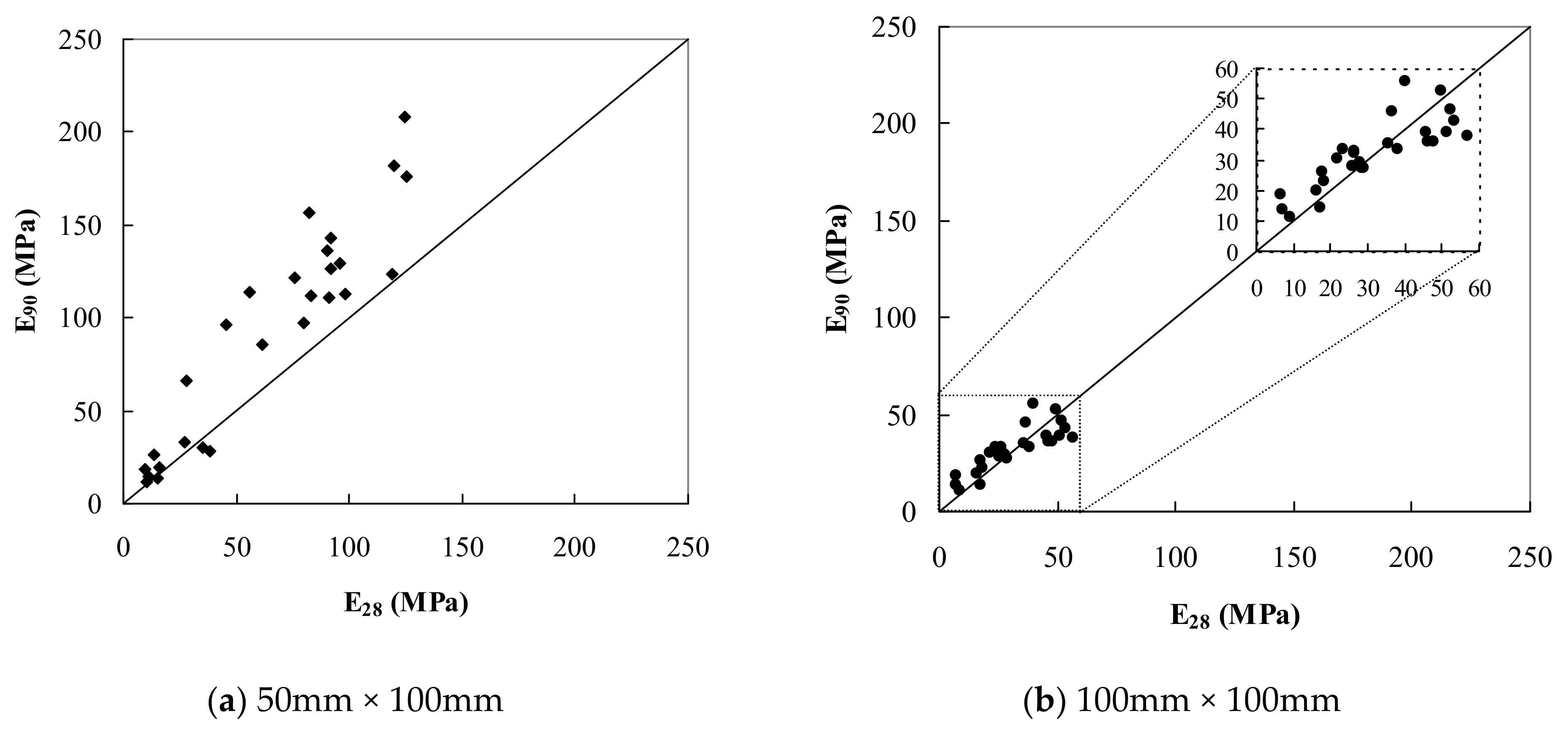
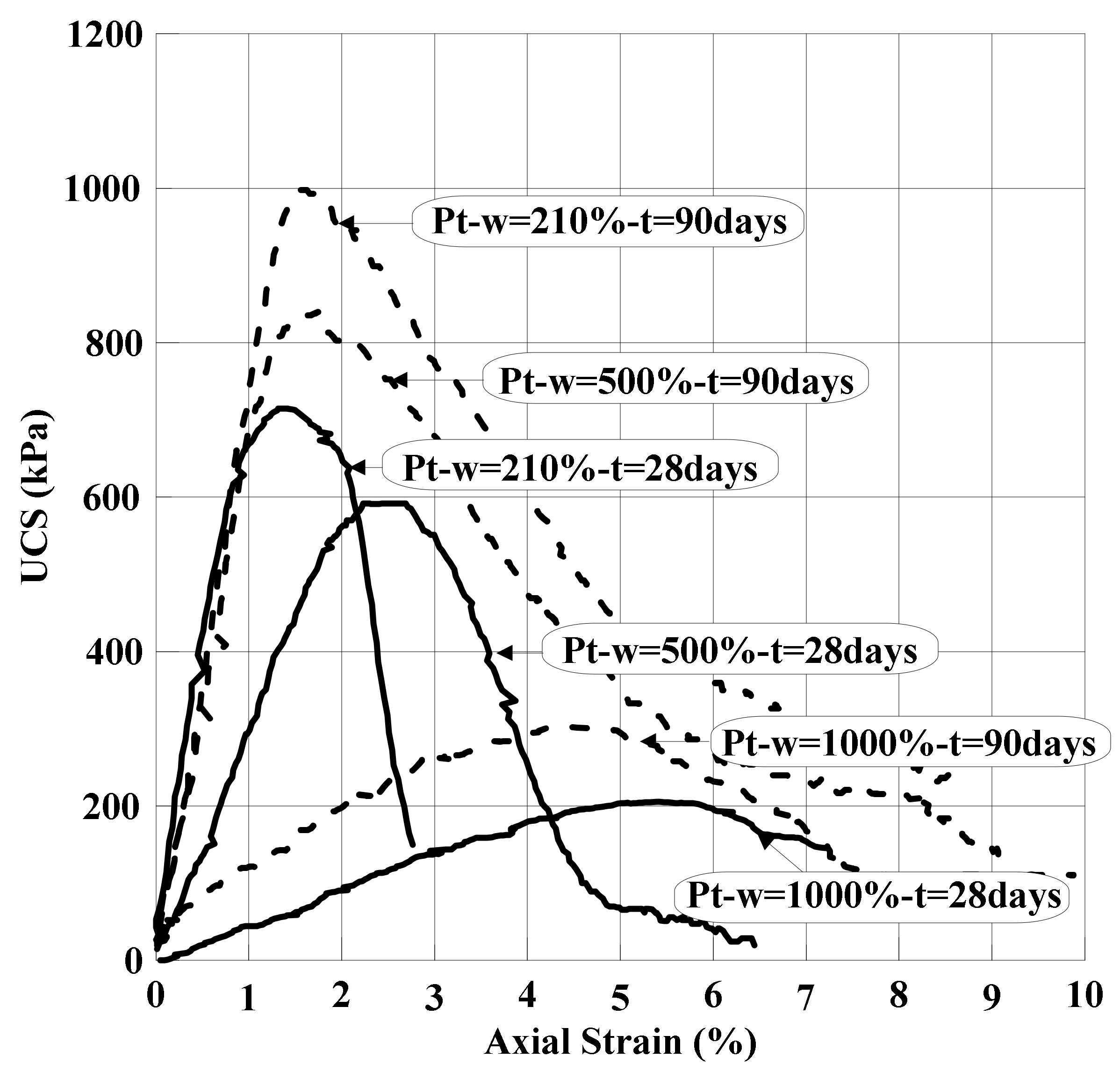

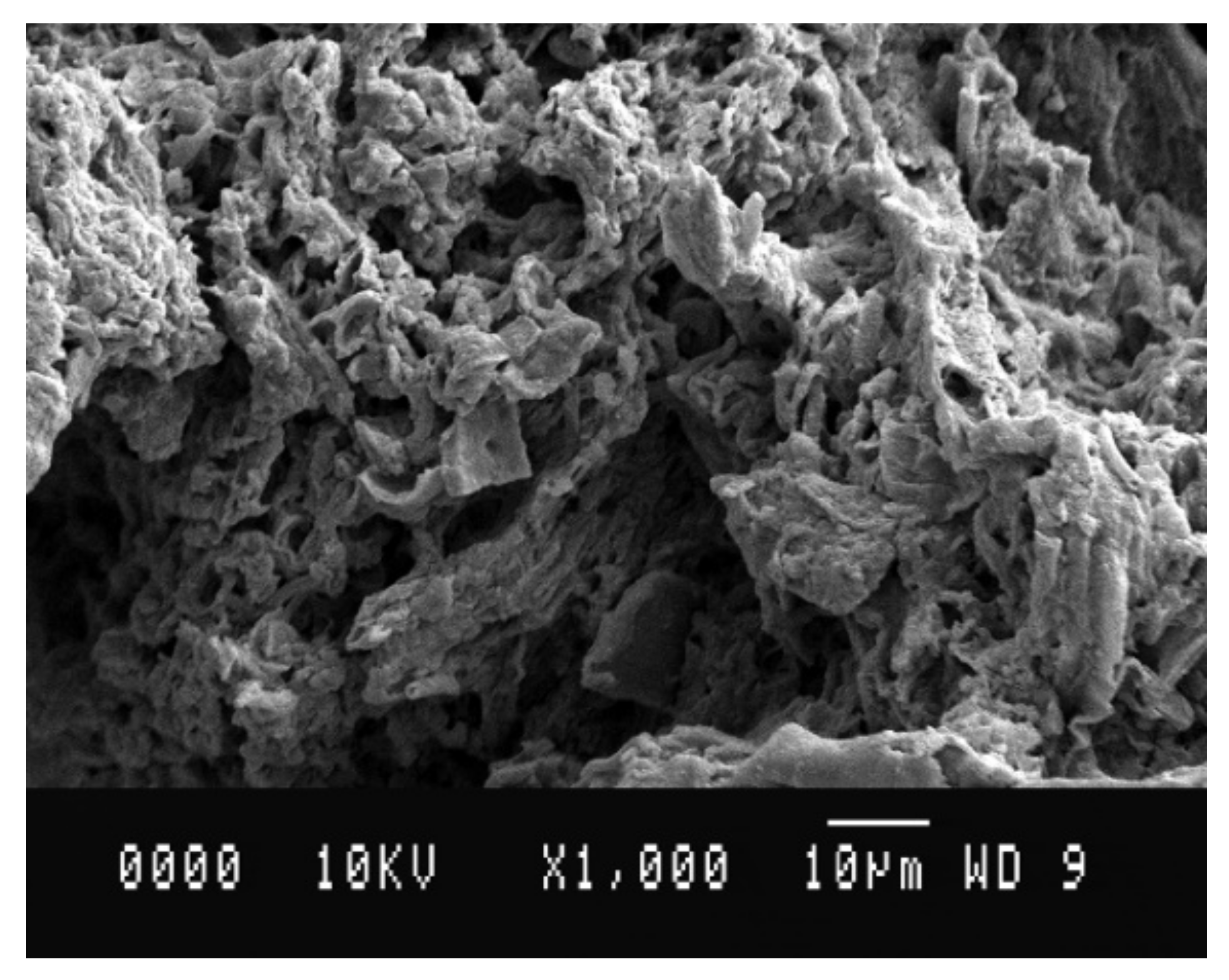

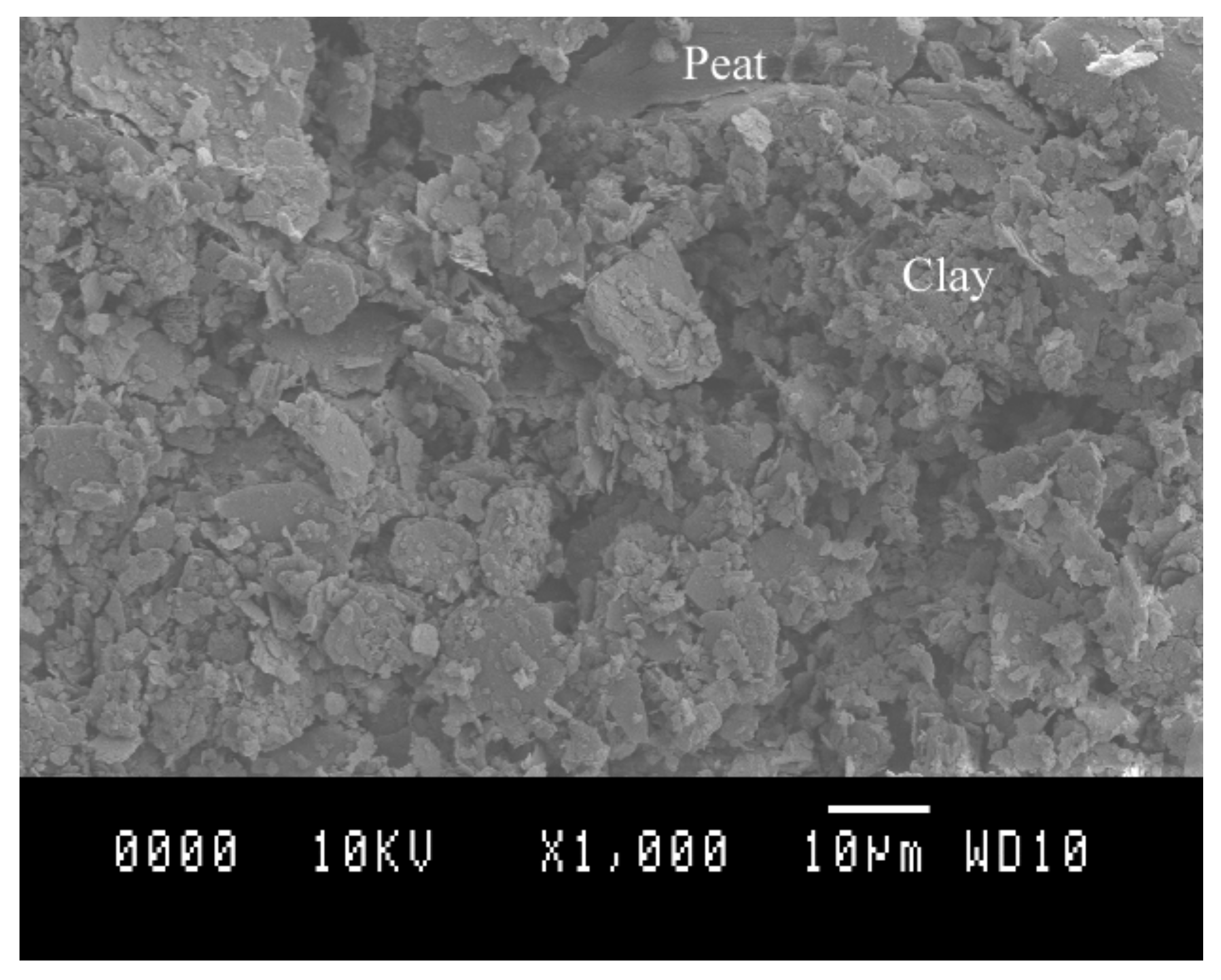

| Clay | Density Kg/m3 | Water Content w (%) | Liquid Limit wL (%) | Plastic Limit wP (%) | Organic Content OC (%) | Soil Classification |
|---|---|---|---|---|---|---|
| Medium-organic clay of extremely high plasticity (OH-1) | 1219 | 180 | 155.7 | 123 | 30 | OH-1 |
| Low-organic clay of high plasticity (OH-2) | 1471 | 85 | 65 | 33.5 | 4 | OH-2 |
| Grout | ||||||||||||
| Admixtures | Peat:Grout Ratios | Binders Ratios | Peat | Water Added % | Binders | |||||||
| Dry Peat % | Water % | PC % | PFA % | BFS % | L % | MgO-C % | G % | |||||
