The Study of Uranium Accumulation in Marine Bottom Sediments: Effect of Redox Conditions at the Time of Sedimentation
Abstract
:1. Introduction
2. Regional Settings
3. Materials and Methods
4. Results of the White Sea Bottom Sediments Investigations
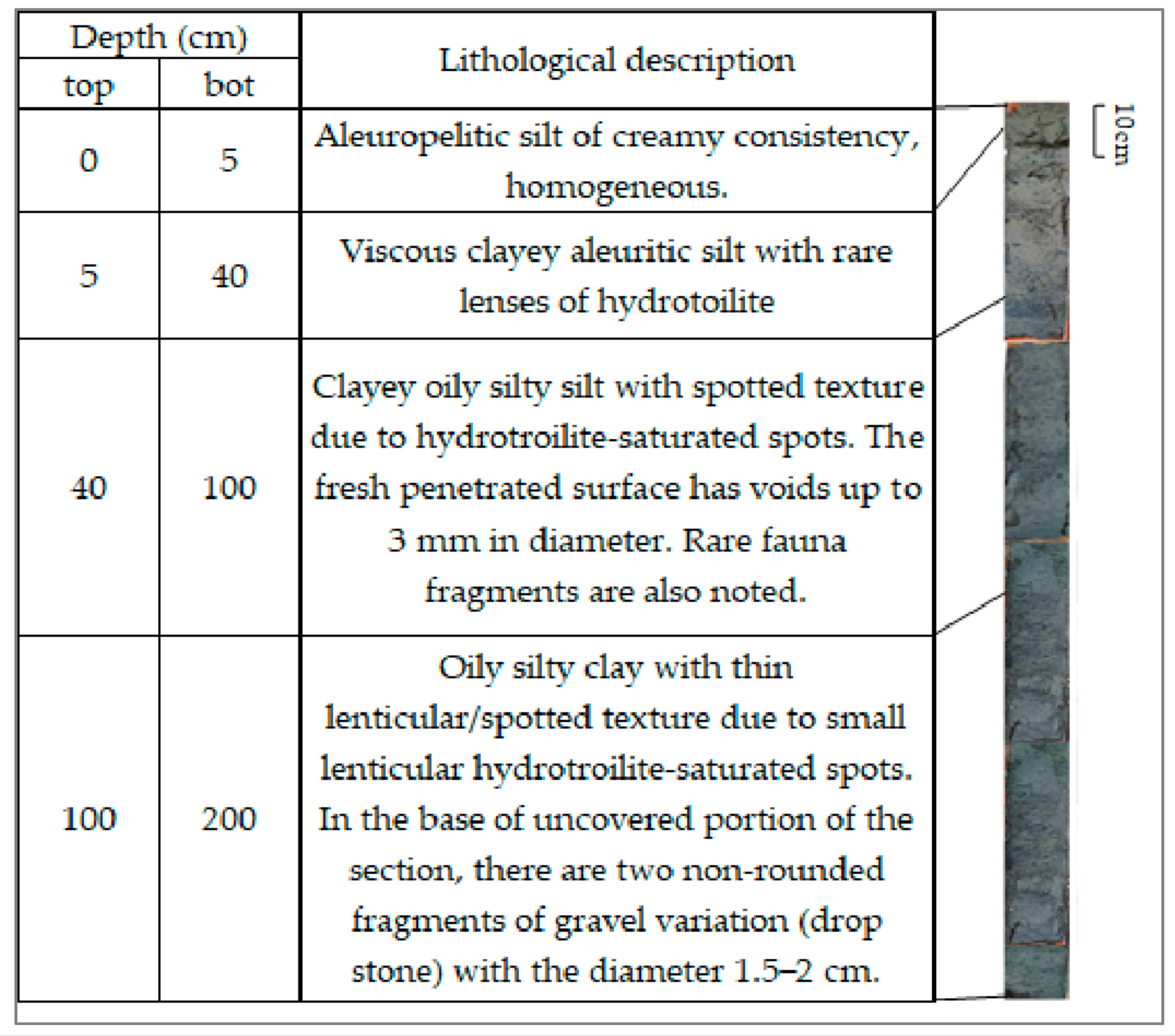
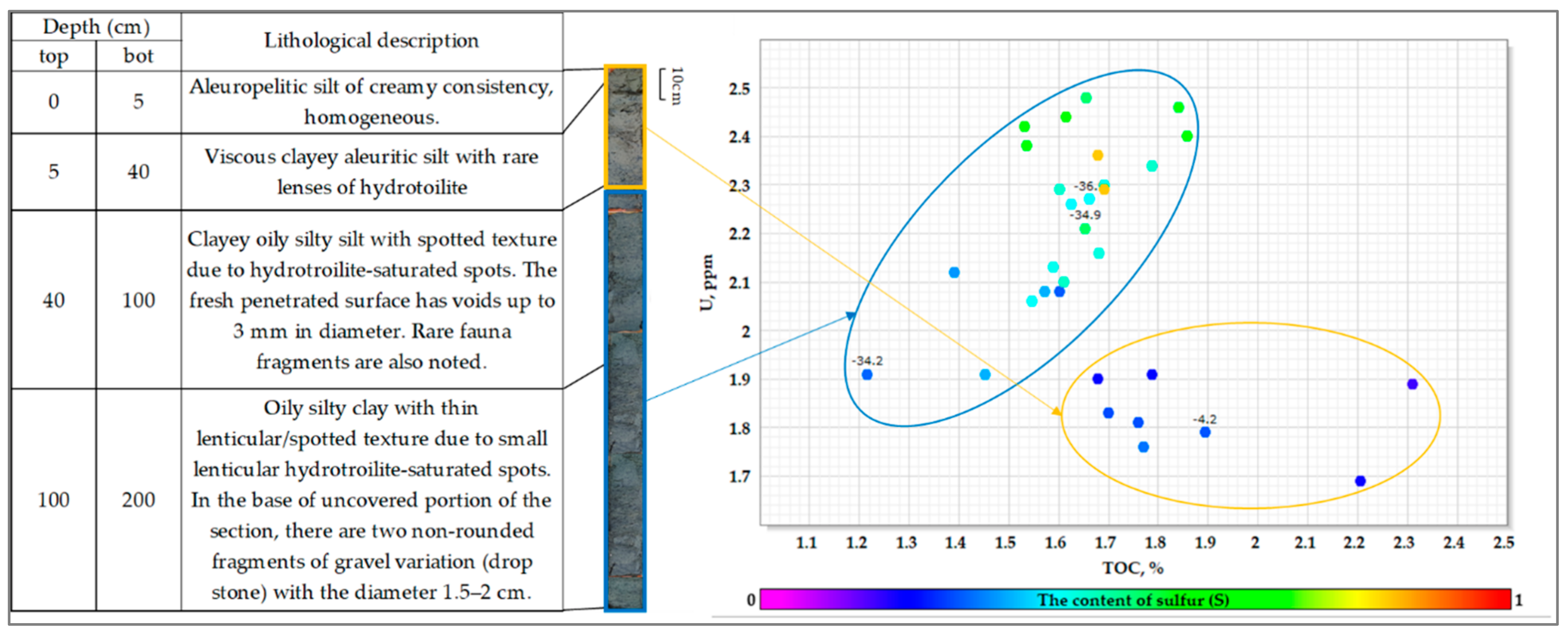
4.1. Lithology
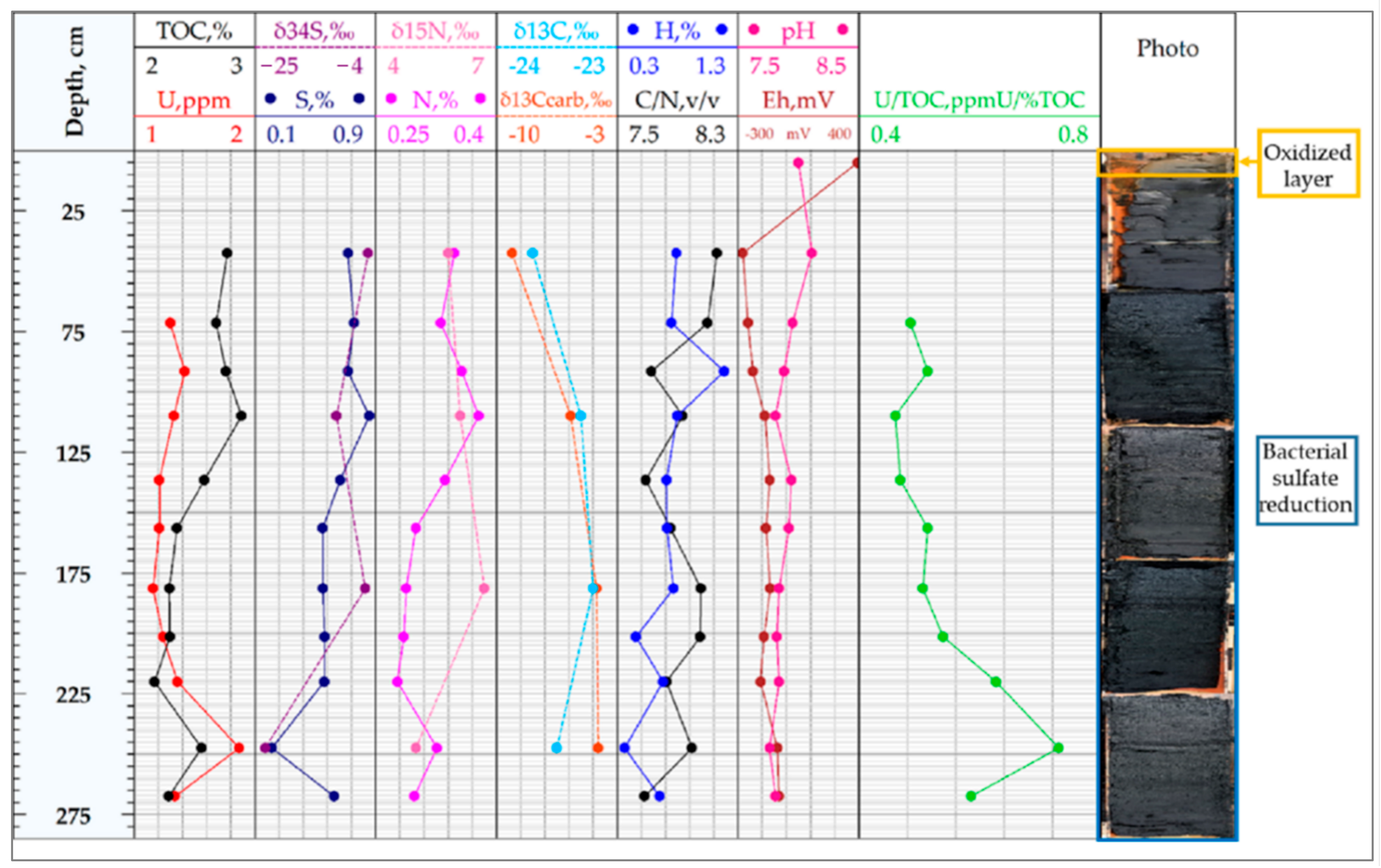
4.2. Eh, pH, and Temperature Values
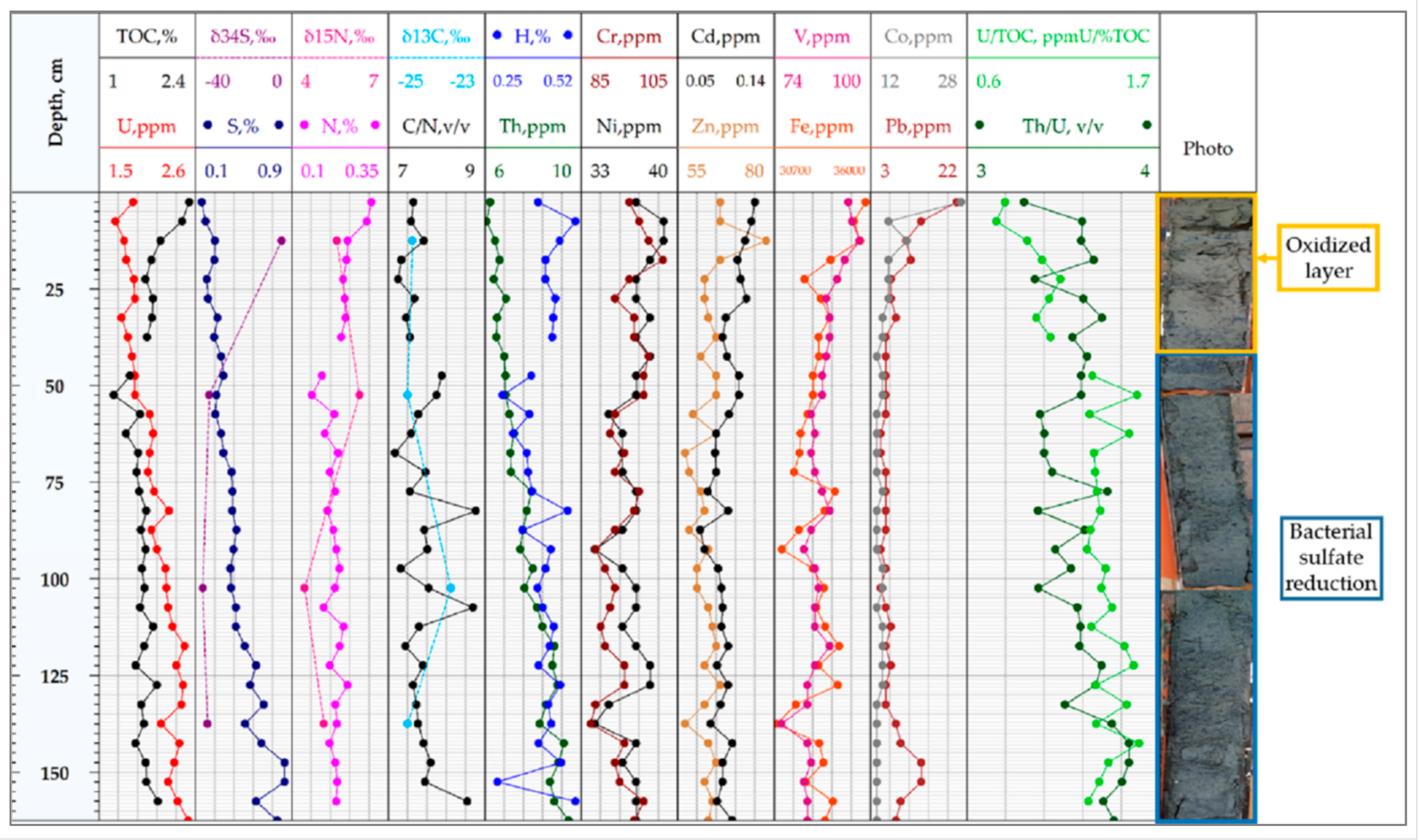
4.3. Uranium and Other Metals Concentrations
4.4. C, H, N, S Element Composition
4.5. C, N, S Isotope Composition
5. Discussion
5.1. The Results of the White Sea Sediments
5.2. Behaviour of Uranium in Bottom Sediments under Reducing Conditions in the Example of Black Sea
5.3. Behavior of Uranium in the Aqueous Solution at Different Eh and pH Conditions: Results of Thermodynamic Modeling
5.4. Comparison of Uranium Accumulation in Oxidizing and Reducing Conditions
6. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swanson, V.E. Geology and Geochemistry of Uranium in Marine Black Shales: A Review; US Government Printing Office: Washington, DC, USA, 1961; pp. 1–110. [Google Scholar]
- Lüning, S.; Kolonic, S. Uranium spectral gamma-ray response as a proxy for organic richness in black shales: Applicability and limitations. J. Pet. Geol. 2003, 26, 153–174. [Google Scholar] [CrossRef]
- Zubkov, M.Y. Peculiarities of uranium distribution in bituminous deposits of the Bazhenov formation (Western Siberia). Logger 2015, 5, 3–32. [Google Scholar]
- Bastrakov, E.; Boreham, C.; Edwards, D.; Jarrett, A. Uranium in organic-rich shales as a tool for predicting hydrocarbon potential: Proterozoic to Cretaceous examples from Australia. In Proceedings of the 20th Australian Organic Geochemistry Conference: Origins of Oil, Old Organics and Organisms, Canberra, Australia, 3–7 December 2018. [Google Scholar]
- Khaustova, N. U/Corg Ratio Within Unconventional Reservoirs: Indicator of Oil Generation Processes and Criteria for Productive Intervals Determination During the Bazhenov Formation Investigation. In Proceedings of the EAGE/SPE Workshop on Shale Science, Moscow, Russia, 8–9 April 2019. [Google Scholar]
- Fertl, W.H. Gamma ray spectral evaluation techniques identify fractured shale reservoirs and source-rock characteristics. J. Pet. Technol. 1980, 32, 11. [Google Scholar] [CrossRef]
