Geochemistry of Sub-Depositional Environments in Estuarine Sediments: Development of an Approach to Predict Palaeo-Environments from Holocene Cores
Abstract
:1. Introduction
- What elements are dominant within the surface sediment in the Ravenglass Estuary?
- What controls elemental abundance and distribution patterns at Ravenglass?
- Do specific estuarine sub-depositional environments have characteristic element concentrations?
- Can surface pXRF data be used to discriminate subsurface estuarine sub-depositional environments?
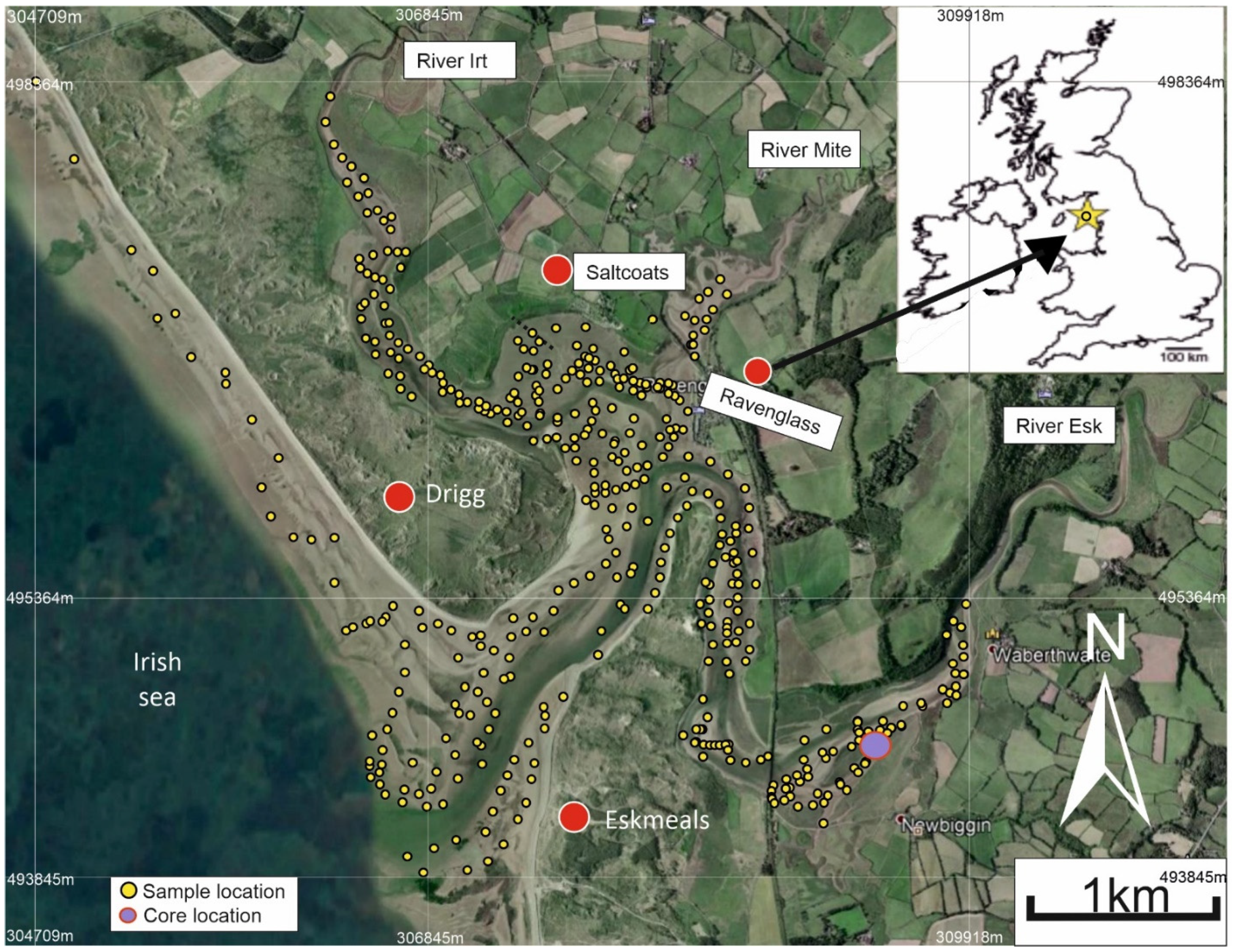
2. Study Area: The Ravenglass Estuary
3. Samples and Methods
3.1. Field-Based Mapping and Samples Collection
3.2. Grain Size Analysis
3.3. Multi-Element Analyses Using Handheld Niton +XL3t GOLDD pXRF Spectrometer
3.4. Spatial Mapping
3.5. Statistical Multivariate Analysis
3.6. ANOVA and Tukey’s Post Hoc Test
3.7. Boxplots and Classification Trees
3.8. Holocene Cores
4. Results
4.1. Sub-Depositional Environments Present across the Estuary
4.2. Element Concentrations in the Ravenglass Estuary
4.3. Relative Element Concentrations
4.4. Holocene Cores
5. Discussion
5.1. Elemental Distribution in the Ravenglass Estuary
5.2. Relationship between Element Indices and Sub-Depositional Environment
5.3. Multi-Element Analyses in Discriminating Estuarine Sub-Depositional Environments
5.4. ANOVA and Tukey’s Post Hoc Test to Differentiate Estuarine Sub-Depositional Environments
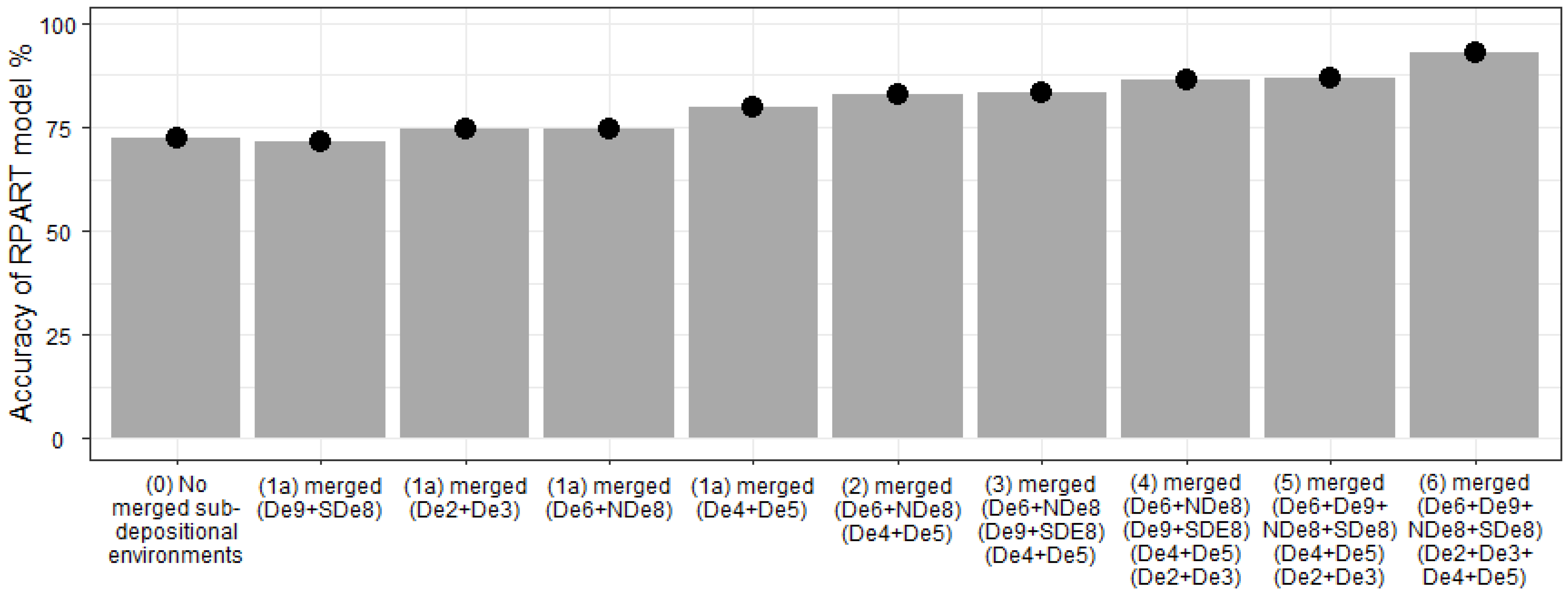
5.5. Development and Application of A Classification Diagram Using a Supervised Machine Learning Approach (RPART)
5.6. Application of Proposed Model for Discrimination of Estuarine Sub-Environments
6. Conclusions
- This work represents a detailed study of sediment, analysed for composition using pXRF analyses, from the Ravenglass Estuary, NW England, United Kingdom.