| Pt | Pt-PC-w= 210% | 1:1.7 | 11.9 | 25.1 | 31.5 | 31.5 | ||||||
| Pt-PC-w= 500% | 1:1.12 | 7.8 | 39.4 | 26.4 | 26.4 | |||||||
| Pt-PC-w= 1000% | 1:0.33 | 6.8 | 68.4 | 8.2 | 16.5 | |||||||
| Pt-PC-w= 500% | 1:1.12 | 7.8 | 39.4 | 26.4 | 26.4 | |||||||
| Pt-PC-BFS-w= 500% | 1:1.12 | 1:2 | 7.8 | 39.4 | 26.4 | 8.8 | 17.6 | |||||
| Pt-PC-PFA-w= 500% | 1:1.12 | 1:1 | 7.8 | 39.4 | 26.4 | 13.2 | 13.2 | |||||
| Pt-PC-PFA-L-w= 500% | 1:1.12 | 3:6:1 | 7.8 | 39.4 | 26.4 | 8 | 15.6 | 2.8 | ||||
| Pt-PC-PFA-MgO-C-w= 500% | 1:1.12 | 2:6:2 | 7.8 | 39.4 | 26.4 | 5.2 | 16 | 5.2 | ||||
| Pt-L-G-BFS-w= 500% | 1:1.12 | 1:1:1 | 7.8 | 39.4 | 26.4 | 8.8 | 8.8 | 8.8 | ||||
| Clay-peat:Grout Ratios | Binders Ratios | Dry Medium Organic Clay % | Water % | Water Added % | Binders | |||||||