- Vosel, J.S. Geohimiya Urana v Sovremennyh Karbonatnyh Otlozheniyah Malyh Ozer (Formy Nahozhdeniya i Izotopnye Otnosheniya 234U/238U). Ph.D. Thesis, V.S. Sobolev Institute of Geology and Mineralogy Siberian Branch Russian Academy of Sciences, Novosibirsk, Russia, 24 February 2016. [Google Scholar]
- Mangini, A.; Jung, M.; Laukenmann, S. What do we learn from peaks of uranium and manganese in deep-sea sediments? Mar. Geol. 2001, 177, 63–78. [Google Scholar] [CrossRef]
- Chappaz, A.; Gobeil, C.; Tessier, A. Controls on uranium distribution in lake sediments. Geochim. Cosmochim. Acta 2010, 74, 203–214. [Google Scholar] [CrossRef]
- Sklyarov, E.V. Klimaticheskaya istoriya golocena Zapadnogo Pribajkal’ya v karbonatnoj osadochnoj letopisi ozera Holbo-Nur. In Doklady Akademii Nauk; Federal’noe Gosudarstvennoe Unitarnoe Predpriyatie Akademicheskij Nauchno-Izdatel’skij, Proizvodstvenno-Poligraficheskij i Knigorasprostranitel’skij Centr Nauka, Izdatel’stvo Nauka: Moscow, Russia, 2010; Volume 431, pp. 668–674. [Google Scholar]
- Rolison, J.M. Uranium stable isotope fractionation in the Black Sea: Modern calibration of the 238U/235U paleo-redox proxy. Geochim. Cosmochim. Acta 2017, 203, 69–88. [Google Scholar] [CrossRef]
- Maslov, A.V. Geohimiya sovremennyh donnyh osadkov belogo, kaspijskogo i chernogo morej: Chto eto nam, “suhoputnym” geologam, daet. In Geologiya, Geoekologiya i Resursnyj Potencial Urala i Sopredel’nyh Territorij; BashNIPIneft: Ufa, Russia, 2016; Volume 4, pp. 129–135. [Google Scholar]
- Demidov, I.N. Kratkie svedeniya o geologii verhnej chasti osadochnogo chekhla Belogo moray. In Sistema Belogo Morya Prirodnaya Sreda Vodosbora Belogo Moray; Izdatel’stvo Nauchnyj mir: Moscow, Russia, 2012; Volume 1, pp. 54–71. [Google Scholar]
- Lein, A.Y.; Lisitsyn, A.P. Processy rannego diageneza arkticheskih morej (na primere Belogo morya). In The White Sea System The Processes of Sedimentation, Geology and History; Izdatel’stvo Nauchnyj mir: Moscow, Russia, 2012; Volume 4, pp. 504–571. [Google Scholar]
- OOO “Izmeritel’naya tekhnika”. Available online: http://www.izmteh.ru/esr/esr-10104/ (accessed on 30 July 2021).
- Coplen, T.B.; Hopple, J.A.; Böhlke, J.K.; Peiser, H.S.; Rieder, S.E.; Krouse, H.R.; Rosman, K.J.R.; Ding, T.; Vocke, R.D., Jr.; Révész, K.M.; et al. Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents. In Water-Resources Investigations Report 01-4222; Joint Research Centre: Geel, Belgium, 2002. [Google Scholar]
- Instrument: CHN628 Series w/Sulfur Add-On Module (S628). In Organic Application Note; Leco Corporation: Saint Joseph, MI, USA, 2014.
