Inclusions of Amorphous and Crystalline SiO2 in Minerals from Itrongay (Madagascar) and Other Evidence for the Natural Occurrence of Hydrosilicate Fluids
Abstract
:1. Introduction
2. Previous Work
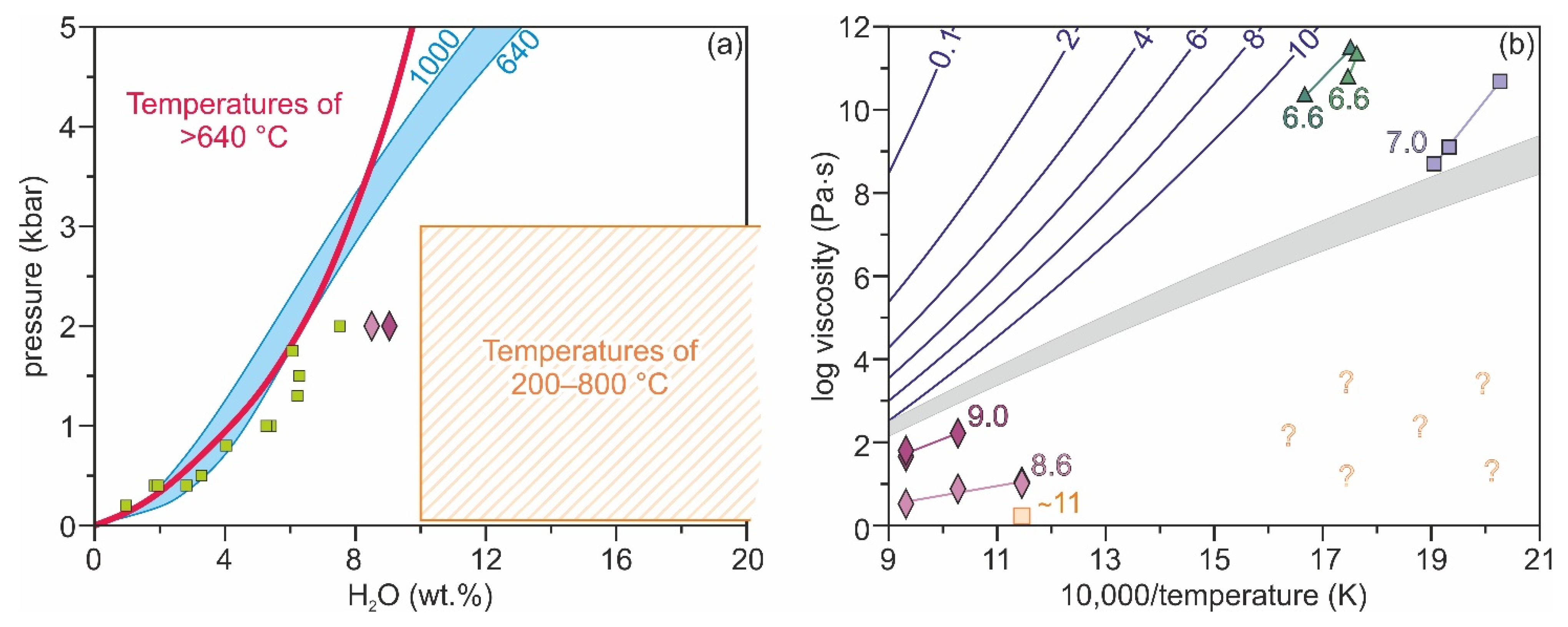
2.1. Experimental Evidence
2.2. Evidence from Inclusions in Minerals
2.3. Evidence from Human-Accessible Environments
3. New Evidence
3.1. Materials
3.2. Methods
3.3. Results
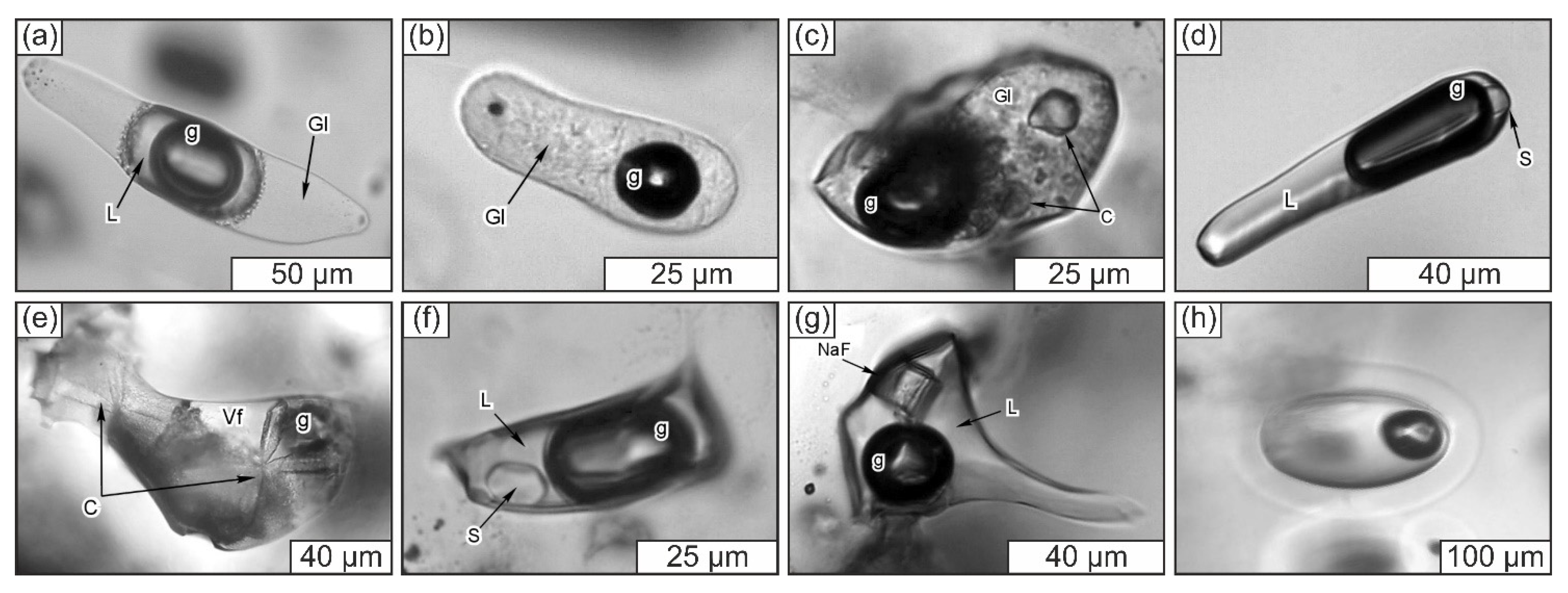
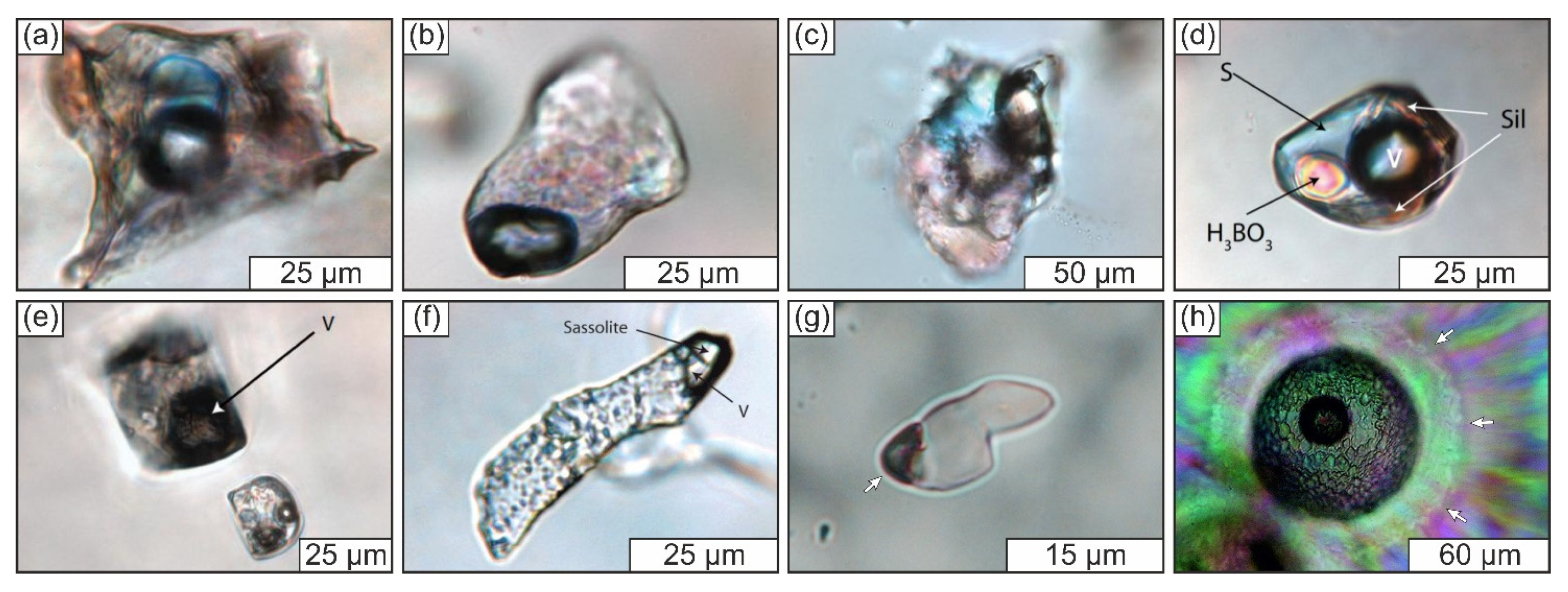
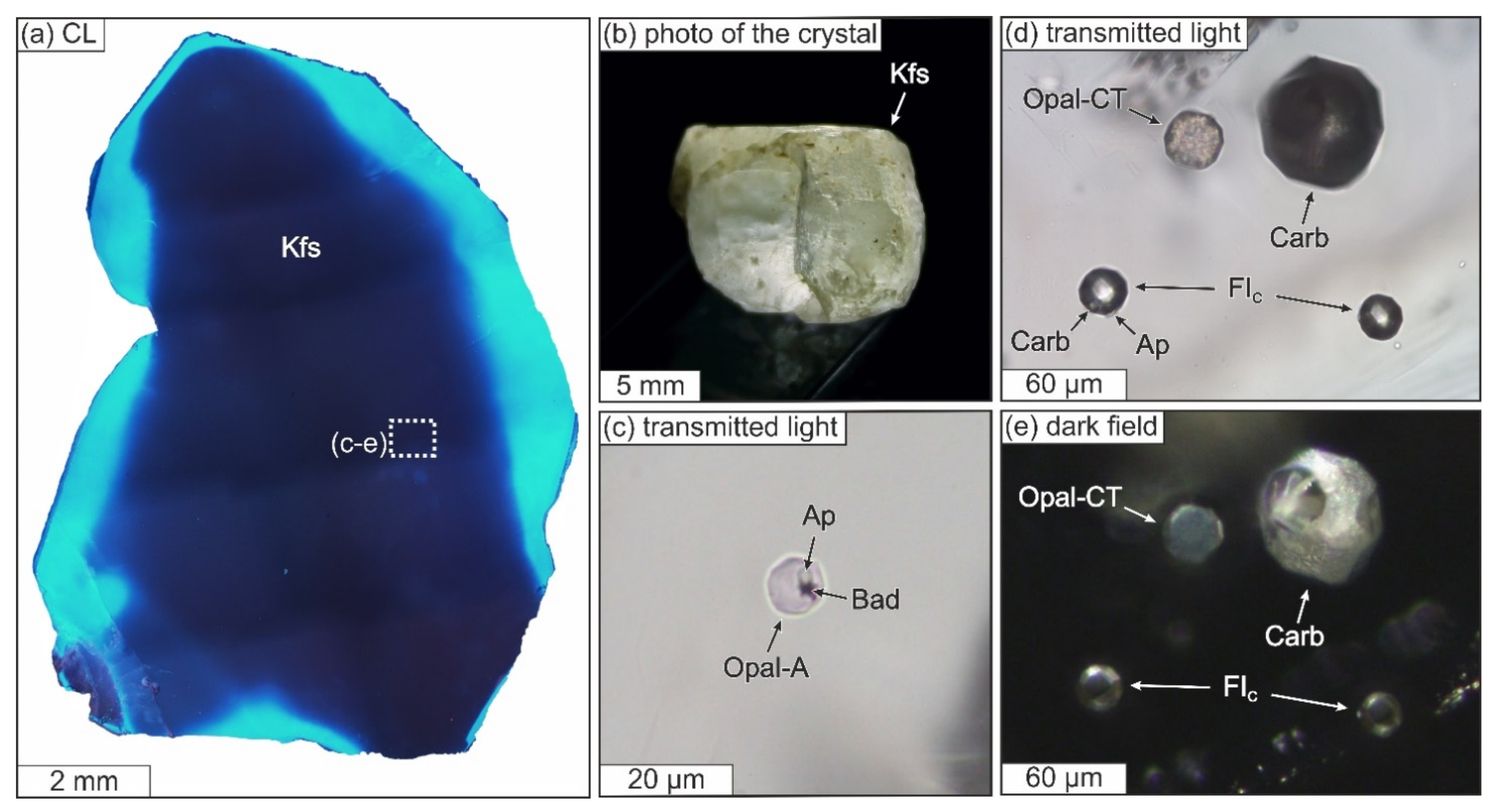
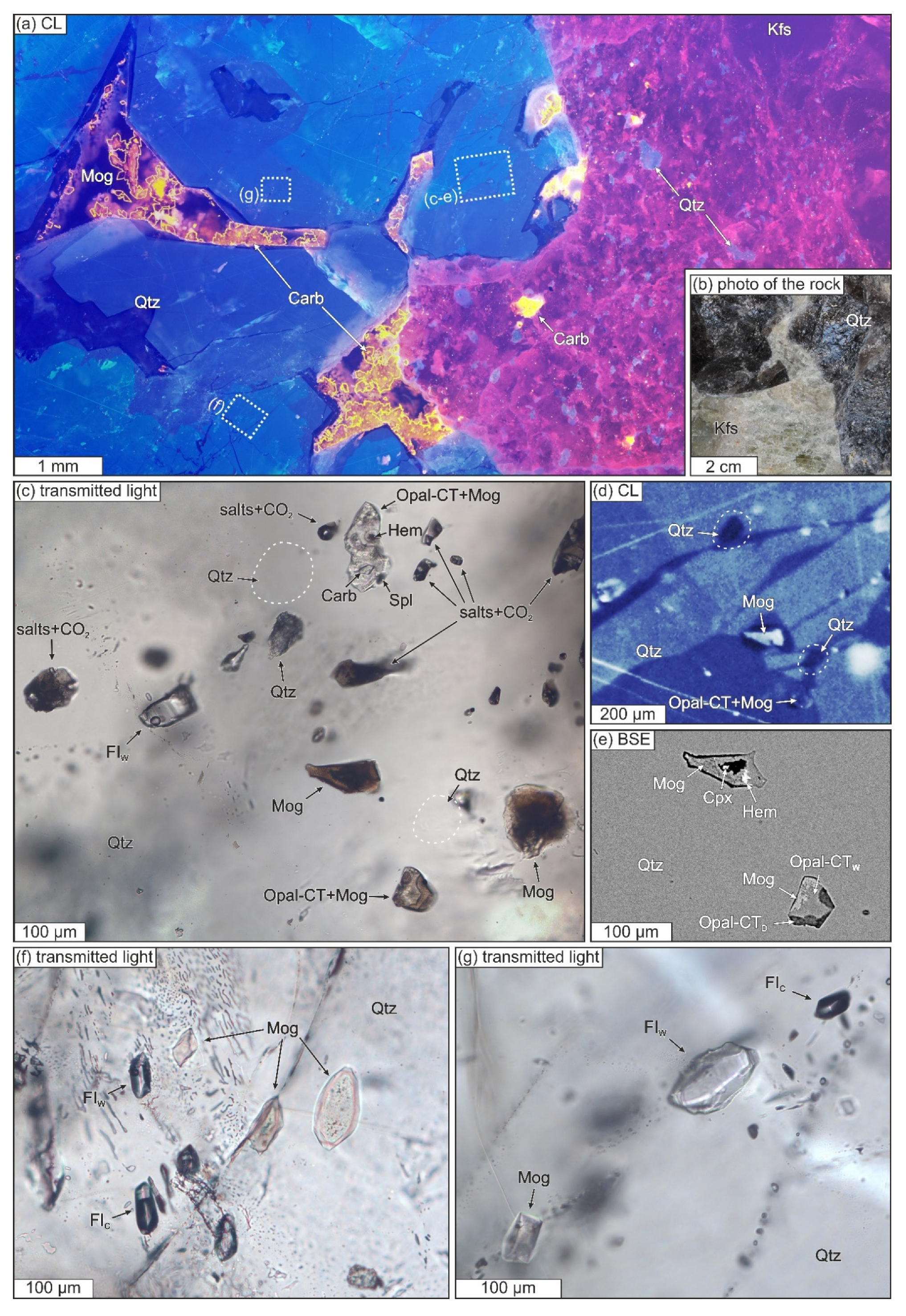
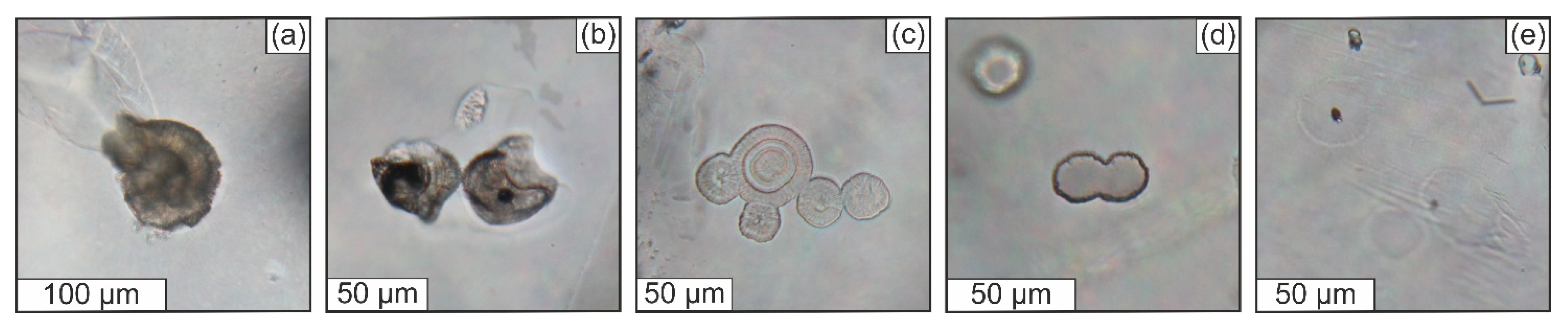
3.3.1. General Description of the Studied Samples
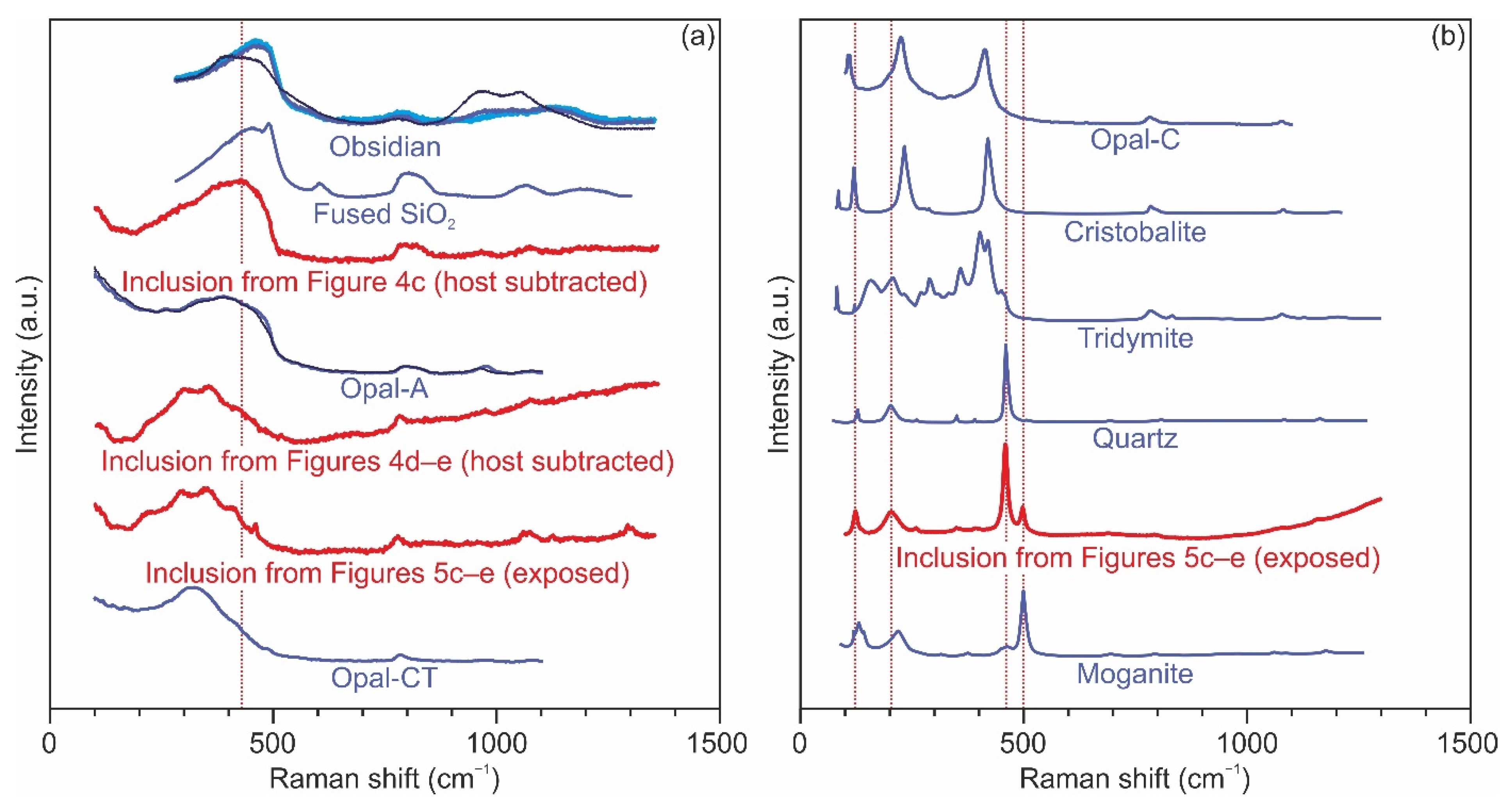
3.3.2. Structural State of SiO2-Rich Fluid Inclusions
3.4. Discussion
4. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuttle, O.F.; Friedman, I.I. Liquid Immiscibility in the System H2O—Na2O—SiO2. J. Am. Chem. Soc. 1948, 70, 919–926. [Google Scholar] [CrossRef]
- Kravchuk, K.G.; Valyashko, V.M. The equilibrium diagram of the system Na₂O–SiO₂–H₂O. In Methods of Experimental Study of Hydrothermal Equilibria; Godovikov, A.A., Ed.; Nauka: Novosibirsk, Russia, 1979; pp. 105–117. [Google Scholar]
- Wilkinson, J.J.; Nolan, J.; Rankin, A.H. Silicothermal fluid: A novel medium for mass transport in the lithosphere. Geology 1996, 24, 1059–1062. [Google Scholar] [CrossRef]
- Veksler, I.V. Liquid immiscibility and its role at the magmatic–hydrothermal transition: A summary of experimental studies. Chem. Geol. 2004, 210, 7–31. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Smirnov, S.Z.; Thomas, V.G.; Zagorsky, V.Y. Gels and Melt-like Gels in High-Temperature Endogeneous Mineral Formation. In Proceedings of the Metallogeny of the Pacific Northwest: Tectonics, Magmatism and Metallogeny of Active Continental Margin; Dalnauka: Vladivostok, Russia, 2004; pp. 306–309. [Google Scholar]