| PC % | PFA % | BFS % | L % | MgO-C % | G % | |||||||
| OH-1 | OH-1-PC-w= 180% | 1:0.41 | 25.4 | 45.6 | 14.5 | 14.5 | ||||||
| OH-1-PC-BFS-w = 180% | 1:0.41 | 1:2 | 25.4 | 45.6 | 14.5 | 4.8 | 9.7 | |||||
| OH-1-PC-PFA-w = 180% | 1:0.41 | 1:1 | 25.4 | 45.6 | 14.5 | 7.25 | 7.25 | |||||
| OH-1-PC-PFA-L-w = 180% | 1:0.41 | 3:6:1 | 25.4 | 45.6 | 14.5 | 4.4 | 8.7 | 1.4 | ||||
| OH-1-PC-PFA-MgO-C-w= 180% | 1:0.41 | 2:6:2 | 25.4 | 45.6 | 14.5 | 2.9 | 8.7 | 2.9 | ||||
| OH-1-L-G-BFS-w= 180% | 1:0.41 | 1:1:1 | 25.4 | 45.6 | 14.5 | 4.8 | 4.8 | 4.8 | ||||
| Clay-Peat:Grout Ratios | Binders Ratios | Dry Low organic Clay % | Water % | Water Added % | Binders | |||||||
| PC % | PFA % | BFS % | L % | MgO-C % | G % | |||||||