- Sub-depositional environments, mapped and defined across the estuary, include gravel beds, salt marsh, mud flats, mixed flats, sand flats, tidal bars, tidal inlet, foreshore, and ebb-tidal delta. The foreshore of the Ravenglass Estuary was subdivided into discrete northern and southern portions as they have distinct textural and elemental attributes.
- Elements concentrations vary throughout the estuary, especially in terms of localised differences of Al, K, Ca, Fe, Mn, Zr, Rb, some of which will be the result of localised dilution due to preferential accumulation of detrital quartz.
- Major, minor and trace element indices, varying between 0 and 1, were employed for the discrimination of sub-depositional environments, instead of raw concentration data, to circumvent the problem of variable dilution by quartz and closed datasets.
- Element indices are heterogeneously distributed throughout the estuary, showing that element concentration patterns are not simply due to variable dilution by quartz.
- There are strong relationships between specific sub-depositional environments and element indices within the estuary.
- Provenance, sediment mineralogy and grain size, controlled by estuarine hydrodynamics, are the dominant controls on the distribution of elements (and their indices) in the Ravenglass Estuary.
- A supervised machine learning method was developed, using the RPART routine in R Statistical Software, for the automatic discrimination of palaeo sub-depositional environments, with the model calibrated using surface sediment element indices. The model was successfully applied to a core drilled through the Holocene succession at Ravenglass to predict palaeo sub-depositional environments over the last 10,000 years.
- This work has proved that there are strong and predictable relationships between estuarine sub-depositional environments and sediment geochemistry.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rui, Z.; Lu, J.; Zhang, Z.; Guo, R.; Ling, K.; Zhang, R.; Patil, S. A quantitative oil and gas reservoir evaluation system for development. J. Nat. Gas Sci. Eng. 2017, 42, 31–39. [Google Scholar] [CrossRef]
- Worden, R.H.; Armitage, P.J.; Butcher, A.R.; Churchill, J.M.; Csoma, A.E.; Hollis, C.; Lander, R.H.; Omma, J.E. Petroleum reservoir quality prediction: Overview and contrasting approaches from sandstone and carbonate communities. Geol. Soc. Lond. Spec. Publ. 2018, 435, 1–31. [Google Scholar] [CrossRef]
- Zahid, M.A.; Chunmei, D.; Lin, C.; Gluyas, J.; Jones, S.; Zhang, X.; Munawar, M.J.; Ma, C. Sequence stratigraphy, sedimentary facies and reservoir quality of Es4s, southern slope of Dongying Depression, Bohai Bay Basin, East China. Mar. Pet. Geol. 2016, 77, 448–470. [Google Scholar] [CrossRef]
- Haile, B.G.; Klausen, T.G.; Czarniecka, U.; Xi, K.; Jahren, J.; Hellevang, H. How are diagenesis and reservoir quality linked to depositional facies? A deltaic succession, Edgeoya, Svalbard. Mar. Pet. Geol. 2018, 92, 519–546. [Google Scholar] [CrossRef]
- Chan, M.A. Correlations of diagenesis with sedimentary facies in Eocene sandstones, western Oregon. J. Sediment. Petrol. 1985, 55, 322–333. [Google Scholar]
- Anderton, R. Clastic facies models and facies analysis. Geol. Soc. Lond. Spec. Publ. 1985, 18, 31–47. [Google Scholar] [CrossRef]
- Dalrymple, R.W.; Choi, K. Morphologic and facies trends through the fluvial–marine transition in tide-dominated depositional systems: A schematic framework for environmental and sequence-stratigraphic interpretation. Earth-Sci. Rev. 2007, 81, 135–174. [Google Scholar] [CrossRef]
- Martinius, A.W.; Ringrose, P.S.; Brostrom, C.; Elfenbein, C.; Naess, A.; Ringas, J.E. Reservoir challenges of heterolithic tidal sandstone reservoirs in the Halten Terrace, mid-Norway. Pet. Geosci. 2005, 11, 3–16. [Google Scholar] [CrossRef]
- Primmer, T.J.; Cade, C.A.; Evans, J.; Gluyas, J.; Hopkins, M.S.; Oxtoby, N.; Smalley, P.C.; Warren, E.A.; Worden, R.H. Global patterns in sandstone diagenesis: Their application to reservoir quality prediction for petroleum exploration. In Reservoir Quality Prediction in Sandstones and Carbonates. AAPG Memoir; Kupecz, J.A., Gluyas, J., Bloch, S., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1997; Volume 69, pp. 61–78. [Google Scholar]
- Berner, E.K.; Berner, R.A. Global Environment: Water, Air, and Geochemical Cycles; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
- Boyle, E.A.; Edmond, J.M.; Sholkovitz, E.R. Mechanism of iron removal in estuaries. Geochim. Cosmochim. Acta 1977, 41, 1313–1324. [Google Scholar] [CrossRef]
- Coynel, A.; Schäfer, J.; Blanc, G.; Bossy, C. Scenario of particulate trace metal and metalloid transport during a major flood event inferred from transient geochemical signals. Appl. Geochem. 2007, 22, 821–836. [Google Scholar] [CrossRef]
- Lanceleur, L.; Schäfer, J.; Blanc, G.; Coynel, A.; Bossy, C.; Baudrimont, M.; Glé, C.; Larrose, A.; Renault, S.; Strady, E. Silver behaviour along the salinity gradient of the Gironde Estuary. Environ. Sci. Pollut. Res. 2013, 20, 1352–1366. [Google Scholar] [CrossRef]