- Smirnov, S.Z.; Thomas, V.G.; Demin, S.P.; Drebushchak, V.A. Experimental study of boron solubility and speciation in the Na₂O–B₂O₃–SiO₂–H₂O system. Chem. Geol. 2005, 223, 16–34. [Google Scholar] [CrossRef]
- Kotelnikova, Z.A.; Kotelnikov, A.R. NaF-bearing fluids: Experimental investigation at 500–800 °C and P = 2000 bar using synthetic fluid inclusions in quartz. Geochem. Int. 2008, 46, 48–61. [Google Scholar] [CrossRef]
- Kotel’Nikova, Z.A.; Kotel’Nikov, A.R. Experimental study of heterogeneous fluid equilibria in silicate-salt-water systems. Geol. Ore Depos. 2010, 52, 154–166. [Google Scholar] [CrossRef]
- Kotel’Nikova, Z.A.; Kotel’Nikov, A.R. Immiscibility in sulfate-bearing fluid systems at high temperatures and pressures. Geochem. Int. 2010, 48, 381–389. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Smirnov, S.Z.; Kotel’Nikov, A.R.; Kotel’Nikova, Z.A. Experimental study of the system H₃BO₃–NaF–SiO₂–H₂O at 350–800 °C and 1–2 kbar by the method of synthetic fluid inclusions. Russ. Geol. Geophys. 2010, 51, 349–368. [Google Scholar] [CrossRef]
- Kotel’Nikova, Z.A.; Kotel’Nikov, A.R. Na2CO3-bearing fluids: Experimental study at 700 °C and under 1, 2, and 3 kbar pressure using synthetic fluid inclusions in quartz. Geol. Ore Depos. 2011, 53, 156–170. [Google Scholar] [CrossRef]
- Kotel’Nikova, Z.A.; Kotel’Nikov, A.R. Unusual phase transformations in synthetic NaF-bearing fluid inclusions in quartz. Dokl. Earth Sci. 2011, 439, 967–969. [Google Scholar] [CrossRef]