| OH-2 | OH-2-PC-w= 85% | 1:0.34 | 40.3 | 34.3 | 12.7 | 12.7 | ||||||
| OH-2-PC-BFS-w = 85% | 1:0.34 | 1:2 | 40.3 | 34.3 | 12.7 | 4.2 | 8.46 | |||||
| OH-2-PC-PFA-w= 85% | 1:0.34 | 1:1 | 40.3 | 34.3 | 12.7 | 6.35 | 6.35 | |||||
| OH-2-PC-PFA-L-w= 85% | 1:0.34 | 3:6:1 | 40.3 | 34.3 | 12.7 | 3.8 | 7.62 | 1.27 | ||||
| OH-2-PC-PFA-MgO-C-w = 85% | 1:0.34 | 2:6:2 | 40.3 | 34.3 | 12.7 | 2.5 | 7.64 | 2.5 | ||||
| OH-2-L-G-BFS-w= 85% | 1:0.34 | 1:1:1 | 40.3 | 34.3 | 12.7 | 4.2 | 4.2 | 4.2 | ||||
| Ref. | Variables | Range of Variation | Mean | Standard Deviation |
|---|---|---|---|---|
| Control Variables (Input Parameters) | ||||
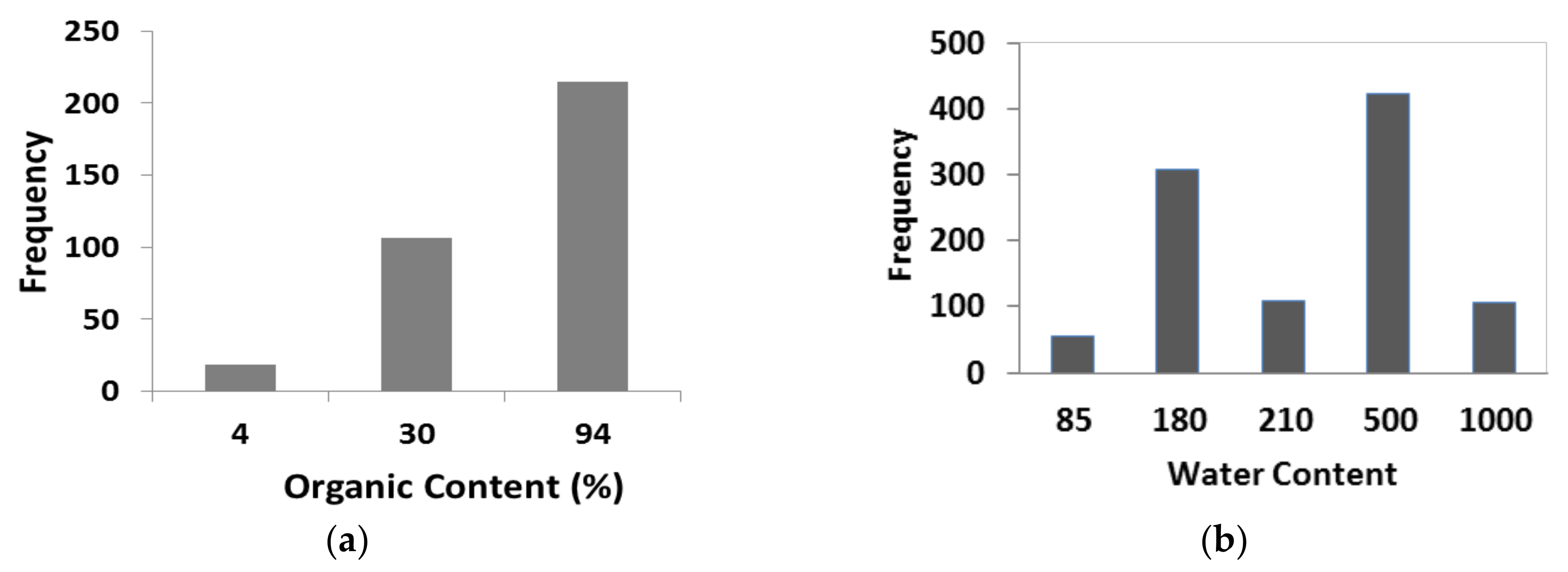
| a | Organic Content of Soil (OC) | 4 (OH-2), 30 (OH-1), 94 (Pt) (%) | 69.2 (%) | 33.2 (%) |