- Elbaz-Poulichet, F.; Martin, J.; Huang, W.; Zhu, J. Dissolved Cd behaviour in some selected French and Chinese estuaries. Consequences on Cd supply to the ocean. Mar. Chem. 1987, 22, 125–136. [Google Scholar] [CrossRef]
- Dabrin, A.; Schäfer, J.; Blanc, G.; Strady, E.; Masson, M.; Bossy, C.; Castelle, S.; Girardot, N.; Coynel, A. Improving estuarine net flux estimates for dissolved cadmium export at the annual timescale: Application to the Gironde Estuary. Estuar. Coast. Shelf Sci. 2009, 84, 429–439. [Google Scholar] [CrossRef]
- Audry, S.; Blanc, G.; Schäfer, J.; Chaillou, G.; Robert, S. Early diagenesis of trace metals (Cd, Cu, Co, Ni, U, Mo, and V) in the freshwater reaches of a macrotidal estuary. Geochim. Cosmochim. Acta 2006, 70, 2264–2282. [Google Scholar] [CrossRef]
- Dyer, K.R. Estuaries: A Physical Introduction; Wiley: New York, NY, USA, 1997; Volume 1. [Google Scholar]
- Zwingmann, H.; Clauer, N.; Gaupp, R. Structure-related geochemical (REE) and isotopic (K-Ar, Rb-Sr, δ18O) characteristics of clay minerals from Rotliegend sandstone reservoirs (Permian, northern Germany). Geochim. Cosmochim. Acta 1999, 63, 2805–2823. [Google Scholar] [CrossRef]
- Smith, D.B.; Woodruff, L.G.; O’Leary, R.M.; Cannon, W.F.; Garrett, R.G.; Kilburn, J.E.; Goldhaber, M.B. Pilot studies for the North American Soil Geochemical Landscapes Project–site selection, sampling protocols, analytical methods, and quality control protocols. Appl. Geochem. 2009, 24, 1357–1368. [Google Scholar] [CrossRef]
- Meinhold, G.; Kostopoulos, D.; Reischmann, T. Geochemical constraints on the provenance and depositional setting of sedimentary rocks from the islands of Chios, Inousses and Psara, Aegean Sea, Greece: Implications for the evolution of Palaeotethys. J. Geol. Soc. 2007, 164, 1145–1163. [Google Scholar] [CrossRef] [Green Version]
- Yardley, B.W.D. An Introduction to Metamorphic Petrology; Longman: Harlow, UK, 1989. [Google Scholar]
- Fralick, P.; Kronberg, B. Geochemical discrimination of clastic sedimentary rock sources. Sediment. Geol. 1997, 113, 111–124. [Google Scholar] [CrossRef]
- Flood, R.P.; Bloemsma, M.R.; Weltje, G.J.; Barr, I.D.; O’Rourke, S.M.; Turner, J.N.; Orford, J.D. Compositional data analysis of Holocene sediments from the West Bengal Sundarbans, India: Geochemical proxies for grain-size variability in a delta environment. Appl. Geochem. 2016, 75, 222–235. [Google Scholar] [CrossRef]
- Dinelli, E.; Tateo, F.; Summa, V. Geochemical and mineralogical proxies for grain size in mudstones and siltstones from the Pleistocene and Holocene of the Po River alluvial plain, Italy. In Proceedings of the Symposium on Sedimentary Provenance and Petrogenesis held at the 32nd International Geological Congress, Florence, Italy, 20–28 August 2004; pp. 25–36. [Google Scholar]
- Folk, R.L. A review of grain-size parameters. Sedimentology 1966, 6, 73–93. [Google Scholar] [CrossRef]
- Folk, R.L. Petrology of sedimentary rocks: Hemphill’s. Austin Tex. 1968, 170, 85. [Google Scholar]
- Rowe, H.; Hughes, N.; Robinson, K. The quantification and application of handheld energy-dispersive x-ray fluorescence (ED-XRF) in mudrock chemostratigraphy and geochemistry. Chem. Geol. 2012, 324, 122–131. [Google Scholar] [CrossRef]
- Morris, P.A. Field-Portable X-ray Fluorescence Analysis and Its Application in GSWA; Geological Survey of Western Australia: Perth, Australia, 2009. [Google Scholar]
- Gazley, M.F.; Vry, J.K.; du Plessis, E.; Handler, M.R. Application of portable X-ray fluorescence analyses to metabasalt stratigraphy, Plutonic Gold Mine, Western Australia. J. Geochem. Explor. 2011, 110, 74–80. [Google Scholar] [CrossRef]
- Weindorf, D.C.; Zhu, Y.; Chakraborty, S.; Bakr, N.; Huang, B. Use of portable X-ray fluorescence spectrometry for environmental quality assessment of peri-urban agriculture. Environ. Monit. Assess. 2012, 184, 217–227. [Google Scholar] [CrossRef]
- McLaren, T.I.; Guppy, C.N.; Tighe, M.K.; Forster, N.; Grave, P.; Lisle, L.M.; Bennett, J.W. Rapid, nondestructive total elemental analysis of vertisol soils using portable X-ray fluorescence. Soil Sci. Soc. Am. J. 2012, 76, 1436–1445. [Google Scholar] [CrossRef] [Green Version]
- Kenna, T.C.; Nitsche, F.O.; Herron, M.M.; Mailloux, B.J.; Peteet, D.; Sritrairat, S.; Sands, E.; Baumgarten, J. Evaluation and calibration of a Field Portable X-Ray Fluorescence spectrometer for quantitative analysis of siliciclastic soils and sediments. J. Anal. At. Spectrom. 2011, 26, 395–405. [Google Scholar] [CrossRef]
- Plourde, A.; Knight, R.; Kjarsgaard, B.; Sharpe, D.; Lesemann, J. Portable XRF spectrometry of surficial sediments, NTS 75-I, 75-J, 75-O, 75-P (Mary Frances Lake–Whitefish Lake–Thelon River area), Northwest Territories. Geol. Surv. Can. Open File 2013, 7408, 25. [Google Scholar]