- Smirnov, S.Z.; Thomas, V.G.; Kamenetsky, V.S.; Kozmenko, O.A.; Large, R. Hydrosilicate liquids in the system Na2O-SiO2-H2O with NaF, NaCl and Ta: Evaluation of their role in ore and mineral formation at high T and P. Petrology 2012, 20, 271–285. [Google Scholar] [CrossRef]
- Thomas, V.G.; Smirnov, S.Z.; Kozmenko, O.A.; Drebushchak, V.A.; Kamenetsky, V.S. Formation and properties of hydrosilicate liquids in the systems Na2O-Al2O3-SiO2-H2O and granite-Na2O-SiO2-H2O at 600 °C and 1.5 kbar. Petrology 2014, 22, 293–309. [Google Scholar] [CrossRef]
- Smirnov, S.Z.; Thomas, V.G.; Kamenetsky, V.; Kozmenko, O.A. Hydrosilicate liquids in the system rare-metal granite–Na2O–SiO2–H2O as accumulators of ore components at high pressure and temperature. Petrology 2017, 25, 625–635. [Google Scholar] [CrossRef]
- Kigai, I.N. The genesis of agates and amethyst geodes. Can. Miner. 2019, 57, 867–883. [Google Scholar] [CrossRef]
- Chukhrov, F.V. Colloids in the Earth’s Crust; USSR Academy of Sciences: Moscow, Russia, 1955. [Google Scholar]
- Williamson, B.J.; Stanley, C.J.; Wilkinson, J.J. Implications from inclusions in topaz for greisenisation and mineralisation in the Hensbarrow topaz granite, Cornwall, England. Contrib. Miner. Pet. 1997, 127, 119–128. [Google Scholar] [CrossRef]
- Williamson, B.J.; Wilkinson, J.J.; Luckham, P.F.; Stanley, C.J. Formation of coagulated colloidal silica in high-temperature mineralizing fluids. Miner. Mag. 2002, 66, 547–553. [Google Scholar] [CrossRef]
- Brotzen, O. Outline of Mineralization in Zoned Granitic Pegmatites: A Qualitative and Comparative Study. Geol. Föreningen Stockh. Förhandlingar 1959, 81, 1–98. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P. Water and Melt/Melt Immiscibility, the Essential Components in the Formation of Pegmatites; Evidence from Melt Inclusions. Z. Geol. Wiss. 2008, 36, 347–364. [Google Scholar]