- Instrument: CHN628. In Organic Application Note; Leco Corporation: Saint Joseph, MI, USA, 2016.
- Gursky, Y.N. Osobennosti himicheskogo sostava ilovyh vod Belogo morya. Okeanologiya 2005, 45, 224–239. [Google Scholar]
- Lein, A.Y. Izuchenie morej rossijskoj Arktiki: Myslitel’nye processy. Priroda 2014, 8, 11–19. [Google Scholar]
- Moiseenko, T.N. Zagryaznenie poverhnostnyh vod vodosbora i osnovnye antropogenno obuslovlennye processy. In Sistema Belogo Morya Prirodnaya Sreda Vodosbora Belogo Moray; Izdatel’stvo Nauchnyj mir: Moscow, Russia, 2012; Volume 1, pp. 297–329. [Google Scholar]
- Lisitsyn, A.P.; Novigatsky, A.N.; Shevchenko, V.P.; Kluvitkin, A.A.; Filippov, A.S.; Kravchishina, M.D.; Politova, N.V. Dispersnye formy osadochnogo veshchestva v okeane i moryah na primere Belogo morya (rezul’taty 12-letnih issledovanij). Dokl. Earth Sci. 2014, 456, 355–359. [Google Scholar]
- Tesdal, J.E.; Galbraith, E.D.; Kienast, M. Nitrogen isotopes in bulk marine sediment: Linking seafloor observations with subseafloor records. Biogeosciences 2013, 10, 101–118. [Google Scholar] [CrossRef] [Green Version]
- Newton, R.; Bottrell, S. Stable isotopes of carbon and sulfur as indicators of environmental change: Past and present. J. Geol. Soc. 2007, 164, 691–708. [Google Scholar] [CrossRef]
- Rozanov, A.G.; Volkov, I.I.; Emelyanov, E.M. Redoks-sistema donnyh otlozhenij Belogo morya: Zhelezo, marganec, sera. In The White Sea System The Processes of Sedimentation, Geology and History; Izdatel’stvo Nauchnyj mir: Moscow, Russia, 2012; Volume 4, pp. 639–661. [Google Scholar]
- Belyaev, N.A. Organic Matter and Hydrocarbon Markers of the White Sea. Ph.D. Thesis, Shirshov Institute of Oceanology of Russian Academy of Sciences, Tomsk Polytechnic University, Moscow, Russia, 2015. [Google Scholar]
- Demaison, G.J.; Moore, G.T. Anoxic environments and oil source bed genesis. AApG Bull. 1980, 8, 1179–1209. [Google Scholar] [CrossRef]
- Rozanov, A.G.; Kokratskaya, N.M.; Gursky, Y.N. Sostav ilovyh vod i formy soedinenij sery v donnyh otlozheniyah severo-vostochnoj chasti CHernogo morya. Litol. Polezn. Iskop. 2017, 4, 291–305. [Google Scholar]
- Baturin, G.N. Uran v Sovremennoj Morskoj Sedimentacii; Atomizdat: Moscow, Russia, 1975. [Google Scholar]
- Gursky, Y.N. Metody izucheniya i processy formirovaniya himicheskogo sostava ilovyh vod otlozhenij Chernogo, Azovskogo, Kaspijskogo, Belogo i Baltijskogo morej. In Geohimiya Litogidrosfery Vnutrennih Morej; GEOS: Moscow, Russia, 2003; Volume 1. [Google Scholar]