| b | Water Content of Soil (w) | 85, 180, 210, 500, 1000 (%) | 400.2 (%) | 258.6 (%) |
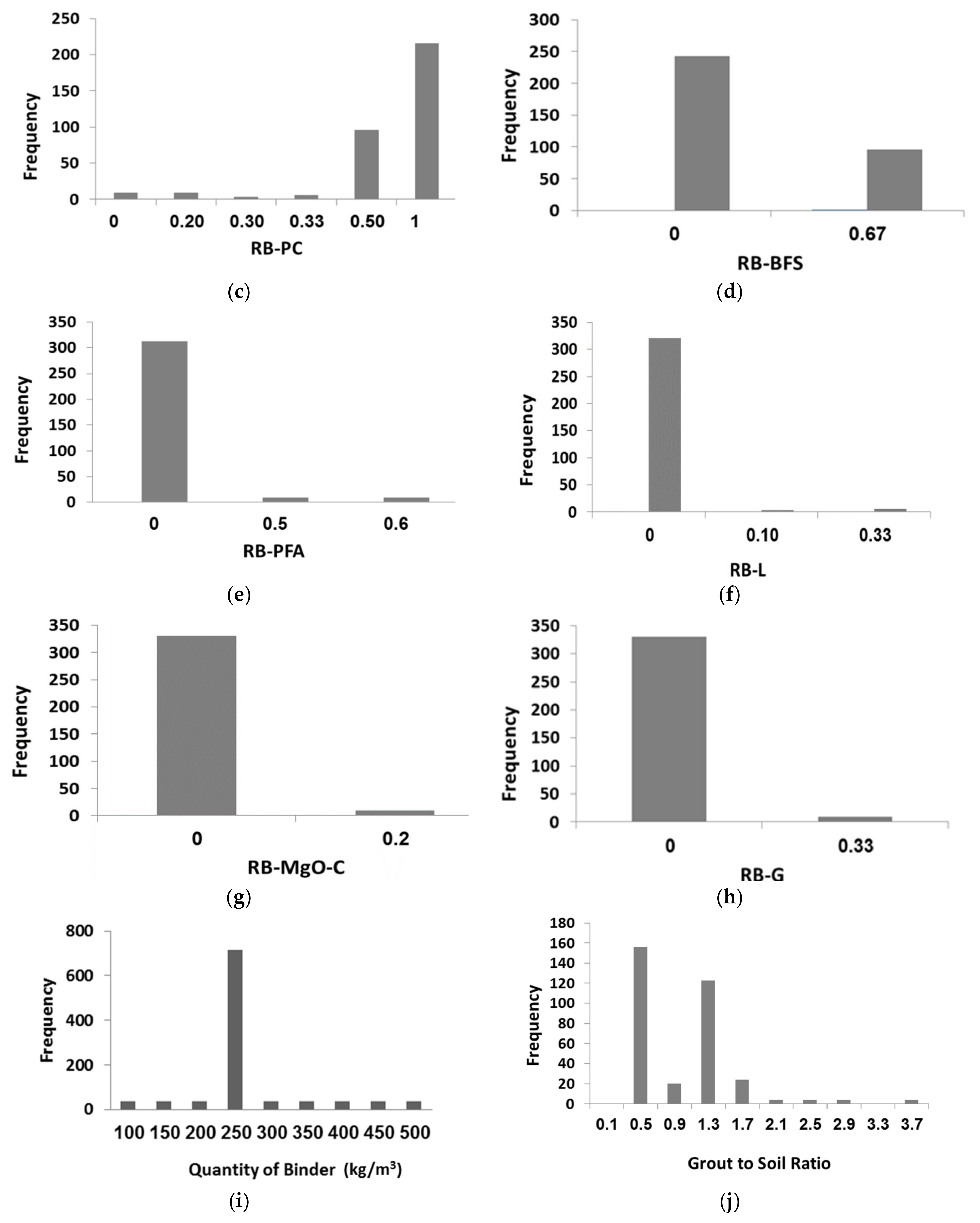
| c | Ratio of Binder for Portland cement (RB-PC) | 0, 0.2, 0.3, 0.33, 0.5, 1 | 0.749 | 0.339 |
| d | Ratio of Binder for Blast Furnace Slag (RB-BFS) | 0, 0.33, 0.67 | 0.180 | 0.292 |
| e | Ratio of Binder for Pulverized Fuel Ash (RB-PFA) | 0, 0.5, 0.6 | 0.045 | 0.154 |
| f | Ratio of Binder for Lime (RB-L) | 0, 0.1, 0.33 | 0.012 | 0.056 |
| g | Ratio of Binder for Magnesium Oxide (RB-MgO-C) | 0, 0.2 | 0.005 | 0.032 |
| h | Ratio of Binder for Gypsum (RB-G) | 0, 0.33 | 0.009 | 0.054 |
| i | Quantity of Binder (QB) | 100–500 (kg/m3) | 265.9 (kg/m3) | 76.6 (kg/m3) |