- Thomas, R.; Davidson, P. Evidence of a water-rich silica gel state during the formation of a simple pegmatite. Miner. Mag. 2012, 76, 2785–2801. [Google Scholar] [CrossRef] [Green Version]
- Peterková, T.; Dolejš, D. Magmatic-hydrothermal transition of Mo-W-mineralized granite-pegmatite-greisen system recorded by trace elements in quartz: Krupka district, Eastern Krušné hory/Erzgebirge. Chem. Geol. 2019, 523, 179–202. [Google Scholar] [CrossRef]
- Smirnov, S.Z. The fluid regime of crystallization of water-saturated granitic and pegmatitic magmas: A physicochemical analysis. Russ. Geol. Geophys. 2015, 56, 1292–1307. [Google Scholar] [CrossRef]
- Vasyukova, O.V. Types and Origin of Quartz and Quartz-Hosted Fluid Inclusions in Mineralised Porphyries. Ph.D. Thesis, University of Tasmania, Hobart, Tasmania, 2011. [Google Scholar]
- Vasyukova, O.V.; Kamenetsky, V.S.; Goemann, K.; Davidson, P. Diversity of primary CL textures in quartz from porphyry environments: Implication for origin of quartz eyes. Contrib. Miner. Pet. 2013, 166, 1253–1268. [Google Scholar] [CrossRef]
- Moxon, T.; Palyanova, G. Agate Genesis: A Continuing Enigma. Minerals 2020, 10, 953. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R.; Pan, Y. Mineralogy, Geochemistry and Genesis of Agate—A Review. Minerals 2020, 10, 1037. [Google Scholar] [CrossRef]
- Korges, M.; Weis, P.; Lüders, V.; Laurent, O. Depressurization and boiling of a single magmatic fluid as a mechanism for tin-tungsten deposit formation. Geology 2017, 46, 75–78. [Google Scholar] [CrossRef]