- Gursky, Y.N. Zakonomernosti povedeniya mikroelementov v sisteme ilovye vody-osadki Chernogo morya. In Proceedings of the XXIII Mezhdunarodnaya Nauchnaya Konferenciya (Shkola) po Morskoj Geologii “Geologiya Morej i Okeanov”, Moscow, Russia, 18–22 November 2019. [Google Scholar]
- Shnyukov, E.F.; Bezborodov, A.A.; Melnik, V.I.; Mitropolsky, A.Y. Geohimicheskaya evolyuciya urana v Chernom more. Geol. Zhurn 1979, 39, 1–9. [Google Scholar]
- Neprochnov, Y.P. Geologicheskaya Istoriya CHernogo Morya po Rezul’tatam Glubokovodnogo Bureniya; Nauka: Moscow, Russia, 1980. [Google Scholar]
- Rozanov, A.G.; Gursky, Y.N. Geohimicheskie osobennosti osadkov v severo-vostochnoj chasti Chernogo morya. Okeanologiya 2016, 56, 919–934. [Google Scholar]
- Anderson, R.F.; Fleisher, M.Q.; LeHuray, A.P. Concentration, oxidation state, and particulate flux of uranium in the Black Sea. Geochim. Cosmochim. Acta 1989, 53, 2215–2224. [Google Scholar] [CrossRef]
- Barnes, C.E.; Cochran, J.K. Geochemistry of uranium in Black Sea sediments. Deep Sea Research Part A. Oceanogr. Res. Pap. 1991, 38, S1237–S1254. [Google Scholar]
- Garrels, R.; Christe, C.L. Solutions, Minerals, and Equilibria, 1st ed.; Harper & Row: New York, NY, USA, 1965. [Google Scholar]
- Wignall, P.B.; Myers, K.J. Interpreting benthic oxygen levels in mudrocks: A new approach. Geology 1988, 16, 452–455. [Google Scholar] [CrossRef]
- Mironenko, M.V.; Akinfiev, N.N.; Melikhova, T.Y. GEOCHEQ—The complex for thermodynamic modeling of geochemical systems. Her. DGGGMS RAS 2000, 5, 96–97. [Google Scholar]
- Bone, S.E.; Dynes, J.J.; Cliff, J.; Bargar, J.R. Uranium (IV) adsorption by natural organic matter in anoxic sediments. Proc. Natl. Acad. Sci. USA 2017, 114, 711–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Station | Easting | Northing | Water Depth m | Column Length cm |
|---|---|---|---|---|
| 1 | 510,868.13 | 7,379,725.5 | 82.9 | 285 |
| 2 | 516,170.33 | 7,377,367.5 | 80.0 | 173 |
| Depth, cm | Quartz % | Albite % | Illite % | Kaolinite % | Pyrite % | Mica % |
|---|---|---|---|---|---|---|
| 47.5 | 56 | 31.2 | 5 | 2.5 | - | 5.2 |
| 112.5 | 46.3 | 37 | 11.3 | 2.2 | 1.8 | 1.3 |
| Depth (cm) | Eh (mV) | pH |
|---|---|---|
| 5 | +392 | 8 |
| 42.5 | −273 | 8.11 |
| 71.5 | −243 | 7.95 |
| 91.5 | −215 | 7.88 |
| 110.0 | −145 | 7.81 |
| 136.5 | −117 | 7.94 |
| 156.5 | −138 | 7.92 |