- Turner, J.N.; Jones, A.F.; Brewer, P.A.; Macklin, M.G.; Rassner, S.M. Micro-XRF applications in fluvial sedimentary environments of Britain and Ireland: Progress and prospects. In Micro-XRF Studies of Sediment Cores; Springer: Berlin/Heidelberg, Germany, 2015; pp. 227–265. [Google Scholar]
- Potts, P.J. Introduction, analytical instrumentation and application overview. In Portable X-ray Fluorescence Spectrometry: Capabilities for In Situ Analysis; The Royal Society of Chemistry: London, UK, 2008; pp. 1–12. [Google Scholar]
- Rollinson, H.R. Using Geochemical Data: Evaluation, Presentation, Interpretation; Routledge: Oxfordshire, UK, 2014. [Google Scholar]
- Benn, C. Lithological discrimination in deeply weathered terrains using multielemental geochemistry–an example from the Yanfolila gold project, Mali. Explore 2012, 156, 1–8. [Google Scholar]
- Marsala, A.; Loermans, T.; Shen, S.; Scheibe, C.; Zereik, R. Portable energy-dispersive X-ray fluorescence integrates mineralogy and chemostratigraphy into real-time formation evaluation. Petrophysics 2012, 53, 102–109. [Google Scholar]
- Le Vaillant, M.; Barnes, S.J.; Fiorentini, M.L.; Santaguida, F.; Törmänen, T. Effects of hydrous alteration on the distribution of base metals and platinum group elements within the Kevitsa magmatic nickel sulphide deposit. Ore Geol. Rev. 2016, 72, 128–148. [Google Scholar] [CrossRef]
- Young, K.E.; Evans, C.A.; Hodges, K.V.; Bleacher, J.E.; Graff, T.G. A review of the handheld X-ray fluorescence spectrometer as a tool for field geologic investigations on Earth and in planetary surface exploration. Appl. Geochem. 2016, 72, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Emmerson, R.; O’Reilly-Wiese, S.; Macleod, C.; Lester, J. A multivariate assessment of metal distribution in inter-tidal sediments of the Blackwater Estuary, UK. Mar. Pollut. Bull. 1997, 34, 960–968. [Google Scholar] [CrossRef]
- Martins, R.; Azevedo, M.; Mamede, R.; Sousa, B.; Freitas, R.; Rocha, F.; Quintino, V.; Rodrigues, A. Sedimentary and geochemical characterization and provenance of the Portuguese continental shelf soft-bottom sediments. J. Mar. Syst. 2012, 91, 41–52. [Google Scholar] [CrossRef]
- Ross, P.-S.; Bourke, A.; Fresia, B. Improving lithological discrimination in exploration drill-cores using portable X-ray fluorescence measurements: (1) testing three Olympus Innov-X analysers on unprepared cores. Geochem. Explor. Environ. Anal. 2014, 14, 171–185. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, Q.; Xia, Q.; Yao, L.; Chen, Z.; Zuo, R.; Xu, D. Spatial patterns of geochemical elements measured on rock surfaces by portable X-ray fluorescence: Application to hand specimens and rock outcrops. Geochem. Explor. Environ. Anal. 2014, 14, 265–276. [Google Scholar] [CrossRef]
- Wooldridge, L.J.; Worden, R.H.; Griffiths, J.; Utley, J.E.P. Clay-coated sand grains in petroleum reservoirs: Understanding their distribution via a modern analogue. J. Sediment. Res. 2017, 87, 338–352. [Google Scholar] [CrossRef]
- Wooldridge, L.J.; Worden, R.H.; Griffiths, J.; Thompson, A.; Chung, P. Biofilm origin of clay-coated sand grains. Geology 2017, 45, 875–878. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, J.; Worden, R.H.; Wooldridge, L.J.; Utley, J.E.; Duller, R.A.; Edge, R.L. Estuarine clay mineral distribution: Modern analogue for ancient sandstone reservoir quality prediction. Sedimentology 2019, 66, 2011–2047. [Google Scholar] [CrossRef]
- Griffiths, J.; Worden, R.H.; Wooldridge, L.J.; Utley, J.E.; Duller, R.A. Compositional variation in modern estuarine sands: Predicting major controls on sandstone reservoir quality. AAPG Bull. 2019, 103, 797–833. [Google Scholar] [CrossRef]
- Griffiths, J.; Worden, R.H.; Wooldridge, L.J.; Utley, J.E.; Duller, R.A. Detrital clay coats, clay minerals, and pyrite: A modern shallow-core analogue for ancient and deeply buried estuarine sandstones. J. Sediment. Res. 2018, 88, 1205–1237. [Google Scholar] [CrossRef] [Green Version]
- Daneshvar, E.; Worden, R.H. Feldspar alteration and Fe minerals: Origin, distribution and implications for sandstone reservoir quality in estuarine sediments. Geol. Soc. Lond. Spec. Publ. 2017, 435, 123–139. [Google Scholar] [CrossRef]
- Daneshvar, E. Dissolved iron behavior in the Ravenglass Estuary waters, an implication on the early diagenesis. Univers. J. Geosci. 2015, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Simon, N.; Worden, R.H.; Muhammed, D.D.; Utley, J.E.P.; Verhagen, I.T.E.; Griffiths, J.; Wooldridge, L.J. Sediment textural characteristics of the Ravenglass Estuary; Development of a method to predict palaeo sub-depositional environments from estuary core samples. Sediment. Geol. 2021, 418, 105906. [Google Scholar] [CrossRef]
- Lloyd, J.M.; Zong, Y.; Fish, P.; Innes, J.B. Holocene and Late-glacial relative sea-level change in north-west England: Implications for glacial isostatic adjustment models. J. Quat. Sci. 2013, 28, 59–70. [Google Scholar] [CrossRef]
- Bousher, A. Ravenglass Estuary: Basic Characteristics and Evaluation of Restoration Options; Westlakes Scientific Consulting: Whitehaven, UK, 1999. [Google Scholar]