- Monnier, L.; Salvi, S.; Jourdan, V.; Sall, S.; Bailly, L.; Melleton, J.; Béziat, D. Contrasting fluid behavior during two styles of greisen alteration leading to distinct wolframite mineralizations: The Echassières district (Massif Central, France). Ore Geol. Rev. 2020, 124, 103648. [Google Scholar] [CrossRef]
- Simmons, W.B.S.; Webber, K.L. Pegmatite genesis: State of the art. Eur. J. Miner. 2008, 20, 421–438. [Google Scholar] [CrossRef]
- London, D.; Morgan, V.G.B. The Pegmatite Puzzle. Elements 2012, 8, 263–268. [Google Scholar] [CrossRef]
- London, D. A petrologic assessment of internal zonation in granitic pegmatites. Lithos 2014, 184–187, 74–104. [Google Scholar] [CrossRef]
- Monecke, T.; Monecke, J.; Reynolds, T.J.; Tsuruoka, S.; Bennett, M.; Skewes, W.B.; Palin, R.M. Quartz Solubility in the H2O-NaCl System: A Framework for Understanding Vein Formation in Porphyry Copper Deposits. Econ. Geol. 2018, 113, 1007–1046. [Google Scholar] [CrossRef]
- Mernagh, T.P.; Leys, C.; Henley, R.W. Fluid inclusion systematics in porphyry copper deposits: The super-giant Grasberg deposit, Indonesia, as a case study. Ore Geol. Rev. 2020, 123, 103570. [Google Scholar] [CrossRef]
- Lee, C.-T.A.; Tang, M. How to make porphyry copper deposits. Earth Planet. Sci. Lett. 2020, 529, 115868. [Google Scholar] [CrossRef]
- Holtz, F.; Johannes, W.; Tamic, N.; Behrens, H. Maximum and minimum water contents of granitic melts generated in the crust: A reevaluation and implications. Lithos 2001, 56, 1–14. [Google Scholar] [CrossRef]
- Newman, S.; Lowenstern, J. VolatileCalc: A silicate melt–H2O–CO2 solution model written in Visual Basic for excel. Comput. Geosci. 2002, 28, 597–604. [Google Scholar] [CrossRef]