| j | Grout to Soil Ratio (G/S) | 0.14–3.38 | 0.856 | 0.568 |
| k | Water to Binder ratio (W/B) | 0.5, 0.8, 1 | 0.937 | 0.157 |
| l | Specimen Diameter/Height (D/H) | 0.5, 1 | 0.920 | 0.183 |
| m | Time (t) | 14–180 (days) | 61 (days) | 36 (days) |
| n | Temperature (T) | 21, 45, 60 (ºC) | 32.9 (ºC) | 15.7 (ºC) |
| o | Relative Humidity (RH) | 70, 80, 90 (%) | 87.6 (%) | 4.9 (%) |
| p | Carbonation (CO2) | 0, 20 (%) | 2.9 (%) | 7.1 (%) |
| Response Variables (Target Parameters) | ||||
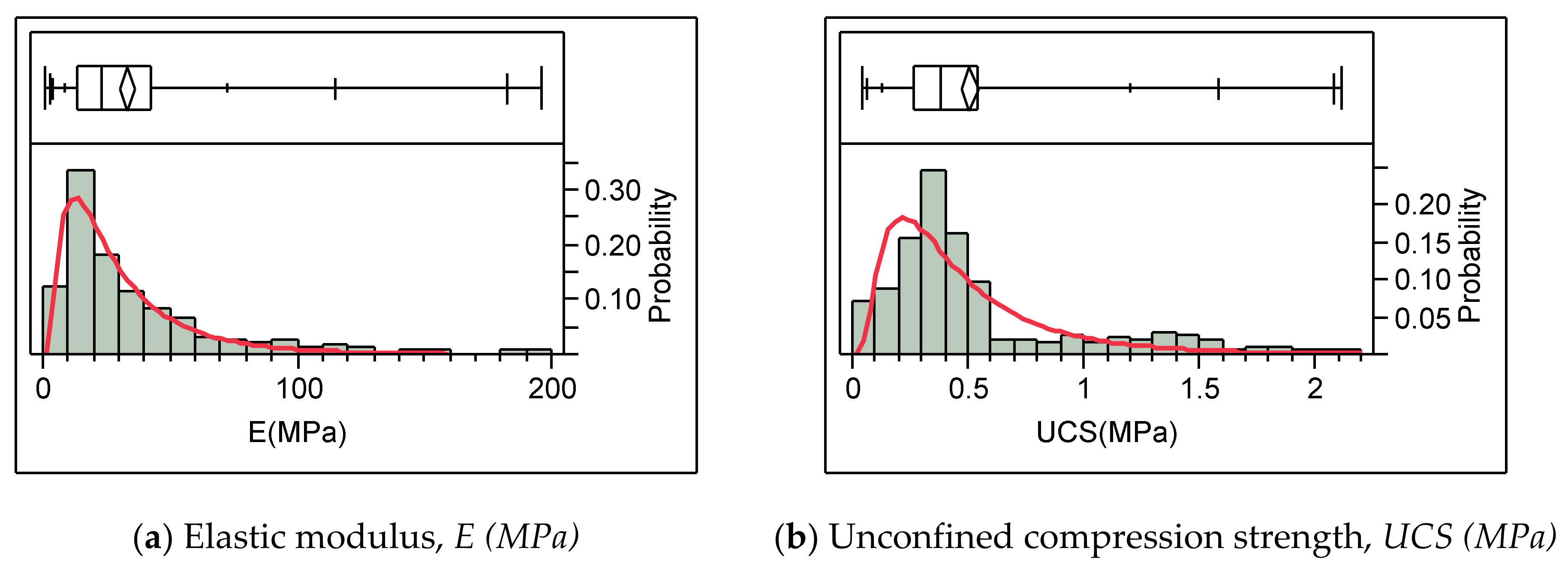
| q | Elastic Modulus (E) | 0.83–214.62 (MPa) | 34.03 (MPa) | 31.44 (MPa) |
| r | Unconfined Compression Strength (UCS) | 0.04–2.09 (MPa) | 0.50 (MPa) | 0.40 (MPa) |
| Statistics | E (MPa) | UCS (MPa) |