- Kelly, M.; Emptage, M.; Mudge, S.; Bradshaw, K.; Hamilton-Taylor, J. The relationship between sediment and plutonium budgets in a small macrotidal estuary—Esk Estuary, Cumbria, UK. J. Environ. Radioact. 1991, 13, 55–74. [Google Scholar] [CrossRef]
- Carr, A.P.; Blackley, M.W.L. Implications of sedimentological and hydrological processes on the distribution of radionuclides: The example of a salt marsh near Ravenglass, Cumbria. Estuar. Coast. Shelf Sci. 1986, 22, 529–543. [Google Scholar] [CrossRef]
- Merritt, J.; Auton, C. An outline of the lithostratigraphy and depositional history of Quaternary deposits in the Sellafield district, west Cumbria. Proc. Yorks Geol. Soc. 2000, 53, 129–154. [Google Scholar] [CrossRef]
- McGhee, C.A.; Muhammed, D.D.; Simon, N.; Acikalin, S.; Utley, J.E.P.; Griffiths, J.; Wooldridge, L.M.; Verhagen, I.T.E.; van der Land, C.; Worden, R.H. Stratigraphy and sedimentary evolution of a modern macro-tidal incised valley—An analogue for reservoir facies and architecture. Sedimentology 2021. [Google Scholar] [CrossRef]
- Brockamp, O.; Zuther, M. Changes in clay mineral content of tidal flat sediments resulting from dike construction along the Lower Saxony coast of the North Sea, Germany. Sedimentology 2004, 51, 591–600. [Google Scholar] [CrossRef]
- Gutiérrez-Ginés, M.J.; Pastor, J.; Hernández, A.J. Assessment of field portable X-ray fluorescence spectrometry for the in situ determination of heavy metals in soils and plants. Environ. Sci. Processes Impacts 2013, 15, 1545–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, J.; Clement, G.; Bursavich, B.; Elbers, D.; Cao, B.; Zhou, W. Rapid detection of toxic metals in non-crushed oyster shells by portable X-ray fluorescence spectrometry. Environ. Pollut. 2010, 158, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.; Zhang, C.; Moles, N.; Harder, M. Identification and mapping of heavy metal pollution in soils of a sports ground in Galway City, Ireland, using a portable XRF analyser and GIS. Environ. Geochem. Health 2008, 30, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Argyraki, A.; Ramsey, M.H.; Potts, P.J. Evaluation of portable X-ray fluorescence instrumentation for in situ measurements of lead on contaminated land. Analyst 1997, 122, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Zarco-Perello, S.; Simões, N. Ordinary kriging vs. inverse distance weighting: Spatial interpolation of the sessile community of Madagascar reef, Gulf of Mexico. PeerJ 2017, 5, e4078. [Google Scholar] [CrossRef] [Green Version]
- Watson, D.F.; Philip, G.M. Comment on “a nonlinear empirical prescription for simultaneously interpolating and smoothing contours over an irregular grid” by F. Duggan. Comput. Methods Appl. Mech. Eng. 1985, 50, 195–198. [Google Scholar] [CrossRef]
- Grunsky, E.C.; Smee, B.W. The differentiation of soil types and mineralization from multi-element geochemistry using multivariate methods and digital topography. J. Geochem. Explor. 1999, 67, 287–299. [Google Scholar] [CrossRef]
- Cheng, Q.; Jing, L.; Panahi, A. Principal component analysis with optimum order sample correlation coefficient for image enhancement. Int. J. Remote Sens. 2006, 27, 3387–3401. [Google Scholar] [CrossRef]
- Michael, D.; Paul, D.; Andreas, S.; Mark, C. Principal component analysis of the geochemistry of soil developed on till in Northern Ireland. J. Maps 2013, 373. [Google Scholar]
- Klovan, J. The use of factor analysis in determining depositional environments from grain-size distributions. J. Sediment. Res. 1966, 36, 115–125. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Scheffe, H. The Analysis of Variance; John Wiley & Sons: Hoboken, NJ, USA, 1999; Volume 72. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Therneau, T.; Atkinson, E. An Introduction to Recursive Partitioning Using Rpart Routines: The Comprehensive R Archive Network. 2019. Available online: https://cran.r-project.org/web/packages/rpart/vignettes/longintro.pdf (accessed on 10 November 2021).
- Reimann, C.; Filzmoser, P.; Garrett, R.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics with R; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Herron, M.M. Geochemical classification of terrigenous sands and shales from core or log data. J. Sediment. Res. 1988, 58, 820–829. [Google Scholar] [CrossRef]
- Michalopoulos, P.; Aller, R.C. Rapid clay mineral transformation in Amazon Delta sediments—Reverse weathering and oceanic element cycles. Science 1995, 270, 614–617. [Google Scholar] [CrossRef]
- Aller, R.C.; Michalopoulos, P. Invited Lecture: Tropical, Mobile Mud Belts as Global Diagenetic Reactors; Balkema: Rotterdam, The Netherlands, 1999; pp. 289–292. [Google Scholar]
- Michalopoulos, P.; Aller, R.C.; Reeder, R.J. Conversion of diatoms to clays during early diagenesis in tropical, continental shelf muds. Geology 2000, 28, 1095–1098. [Google Scholar] [CrossRef]