- Dingwell, D.B.; Holtz, F.; Behrens, H. The solubility of H2O in peralkaline and peraluminous granitic melts. Am. Miner. 1997, 82, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Bureau, H. Complete miscibility between silicate melts and hydrous fluids in the upper mantle: Experimental evidence and geochemical implications. Earth Planet. Sci. Lett. 1999, 165, 187–196. [Google Scholar] [CrossRef]
- Kennedy, G.C.; Wasserburg, G.J.; Heard, H.C.; Newton, R.C. The upper three-phase region in the system SiO2–H2O. Am. J. Sci. 1962, 260, 501–521. [Google Scholar] [CrossRef]
- Sowerby, J.R.; Keppler, H. The effect of fluorine, boron and excess sodium on the critical curve in the albite–H2O system. Contrib. Miner. Pet. 2002, 143, 32–37. [Google Scholar] [CrossRef]
- Giordano, D.; Russell, J.K.; Dingwell, D.B. Viscosity of magmatic liquids: A model. Earth Planet. Sci. Lett. 2008, 271, 123–134. [Google Scholar] [CrossRef]
- Bartels, A.; Vetere, F.; Holtz, F.; Behrens, H.; Linnen, R.L. Viscosity of flux-rich pegmatitic melts. Contrib. Miner. Pet. 2011, 162, 51–60. [Google Scholar] [CrossRef]
- Tuttle, O.F.; Bowen, N.L. Origin of Granite in the Light of Experimental Studies in the System NaAlSi3O8–KAlSi3O8–SiO2–H2O; The Geological Society of America Memoir; Geological Society of America: New York, NY, USA, 1958; Volume 74. [Google Scholar]
- Di Matteo, V.; Carroll, M.; Behrens, H.; Vetere, F.; Brooker, R. Water solubility in trachytic melts. Chem. Geol. 2004, 213, 187–196. [Google Scholar] [CrossRef]
- Popov, D.V.; Nekrylov, N.; Plechov, P.; Shcherbakov, V.D.; Portnyagin, M.; Serova, M.S. Composition and conditions of formation of the parental melts of Jurassic dolerites of southwestern Crimea: Evidence from melt inclusions in olivine phenocrysts. Petrology 2017, 25, 272–303. [Google Scholar] [CrossRef] [Green Version]