| 181.5 | −115 | 7.84 |
| 201.5 | −150 | 7.82 |
| 220.0 | −170 | 7.84 |
| 247.5 | −73 | 7.76 |
| 267.5 | −63 | 7.81 |
| Depth, cm | U, ppm | |
|---|---|---|
| 1 | 71.5 | 1.3 |
| 91.5 | 1.42 | |
| 110 | 1.33 | |
| 136.5 | 1.21 | |
| 156.5 | 1.21 | |
| 181.5 | 1.16 | |
| 201.5 | 1.24 | |
| 220 | 1.36 | |
| 247.5 | 1.87 | |
| 267.5 | 1.34 |
| Depth, cm | U | Fe | Cr | V | Zn | Ni | Co | Pb | Th | Cd |
|---|---|---|---|---|---|---|---|---|---|---|
| Instrumental detection limit | 0.032 | 0.300 | 0.024 | 0.021 | 0.065 | 0.015 | 0.016 | 0.019 | 0.029 | 0.013 |
| 2.5 | 1.89 | 35,721 | 95 | 94 | 66 | 37 | 27 | 20 | 6.23 | 0.122 |
| 7.5 | 1.69 | 35,078 | 97 | 95 | 66 | 39 | 15 | 13 | 6.08 | 0.119 |
| 12.5 | 1.79 | 35,430 | 99 | 97 | 78 | 39 | 18 | 10 | 6.43 | 0.113 |
| 17.5 | 1.81 | 33,801 | 102 | 93 | 66 | 38 | 15 | 11 | 6.62 | 0.106 |
| 22.5 | 1.9 | 32,372 | 95 | 91 | 62 | 37 | 15 | 7 | 6.37 | 0.109 |
| 27.5 | 1.91 | 33,269 | 92 | 88 | 62 | 37 | 15 | 7 | 6.88 | 0.114 |
| 32.5 | 1.76 | 33,751 | 96 | 89 | 63 | 38 | 14 | 8 | 6.51 | 0.095 |
| 37.5 | 1.83 | 33,153 | 96 | 89 | 65 | 37 | 14 | 6 | 6.49 | 0.092 |
| 42.5 | 1.88 | 33,165 | 99 | 88 | 61 | 38 | 13 | 6 | 6.81 | 0.096 |
| 47.5 | 1.91 | 32,839 | 98 | 87 | 65 | 37 | 14 | 6 | 6.86 | 0.107 |
| 52.5 | 1.91 | 32,839 | 98 | 87 | 65 | 37 | 14 | 6 | 6.86 | 0.107 |
| 57.5 | 2.08 | 32,551 | 92 | 84 | 59 | 35 | 13 | 6 | 7.03 | 0.098 |
| 62.5 | 2.12 | 32,152 | 91 | 85 | 65 | 36 | 13 | 5 | 7.21 | 0.086 |
| 67.5 | 2.08 | 32,100 | 94 | 84 | 57 | 36 | 13 | 5 | 7.07 | 0.085 |
| 72.5 | 2.06 | 31,789 | 92 | 85 | 58 | 36 | 13 | 6 | 7.09 | 0.086 |
| 77.5 | 2.13 | 34,040 | 97 | 87 | 61 | 37 | 14 | 6 | 7.94 | 0.078 |
| 82.5 | 2.3 | 33,484 | 96 | 89 | 62 | 37 | 13 | 6 | 7.75 | 0.097 |
| 87.5 | 2.1 | 32,074 | 92 | 84 | 58 | 36 | 13 | 6 | 7.58 | 0.071 |
| 92.5 | 2.16 | 31,134 | 88 | 82 | 63 | 34 | 13 | 5 | 7.47 | 0.075 |
| 97.5 | 2.26 | 32,848 | 90 | 85 | 60 | 36 | 14 | 6 | 8 | 0.088 |
| 102.5 | 2.27 | 33,454 | 92 | 86 | 60 | 37 | 14 | 5 | 7.65 | 0.091 |
| 107.5 | 2.29 | 33,027 | 91 | 85 | 63 | 37 | 13 | 6 | 8.18 | 0.092 |
| 112.5 | 2.34 | 33,503 | 89 | 85 | 64 | 36 | 14 | 7 | 8.4 | 0.091 |
| 117.5 | 2.48 | 34,274 | 90 | 89 | 65 | 37 | 14 | 6 | 8.89 | 0.097 |
| 122.5 | 2.38 | 33,132 | 94 | 85 | 62 | 38 | 14 | 7 | 8.8 | 0.087 |
| 127.5 | 2.46 | 34,200 | 94 | 83 | 66 | 38 | 14 | 6 | 9.02 | 0.097 |
| 132.5 | 2.44 | 31,894 | 88 | 83 | 62 | 35 | 13 | 6 | 8.56 | 0.09 |
| 137.5 | 2.21 | 30,840 | 87 | 76 | 57 | 34 | 13 | 8 | 8.29 | 0.081 |