- Nelson, B.W. Clay mineralogy of the bottom sediments, Rappahannock River, Virginia. In Clays and Clay Minerals, Proceedings of the Seventh National Conference on Clays and Clay Minerals, Washington, DC, USA, 20–23 Octorber 1958; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–148. [Google Scholar]
- Krauskopf, K.B.; Bird, D.K. Introduction to Geochemistry; McGraw-Hill: New York, NY, USA, 1967; Volume 721. [Google Scholar]
- Worden, R.H.; Griffiths, J.; Wooldridge, L.J.; Utley, J.E.P.; Lawan, A.Y.; Muhammed, D.D.; Simon, N.; Armitage, P.J. Chlorite in sandstones. Earth-Sci. Rev. 2020, 204, 103105. [Google Scholar] [CrossRef]
- Wooldridge, L.J.; Worden, R.H.; Griffiths, J.; Utley, J.E.P. Clay-coat diversity in marginal marine sediments. Sedimentology 2019, 66, 1118–1138. [Google Scholar] [CrossRef]
- Baker, P.A.; Gieskes, J.M.; Elderfield, H. Diagenesis of carbonates in deep-sea sediments; evidence from Sr/Ca ratios and interstitial dissolved Sr2+ data. J. Sediment. Res. 1982, 52, 71–82. [Google Scholar]
- Brookins, D.G. Strontium. In Eh-pH Diagrams for Geochemistry; Springer: Berlin/Heidelberg, Germany, 1988; pp. 166–167. [Google Scholar]
- Andrew-Oha, I.; Mosto-Onuoha, K.; Sunday-Dada, S. Contrasting styles of lead-zinc-barium mineralization in the Lower Benue Trough, Southeastern Nigeria. Earth Sci. Res. J. 2017, 21, 7–16. [Google Scholar]
- Rothwell, R.G.; Croudace, I.W. Twenty years of XRF core scanning marine sediments: What do geochemical proxies tell us? In Micro-XRF Studies of Sediment Cores: Applications of a Non-Destructive Tool Foe the Environmental Sciences; Developments in Paleoenvironmental Research; Croudace, I.W., Rothwell, R.G., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 17, pp. 65–82. [Google Scholar]
- Hatch, J.; Leventhal, J. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) Stark Shale Member of the Dennis Limestone, Wabaunsee County, Kansas, USA. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Vaalgamaa, S.; Korhola, A. Geochemical signatures of two different coastal depositional environments within the same catchment. J. Paleolimnol. 2007, 38, 241–260. [Google Scholar] [CrossRef]
- Driskill, B.; Pickering, J.; Rowe, H. Interpretation of high resolution XRF data from the Bone Spring and Upper Wolfcamp, Delaware Basin, USA. In Proceedings of the Unconventional Resources Technology Conference, Houston, TX, USA, 23–25 July 2018; pp. 2861–2888. [Google Scholar]
- Calvert, S.; Pedersen, T. Chapter fourteen elemental proxies for palaeoclimatic and palaeoceanographic variability in marine sediments: Interpretation and application. Dev. Mar. Geol. 2007, 1, 567–644. [Google Scholar]
- Doerner, M.; Berner, U.; Erdmann, M.; Barth, T. Geochemical characterization of the depositional environment of Paleocene and Eocene sediments of the Tertiary Central Basin of Svalbard. Chem. Geol. 2020, 542, 119587. [Google Scholar] [CrossRef]
- Rübsam, W. The Early Toarcian Environmental Crisis: Mechanisms and Consequences of an Icehouse-Greenhouse Transition; Universitätsbibliothek Kiel: Kiel, Germany, 2019. [Google Scholar]
- Armstrong-Altrin, J.S.; Machain-Castillo, M.L.; Rosales-Hoz, L.; Carranza-Edwards, A.; Sanchez-Cabeza, J.-A.; Ruíz-Fernández, A.C. Provenance and depositional history of continental slope sediments in the Southwestern Gulf of Mexico unraveled by geochemical analysis. Cont. Shelf Res. 2015, 95, 15–26. [Google Scholar] [CrossRef]
- Mountney, N.P.; Thompson, D.B. Stratigraphic evolution and preservation of aeolian dune and damp/wet interdune strata: An example from the Triassic Helsby Sandstone Formation, Cheshire Basin, UK. Sedimentology 2002, 49, 805–833. [Google Scholar] [CrossRef]
- Hullman, J.; Resnick, P.; Adar, E. Hypothetical outcome plots outperform error bars and violin plots for inferences about reliability of variable ordering. PLoS ONE 2015, 10, e0142444. [Google Scholar]
- Hintze, J.L.; Nelson, R.D. Violin plots: A box plot-density trace synergism. Am. Stat. 1998, 52, 181–184. [Google Scholar]
- Aitchison, J. The Statistical Analysis of Compositional Data. J. R. Stat. Soc. Ser. B 1982, 44, 139–160. [Google Scholar] [CrossRef]










| Element | Reported Detection Limit (ppm) | Mean of 30 Repeat Analyses from One Sample (ppm) | Standard Deviation of 30 Repeat Analyses from One Sample (ppm) |
|---|---|---|---|
| Al | 2000 | 64,099 | 1685 |
| K | 250 | 18,234 | 145 |
| Ca | 70 | 2610 | 46 |
| Ti | 6 | 2477 | 92 |
| Fe | 25 | 11,837 | 90 |
| Mn | 30 | 172 | 19 |
| Rb | 6 | 70 | 1 |
| Sr | 8 | 73 | 2 |
| Zr | 3 | 352 | 3 |
| Ba | 50 | 487 | 18 |
| Cs | 12 | 85 | 4 |
| Sub-Environment | Samples | Al | Si | P | S | Cl | K | Ca | Sc | Ti | V | Cr | Mn |
| Foreshore | 69 | 69 | 69 | 17 | 24 | 69 | 69 | 69 | 3 | 69 | 48 | 35 | 67 |
| Gravel bed | 28 | 28 | 28 | 10 | 18 | 28 | 28 | 28 | 4 | 28 | 17 | 19 | 26 |
| Mixed flat | 94 | 94 | 94 | 1 | 54 | 94 | 94 | 94 | 2 | 94 | 51 | 66 | 93 |
| Mud flat | 55 | 55 | 55 | 1 | 52 | 55 | 55 | 55 | 16 | 55 | 33 | 52 | 54 |
| Ebb-tidal delta | 21 | 21 | 21 | 9 | 20 | 21 | 21 | 21 | 2 | 21 | 6 | 7 | 20 |