- Hess, K.-U.; Dingwell, D.B. Viscosities of Hydrous Leucogranitic Melts: A Non-Arrhenian Model. Am. Mineral. 1996, 81, 1297–1300. [Google Scholar]
- Dingwell, D.; Hess, K.-U.; Romano, C. Extremely fluid behavior of hydrous peralkaline rhyolites. Earth Planet. Sci. Lett. 1998, 158, 31–38. [Google Scholar] [CrossRef]
- Sirbescu, M.-L.C.; Schmidt, C.; Veksler, I.V.; Whittington, A.G.; Wilke, M. Experimental Crystallization of Undercooled Felsic Liquids: Generation of Pegmatitic Texture. J. Pet. 2017, 58, 539–568. [Google Scholar] [CrossRef]
- Ermakov, N.P. Geochemical Systems of Inclusions in Minerals; Nedra: Moscow, Russia, 1972. [Google Scholar]
- Roedder, E. Fluid Inclusions. Rev. Mineral. 1984, 12, 1–644. [Google Scholar]
- Bakker, R.; Jansen, J.H. Experimental post-entrapment water loss from synthetic CO₂–H₂O inclusions in natural quartz. Geochim. Cosmochim. Acta 1991, 55, 2215–2230. [Google Scholar] [CrossRef]
- Bodnar, R.J. Reequilibration of Fluid Inclusions. In Fluid Inclusions: Analysis and Interpretation; Samson, I., Anderson, A., Marshall, D., Eds.; Short Course Series; Mineralogical Association of Canada: Quebec, QC, Canada, 2003; pp. 213–230. [Google Scholar]
- Danyushevsky, L.; Della-Pasqua, F.N.; Sokolov, S. Re-equilibration of melt inclusions trapped by magnesian olivine phenocrysts from subduction-related magmas: Petrological implications. Contrib. Miner. Pet. 2000, 138, 68–83. [Google Scholar] [CrossRef] [Green Version]