| 142.5 | 2.42 | 33,186 | 94 | 83 | 63 | 37 | 13 | 9 | 9.29 | 0.101 |
| 147.5 | 2.36 | 33,411 | 92 | 84 | 65 | 36 | 13 | 13 | 9.06 | 0.092 |
| 152.5 | 2.29 | 32,458 | 93 | 82 | 62 | 37 | 13 | 13 | 8.71 | 0.092 |
| 157.5 | 2.4 | 33,932 | 98 | 83 | 64 | 37 | 13 | 9 | 8.9 | 0.087 |
| 162.5 | 2.52 | 33,502 | 96 | 83 | 63 | 37 | 13 | 8 | 9.48 | 0.101 |
| Station | Depth, cm | Elemental Composition | ||||
|---|---|---|---|---|---|---|
| N, % | C, % | H, % | S, % | C/N | ||
| 1 | 42.5 | 0.35 | 2.77 | 0.79 | 0.72 | 7.91 |
| 71.5 | 0.33 | 2.68 | 0.75 | 0.76 | 8.12 | |
| 91.5 | 0.36 | 2.76 | 1.19 | 0.72 | 7.67 | |
| 110 | 0.38 | 2.89 | 0.80 | 0.86 | 7.61 | |
| 136.5 | 0.34 | 2.58 | 0.71 | 0.67 | 7.59 | |
| 156.5 | 0.30 | 2.36 | 0.71 | 0.55 | 7.87 | |
| 181.5 | 0.29 | 2.29 | 0.76 | 0.55 | 7.90 | |
| 201.5 | 0.29 | 2.30 | 0.46 | 0.56 | 7.93 | |
| 220 | 0.28 | 2.17 | 0.68 | 0.56 | 7.75 | |
| 247.5 | 0.33 | 2.56 | 0.36 | 0.21 | 7.76 | |
| 267.5 | 0.30 | 2.29 | 0.65 | 0.63 | 7.63 | |
| 2 | 2.5 | 0.31 | 2.31 | 0.40 | 0.15 | 7.45 |
| 7.5 | 0.30 | 2.21 | 0.50 | 0.18 | 7.37 | |
| 12.5 | 0.25 | 1.90 | 0.46 | 0.26 | 7.60 | |
| 17.5 | 0.24 | 1.76 | 0.42 | 0.26 | 7.33 | |
| 22.5 | 0.23 | 1.68 | 0.42 | 0.20 | 7.30 | |
| 27.5 | 0.24 | 1.79 | 0.45 | 0.21 | 7.46 | |
| 32.5 | 0.24 | 1.77 | 0.44 | 0.29 | 7.38 | |
| 37.5 | 0.23 | 1.70 | 0.44 | 0.26 | 7.39 | |
| 47.5 | 0.18 | 1.45 | 0.38 | 0.33 | 8.06 | |
| 52.5 | 0.15 | 1.22 | 0.30 | 0.28 | 8.13 | |
| 57.5 | 0.21 | 1.60 | 0.38 | 0.27 | 7.62 | |
| 62.5 | 0.19 | 1.39 | 0.33 | 0.32 | 7.32 | |
| 67.5 | 0.22 | 1.57 | 0.37 | 0.33 | 7.14 | |
| 72.5 | 0.20 | 1.55 | 0.37 | 0.40 | 7.75 | |
| 77.5 | 0.21 | 1.59 | 0.38 | 0.41 | 7.57 | |
| 82.5 | 0.19 | 1.69 | 0.48 | 0.41 | 8.89 | |
| 87.5 | 0.21 | 1.61 | 0.36 | 0.44 | 7.67 | |
| 92.5 | 0.22 | 1.68 | 0.44 | 0.42 | 7.64 | |
| 97.5 | 0.22 | 1.63 | 0.42 | 0.39 | 7.41 | |
| 102.5 | 0.21 | 1.66 | 0.40 | 0.40 | 7.90 | |
| 107.5 | 0.18 | 1.60 | 0.41 | 0.44 | 8.89 | |
| 112.5 | 0.23 | 1.79 | 0.44 | 0.44 | 7.78 | |
| 117.5 | 0.23 | 1.66 | 0.43 | 0.51 | 7.22 | |
| 122.5 | 0.20 | 1.54 | 0.40 | 0.61 | 7.70 | |
| 127.5 | 0.25 | 1.84 | 0.46 | 0.56 | 7.36 | |
| 132.5 | 0.21 | 1.62 | 0.43 | 0.67 | 7.71 | |
| 137.5 | 0.22 | 1.65 | 0.44 | 0.51 | 7.50 | |
| 142.5 | 0.20 | 1.53 | 0.40 | 0.65 | 7.65 | |
| 147.5 | 0.21 | 1.68 | 0.46 | 0.84 | 8.00 | |
| 152.5 | 0.22 | 1.69 | 0.29 | 0.84 | 7.68 | |
| 157.5 | 0.22 | 1.86 | 0.50 | 0.61 | 8.45 | |