|---|---|---|
| Minimum | 0.83 | 0.04 |
| Median | 24.32 | 0.39 |
| Maximum | 214.62 | 2.09 |
| Mean | 34.03 | 0.50 |
| Standard Deviation | 31.49 | 0.41 |
| Number of Data Points | 339 | 339 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Martinez, F.G.; Al-Tabbaa, A.; Medina-Cetina, Z.; Yousefpour, N. Stiffness and Strength of Stabilized Organic Soils—Part I/II: Experimental Database and Statistical Description for Machine Learning Modelling. Geosciences 2021, 11, 243. https://doi.org/10.3390/geosciences11060243
Hernandez-Martinez FG, Al-Tabbaa A, Medina-Cetina Z, Yousefpour N. Stiffness and Strength of Stabilized Organic Soils—Part I/II: Experimental Database and Statistical Description for Machine Learning Modelling. Geosciences. 2021; 11(6):243. https://doi.org/10.3390/geosciences11060243
Chicago/Turabian StyleHernandez-Martinez, Francisco G., Abir Al-Tabbaa, Zenon Medina-Cetina, and Negin Yousefpour. 2021. "Stiffness and Strength of Stabilized Organic Soils—Part I/II: Experimental Database and Statistical Description for Machine Learning Modelling" Geosciences 11, no. 6: 243. https://doi.org/10.3390/geosciences11060243
APA StyleHernandez-Martinez, F. G., Al-Tabbaa, A., Medina-Cetina, Z., & Yousefpour, N. (2021). Stiffness and Strength of Stabilized Organic Soils—Part I/II: Experimental Database and Statistical Description for Machine Learning Modelling. Geosciences, 11(6), 243. https://doi.org/10.3390/geosciences11060243