| Sand flat | 120 | 120 | 120 | 0 | 28 | 120 | 120 | 120 | 1 | 120 | 102 | 40 | 113 |
| Tidal bars | 53 | 53 | 53 | 0 | 12 | 53 | 53 | 53 | 1 | 53 | 43 | 18 | 50 |
| Tidal inlet | 25 | 25 | 25 | 5 | 8 | 25 | 25 | 25 | 0 | 25 | 20 | 6 | 24 |
| Salt marsh | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 5 | 17 | 14 | 11 | 17 |
| Sub-Environment | Samples | Fe | Ni | Cu | Zn | As | Rb | Sr | Zr | Nb | Pd | Ag | |
| Foreshore | 69 | 69 | 0 | 0 | 28 | 15 | 69 | 69 | 69 | 25 | 3 | 0 | |
| Gravel bed | 28 | 27 | 0 | 1 | 23 | 13 | 28 | 28 | 28 | 14 | 0 | 0 | |
| Mixed flat | 94 | 94 | 0 | 0 | 93 | 20 | 94 | 94 | 94 | 88 | 0 | 2 | |
| Mud flat | 55 | 54 | 0 | 0 | 55 | 19 | 55 | 55 | 55 | 55 | 0 | 0 | |
| Ebb-tidal delta | 21 | 21 | 0 | 0 | 14 | 2 | 21 | 21 | 21 | 4 | 4 | 0 | |
| Sand flat | 120 | 119 | 0 | 0 | 76 | 12 | 120 | 120 | 120 | 69 | 0 | 0 | |
| Tidal bars | 53 | 52 | 1 | 0 | 39 | 6 | 53 | 53 | 53 | 31 | 0 | 2 | |
| Tidal inlet | 25 | 25 | 1 | 1 | 14 | 6 | 25 | 25 | 25 | 7 | 2 | 1 | |
| Salt marsh | 17 | 17 | 8 | 1 | 17 | 16 | 17 | 17 | 17 | 13 | 2 | 3 | |
| Sub-Environment | Samples | Cd | Sn | Sb | Te | Cs | Ba | Hg | Pb | Bi | Th | U | |
| Foreshore | 69 | 13 | 32 | 20 | 60 | 65 | 69 | 3 | 13 | 0 | 14 | 8 | |
| Gravel bed | 28 | 11 | 17 | 10 | 28 | 28 | 28 | 0 | 13 | 1 | 14 | 3 | |
| Mixed flat | 94 | 0 | 51 | 15 | 92 | 93 | 94 | 6 | 3 | 1 | 46 | 5 | |
| Mud flat | 55 | 0 | 28 | 4 | 48 | 54 | 55 | 3 | 11 | 8 | 41 | 1 | |
| Ebb-tidal delta | 21 | 17 | 21 | 19 | 21 | 21 | 21 | 0 | 19 | 0 | 5 | 1 | |
| Sand flat | 120 | 0 | 64 | 27 | 106 | 118 | 120 | 2 | 2 | 1 | 7 | 6 | |
| Tidal bars | 53 | 0 | 35 | 8 | 49 | 53 | 53 | 2 | 2 | 1 | 3 | 1 | |
| Tidal inlet | 25 | 4 | 17 | 9 | 25 | 25 | 25 | 3 | 5 | 0 | 8 | 1 | |
| Salt marsh | 17 | 16 | 17 | 17 | 17 | 17 | 17 | 1 | 17 | 3 | 15 | 7 |
| Elements | Al | Si | P | S | Cl | K | Ca | Sc | Ti |
| Minimum value (ppm) | 1246 | 56,322 | 119 | 90 | 266 | 2189 | 73 | 6 | 257 |
| Samples above minimum value | 100% | 100% | 12% | 48% | 100% | 100% | 100% | 7% | 100% |
| Elements | V | Cr | Mn | Fe | Ni | Cu | Zn | As | Rb |
| Minimum value (ppm) | 44 | 20 | 52 | 2245 | 18 | 17 | 9 | 4 | 9 |
| Samples above minimum value | 69% | 53% | 96% | 99% | 2% | 1% | 74% | 23% | 100% |
| Elements | Sr | Zr | Nb | Pd | Ag | Cd | Sn | Sb | |
| Minimum value (ppm) | 28 | 29 | 2 | 4 | 100 | 10 | 13 | 12 | |
| Samples above minimum value | 100% | 100% | 63% | 2% | 2% | 13% | 59% | 27% | |
| Elements | Te | Cs | Ba | Hg | Pb | Bi | Th | U | |
| Minimum value (ppm) | 30 | 10 | 93 | 6 | 5 | 5 | 3 | 6 | |
| Samples above minimum value | 93% | 98% | 100% | 4% | 18% | 3% | 32% | 7% |
| Sub-Environment | Variable | p-Value | Sub-Environment | Variable | p-Value |
|---|---|---|---|---|---|
| De3-De2 | K/(K + Si) | 0.0000000 | De9-De4 | K/(K + Ca) | 0.0000007 |
| De4-De2 | K/(K + Si) | 0.0000000 | N-De8-De4 | K/(K + Ca) | 0.0000000 |
| De5-De2 | K/(K + Si) | 0.0000000 | S-De8-De4 | K/(K + Ca) | 0.0000000 |
| De6-De2 | K/(K + Si) | 0.0000000 | De6-De5 | K/(K + Ca) | 0.0000012 |
| De9-De2 | K/(K + Si) | 0.0000000 | De9-De5 | K/(K + Ca) | 0.0000044 |
| N-De8-De2 | K/(K + Si) | 0.0000000 | N-De8-De5 | K/(K + Ca) | 0.0000000 |
| S-De8-De2 | K/(K + Si) | 0.0000000 | S-De8-De5 | K/(K + Ca) | 0.0000000 |
| De4-De3 | K/(K + Si) | 0.0000000 | S-De8-De6 | K/(K + Ca) | 0.0041914 |
| De5-De3 | K/(K + Si) | 0.0000000 | S-De8-De9 | K/(K + Ca) | 0.0112902 |
| De6-De3 | K/(K + Si) | 0.0000000 | S-De8-N-De8 | K/(K + Ca) | 0.0001769 |
| De9-De3 | K/(K + Si) | 0.0000000 | De3-De2 | K/(K + Ti) | 0.0000007 |
| N-De8-De3 | K/(K + Si) | 0.0000000 | De4-De2 | K/(K + Ti) | 0.0000000 |
| S-De8-De3 | K/(K + Si) | 0.0000000 | De5-De2 | K/(K + Ti) | 0.0000000 |
| De6-De4 | K/(K + Si) | 0.0033850 | De6-De2 | K/(K + Ti) | 0.0000000 |
| N-De8-De4 | K/(K + Si) | 0.0000060 | De9-De2 | K/(K + Ti) | 0.0002649 |
| N-De8-De5 | K/(K + Si) | 0.0000939 | N-De8-De2 | K/(K + Ti) | 0.0000000 |
| De4-De2 | K/(K + Al) | 0.0000000 | S-De8-De2 | K/(K + Ti) | 0.0000084 |
| De5-De2 | K/(K + Al) | 0.0000005 | De4-De3 | K/(K + Ti) | 0.0000000 |
| N-De8-De2 | K/(K + Al) | 0.0000000 | De5-De3 | K/(K + Ti) | 0.0004749 |
| De4-De3 | K/(K + Al) | 0.0000000 | De6-De3 | K/(K + Ti) | 0.0018964 |
| De5-De3 | K/(K + Al) | 0.0000132 | N-De8-De3 | K/(K + Ti) | 0.0035633 |
| De9-De3 | K/(K + Al) | 0.0039216 | De3-De2 | K/(K + Mn) | 0.0000000 |
| N-De8-De3 | K/(K + Al) | 0.0000000 | De4-De2 | K/(K + Mn) | 0.0000000 |
| De6-De4 | K/(K + Al) | 0.0000027 | De5-De2 | K/(K + Mn) | 0.0000000 |
| De9-De4 | K/(K + Al) | 0.0000000 | De6-De2 | K/(K + Mn) | 0.0000000 |
| S-De8-De4 | K/(K + Al) | 0.0000000 | De9-De2 | K/(K + Mn) | 0.0000000 |
| De9-De5 | K/(K + Al) | 0.0000000 | N-De8-De2 | K/(K + Mn) | 0.0000000 |
| S-De8-De5 | K/(K + Al) | 0.0000044 | S-De8-De2 | K/(K + Mn) | 0.0000000 |
| N-De8-De6 | K/(K + Al) | 0.0006158 | De4-De3 | K/(K + Mn) | 0.0000000 |
| N-De8-De9 | K/(K + Al) | 0.0000000 | De5-De3 | K/(K + Mn) | 0.0086938 |