| Station | Depth, cm | δ15N Air, ‰ | δ13C PDB | δ13C PDB | δ34S CDT, ‰ |
|---|---|---|---|---|---|
| org., ‰ | carb.,‰ | ||||
| 1 | 42.5 | 5.8 | −23.7 | −9.1 | −5.3 |
| 110 | 6.1 | −23.3 | −5.7 | −10.8 | |
| 181.5 | 6.7 | −23.2 | −4.2 | −5.8 | |
| 247.5 | 5.0 | −23.5 | −4.1 | −23.2 | |
| 2 | 12.5 | 5.4 | −24.5 | −4.2 | |
| 52.5 | 6.1 | −24.6 | −34.2 | ||
| 102.5 | 4.4 | −23.7 | −36.9 | ||
| 137.5 | 5.0 | −24.6 | −34.9 |
| Depth, m | pH | Eh, mV | H2S, mg/L | Compounds and Concentration of Uranium | ||
|---|---|---|---|---|---|---|
| Seawater | 0–200 | 7.85–7.95 | −140...−160 | 0.08–0.83 | UO2(CO3)22−, UO2(CO3)34-, Uconcentration in seawater = 0.00093–0.00324 ppm | |
| 200–1500 | 7.74–7.8 | −176…−194 | 2.39–10.18 | [UO2(CO3)3]4−, Uconcentration in seawater = 0.00095–0.00261 ppm | ||
| 4 | 1500–2000 | 7.64–7.73 | −200…−203 | 10.40–11.66 | [UO2(CO3)3]4−, U(OH)4, UO2cr, the concentration of uranium in water decreases U concentration in seawater = 0.00029–0.00299 ppm | |
| Sediments | Modern | 6.4–8.5 | −80...−230 | 0–121.24 | U(OH)4, UO2cr, Uconcentration = 1.7–20 ppm | |
| Ancient | 6.2–8.2 | −80...−220 | 50–60 | Maximum uranium concentration in sapropel, Uconcentration = 1.1–35 ppm | ||
| 1 | Novoeuxinian | 6.2–8.0 | −198 | 0 | Minimum uranium concentration, Uconcentration = 0.3–4 ppm |
| Depth, cm | pH | Eh, mV | pO2 | The Calculated Concentration of Uranium in Pore Water, mol/L | The Proportion of the Total Uranium Contained by Pore Water, % | The Calculated Concentration of Uranium in the Solid Phase UO2 (cr), mol/L | The Proportion of the Total Uranium Contained by the Solid Phase, % | The Total Amount of Uranium, mol/L |
|---|---|---|---|---|---|---|---|---|
| 5 | 8 | 392 | 1.23 × 10−30 | 1.00 × 10−5 | 1.00 × 10+2 | 0 | 0 | 1.00 × 10−5 |
| 42.5 | 8.11 | −273 | 4.28 × 10−79 | 3.99 × 10−10 | 3.99 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 71.5 | 7.95 | −243 | 1.57 × 10−77 | 3.99 × 10−10 | 3.99 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 91.5 | 7.88 | −215 | 9.46 × 10−76 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 110 | 7.81 | −145 | 6.97 × 10−71 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 136.5 | 7.94 | −117 | 2.64 × 10−68 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 156.5 | 7.92 | −138 | 6.28 × 10−70 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 181.5 | 7.84 | −115 | 1.48 × 10−68 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 201.5 | 7.82 | −150 | 3.28 × 10−71 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 220 | 7.84 | −170 | 1.33 × 10−72 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 247.5 | 7.76 | −73 | 8.65 × 10−66 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 267.5 | 7.81 | −63 | 7.45 × 10−65 | 3.98 × 10−10 | 3.98 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