| S-De8-N-De8 | K/(K + Al) | 0.0000001 | De6-De3 | K/(K + Mn) | 0.0004807 |
| De3-De2 | K/(K + Ca) | 0.0320513 | N-De8-De3 | K/(K + Mn) | 0.0000001 |
| De4-De2 | K/(K + Ca) | 0.0000000 | De6-De2 | K/(K + Sr) | 0.0000000 |
| De5-De2 | K/(K + Ca) | 0.0000000 | De9-De2 | K/(K + Sr) | 0.0000000 |
| De4-De3 | K/(K + Ca) | 0.0000000 | N-De8-De2 | K/(K + Sr) | 0.0000000 |
| De5-De3 | K/(K + Ca) | 0.0000000 | S-De8-De2 | K/(K + Sr) | 0.0000000 |
| S-De8-De3 | K/(K + Ca) | 0.0000884 | De6-De3 | K/(K + Sr) | 0.0000000 |
| De6-De4 | K/(K + Ca) | 0.0000001 | De9-De3 | K/(K + Sr) | 0.0000000 |
| N-De8-De3 | K/(K + Sr) | 0.0000000 | S-De8-De4 | Ca/(Ca + Fe) | 0.0000000 |
| S-De8-De3 | K/(K + Sr) | 0.0000000 | De6-De5 | Ca/(Ca + Fe) | 0.0002164 |
| De6-De4 | K/(K + Sr) | 0.0000000 | De9-De5 | Ca/(Ca + Fe) | 0.0000749 |
| De9-De4 | K/(K + Sr) | 0.0000000 | N-De8-De5 | Ca/(Ca + Fe) | 0.0000170 |
| N-De8-De4 | K/(K + Sr) | 0.0000000 | S-De8-De5 | Ca/(Ca + Fe) | 0.0000000 |
| S-De8-De4 | K/(K + Sr) | 0.0000000 | S-De8-De6 | Ca/(Ca + Fe) | 0.0013038 |
| De6-De5 | K/(K + Sr) | 0.0000000 | S-De8-De9 | Ca/(Ca + Fe) | 0.0116983 |
| De9-De5 | K/(K + Sr) | 0.0000000 | S-De8-N-De8 | Ca/(Ca + Fe) | 0.0000284 |
| N-De8-De5 | K/(K + Sr) | 0.0000000 | De3-De2 | Mn/(Mn + Sr) | 0.0059663 |
| S-De8-De5 | K/(K + Sr) | 0.0000000 | De4-De2 | Mn/(Mn + Sr) | 0.0000000 |
| De6-De2 | Sr/(Sr + Rb) | 0.0015649 | De5-De2 | Mn/(Mn + Sr) | 0.0000001 |
| De9-De2 | Sr/(Sr + Rb) | 0.0000001 | De6-De2 | Mn/(Mn + Sr) | 0.0000000 |
| N-De8-De2 | Sr/(Sr + Rb) | 0.0000002 | De9-De2 | Mn/(Mn + Sr) | 0.0000000 |
| S-De8-De2 | Sr/(Sr + Rb) | 0.0063469 | N-De8-De2 | Mn/(Mn + Sr) | 0.0000000 |
| De6-De3 | Sr/(Sr + Rb) | 0.0002659 | S-De8-De2 | Mn/(Mn + Sr) | 0.0000000 |
| De9-De3 | Sr/(Sr + Rb) | 0.0000000 | De4-De3 | Mn/(Mn + Sr) | 0.0000000 |
| N-De8-De3 | Sr/(Sr + Rb) | 0.0000000 | De5-De3 | Mn/(Mn + Sr) | 0.0220222 |
| S-De8-De3 | Sr/(Sr + Rb) | 0.0019248 | De6-De3 | Mn/(Mn + Sr) | 0.0000000 |
| De6-De4 | Sr/(Sr + Rb) | 0.0000004 | De9-De3 | Mn/(Mn + Sr) | 0.0000003 |
| De9-De4 | Sr/(Sr + Rb) | 0.0000005 | N-De8-De3 | Mn/(Mn + Sr) | 0.0000000 |
| N-De8-De4 | Sr/(Sr + Rb) | 0.0000000 | S-De8-De3 | Mn/(Mn + Sr) | 0.0000000 |
| S-De8-De4 | Sr/(Sr + Rb) | 0.0367545 | De5-De4 | Mn/(Mn + Sr) | 0.0105295 |
| De6-De5 | Sr/(Sr + Rb) | 0.0000033 | De6-De4 | Mn/(Mn + Sr) | 0.0009477 |
| De9-De5 | Sr/(Sr + Rb) | 0.0000104 | N-De8-De4 | Mn/(Mn + Sr) | 0.0000000 |
| N-De8-De5 | Sr/(Sr + Rb) | 0.0000000 | S-De8-De4 | Mn/(Mn + Sr) | 0.0001308 |
| De9-De6 | Sr/(Sr + Rb) | 0.0000000 | De6-De5 | Mn/(Mn + Sr) | 0.0000000 |
| S-De8-De6 | Sr/(Sr + Rb) | 0.0000000 | De9-De5 | Mn/(Mn + Sr) | 0.0238969 |
| N-De8-De9 | Sr/(Sr + Rb) | 0.0000000 | N-De8-De5 | Mn/(Mn + Sr) | 0.0000000 |
| S-De8-N-De8 | Sr/(Sr + Rb) | 0.0000000 | S-De8-De5 | Mn/(Mn + Sr) | 0.0000000 |
| De4-De2 | Ca/(Ca + Fe) | 0.0000000 | N-De8-De9 | Mn/(Mn + Sr) | 0.0161029 |
| De5-De2 | Ca/(Ca + Fe) | 0.0000000 | De3-De2 | Ti/(Ti + Mn) | 0.0135132 |
| S-De8-De2 | Ca/(Ca + Fe) | 0.0041582 | De4-De2 | Ti/(Ti + Mn) | 0.0000000 |
| De4-De3 | Ca/(Ca + Fe) | 0.0000000 | De5-De2 | Ti/(Ti + Mn) | 0.0064950 |
| De5-De3 | Ca/(Ca + Fe) | 0.0000000 | De6-De2 | Ti/(Ti + Mn) | 0.0016346 |
| S-De8-De3 | Ca/(Ca + Fe) | 0.0001215 | N-De8-De2 | Ti/(Ti + Mn) | 0.0000000 |
| De6-De4 | Ca/(Ca + Fe) | 0.0002233 | S-De8-De2 | Ti/(Ti + Mn) | 0.0473812 |
| De9-De4 | Ca/(Ca + Fe) | 0.0000826 | De4-De3 | Ti/(Ti + Mn) | 0.0085386 |
| N-De8-De4 | Ca/(Ca + Fe) | 0.0000040 | N-De8-De3 | Ti/(Ti + Mn) | 0.0076982 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammed, D.D.; Simon, N.; Utley, J.E.P.; Verhagen, I.T.E.; Duller, R.A.; Griffiths, J.; Wooldridge, L.J.; Worden, R.H. Geochemistry of Sub-Depositional Environments in Estuarine Sediments: Development of an Approach to Predict Palaeo-Environments from Holocene Cores. Geosciences 2022, 12, 23. https://doi.org/10.3390/geosciences12010023
Muhammed DD, Simon N, Utley JEP, Verhagen ITE, Duller RA, Griffiths J, Wooldridge LJ, Worden RH. Geochemistry of Sub-Depositional Environments in Estuarine Sediments: Development of an Approach to Predict Palaeo-Environments from Holocene Cores. Geosciences. 2022; 12(1):23. https://doi.org/10.3390/geosciences12010023
Chicago/Turabian StyleMuhammed, Dahiru D., Naboth Simon, James E. P. Utley, Iris T. E. Verhagen, Robert A. Duller, Joshua Griffiths, Luke J. Wooldridge, and Richard H. Worden. 2022. "Geochemistry of Sub-Depositional Environments in Estuarine Sediments: Development of an Approach to Predict Palaeo-Environments from Holocene Cores" Geosciences 12, no. 1: 23. https://doi.org/10.3390/geosciences12010023
APA StyleMuhammed, D. D., Simon, N., Utley, J. E. P., Verhagen, I. T. E., Duller, R. A., Griffiths, J., Wooldridge, L. J., & Worden, R. H. (2022). Geochemistry of Sub-Depositional Environments in Estuarine Sediments: Development of an Approach to Predict Palaeo-Environments from Holocene Cores. Geosciences, 12(1), 23. https://doi.org/10.3390/geosciences12010023