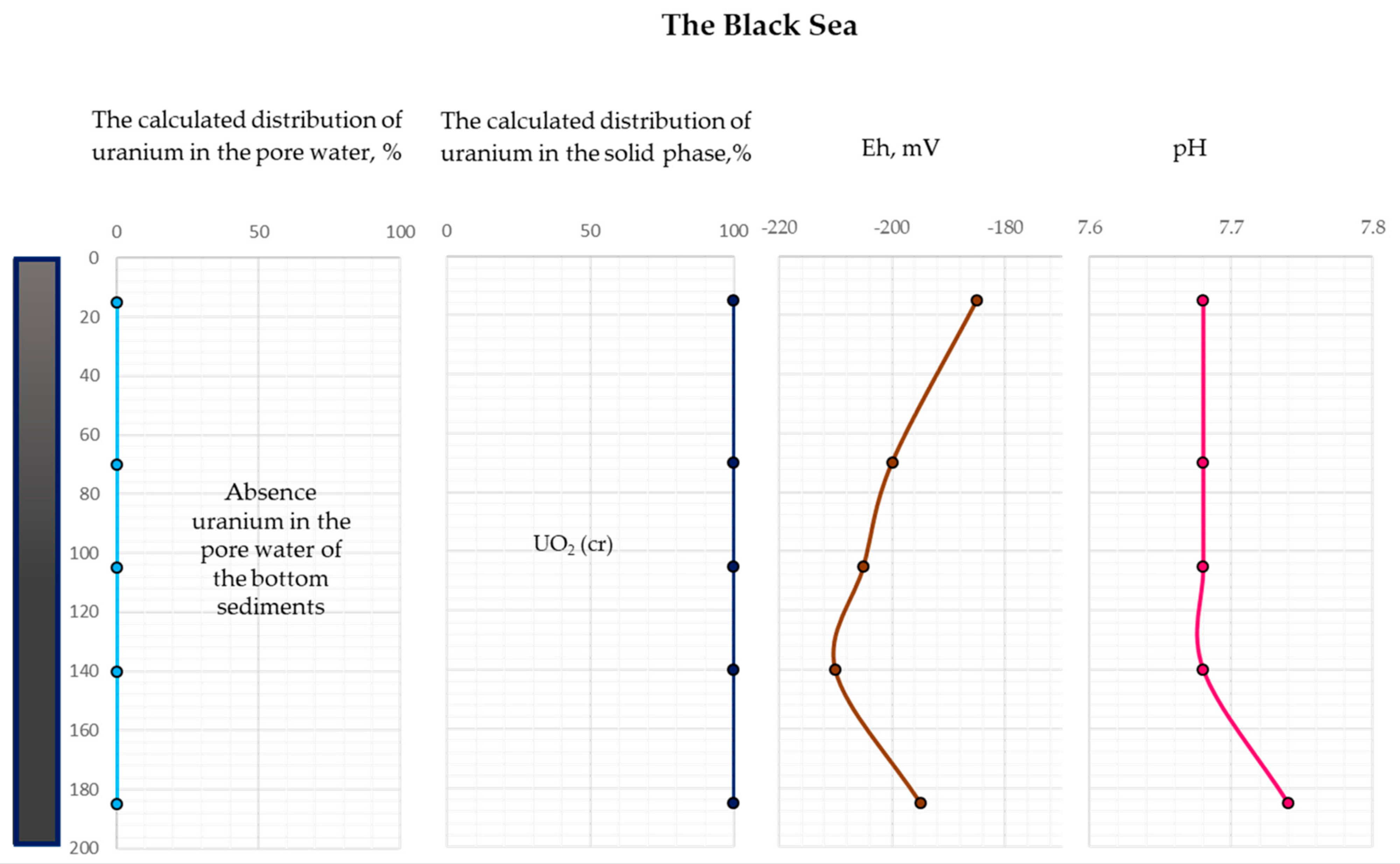
| Depth, cm | pH | Eh, mV | pO2 | The Calculated Concentration of Uranium in Pore Water, mol/L | The Proportion of Uranium in the Pore Water, % | The Calculated Concentration of Uranium in the Solid Phase UO2 (cr), mol/L | The Proportion of Uranium in the Solid Phase, % | The Total Amount of Uranium, mol/L |
|---|---|---|---|---|---|---|---|---|
| 15 | 7.68 | −185 | 2.41 × 10−74 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 70 | 7.68 | −200 | 1.90 × 10−75 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 105 | 7.68 | −205 | 8.15 × 10−76 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 140 | 7.68 | −210 | 3.50 × 10−76 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
| 185 | 7.74 | −195 | 7.70 × 10−75 | 3.97 × 10−10 | 3.97 × 10−3 | 1.00 × 10−5 | 1.00 × 102 | 1.00 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaustova, N.; Tikhomirova, Y.; Korost, S.; Poludetkina, E.; Voropaev, A.; Mironenko, M.; Spasennykh, M. The Study of Uranium Accumulation in Marine Bottom Sediments: Effect of Redox Conditions at the Time of Sedimentation. Geosciences 2021, 11, 332. https://doi.org/10.3390/geosciences11080332
Khaustova N, Tikhomirova Y, Korost S, Poludetkina E, Voropaev A, Mironenko M, Spasennykh M. The Study of Uranium Accumulation in Marine Bottom Sediments: Effect of Redox Conditions at the Time of Sedimentation. Geosciences. 2021; 11(8):332. https://doi.org/10.3390/geosciences11080332
Chicago/Turabian StyleKhaustova, Nadezhda, Yulia Tikhomirova, Svetlana Korost, Elena Poludetkina, Andrey Voropaev, Mikhail Mironenko, and Mikhail Spasennykh. 2021. "The Study of Uranium Accumulation in Marine Bottom Sediments: Effect of Redox Conditions at the Time of Sedimentation" Geosciences 11, no. 8: 332. https://doi.org/10.3390/geosciences11080332
APA StyleKhaustova, N., Tikhomirova, Y., Korost, S., Poludetkina, E., Voropaev, A., Mironenko, M., & Spasennykh, M. (2021). The Study of Uranium Accumulation in Marine Bottom Sediments: Effect of Redox Conditions at the Time of Sedimentation. Geosciences, 11(8), 332. https://doi.org/10.3390/geosciences11080332