- Portnyagin, M.; Mironov, N.; Botcharnikov, R.; Gurenko, A.; Almeev, R.; Luft, C.; Holtz, F. Dehydration of melt inclusions in olivine and implications for the origin of silica-undersaturated island-arc melts. Earth Planet. Sci. Lett. 2019, 517, 95–105. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica; John Wiley & Sons: New York, NY, USA, 1979. [Google Scholar]
- Scherer, G.W. Aging and drying of gels. J. Non-Cryst. Solids 1988, 100, 77–92. [Google Scholar] [CrossRef]
- Smirnov, S.Z.; Peretyazhko, I.S.; Zagorsky, V.E.; Mikhailov, M.Y. Inclusions of Unusual Late Magmatic Melts in Quartz from the Oktyabr’skaya Pegmatite Vein, Malkhan Field (Central Transbaikal Region). Dokl. Earth Sci. 2003, 392, 999–1003. [Google Scholar]
- Peretyazhko, I.; Zagorsky, V.Y.; Smirnov, S.Z.; Mikhailov, M.Y. Conditions of pocket formation in the Oktyabrskaya tourmaline-rich gem pegmatite (the Malkhan field, Central Transbaikalia, Russia). Chem. Geol. 2004, 210, 91–111. [Google Scholar] [CrossRef]
- Touret, J.L.R.; Smirnov, S.Z.; Peretyazhko, I.S.; Zagorsky, V.Y.; Thomas, V.G. Magmatic-Hydrothermal Transition in Tourmaline-Bearing Miarolitic Pegmatites: Hydrosaline Fluids or Silica Gels? In Proceedings of the International Symposium. Granitic Pegmatites: The State of the Art, Porto, Portugal, 6–12 May 2007. [Google Scholar]
- Thomas, R.; Davidson, P.; Badanina, E. Water- and Boron-Rich Melt Inclusions in Quartz from the Malkhan Pegmatite, Transbaikalia, Russia. Minerals 2012, 2, 435–458. [Google Scholar] [CrossRef]
- London, D. Reply to Thomas and Davidson on “A petrologic assessment of internal zonation in granitic pegmatites” (London, 2014a). Lithos 2015, 212–215, 469–484. [Google Scholar] [CrossRef]
- Popov, D.V.; Spikings, R.A.; Scaillet, S.; O’Sullivan, G.; Chew, D.; Badenszki, E.; Daly, J.S.; Razakamanana, T.; Davies, J.H.F.L. Diffusion and fluid interaction in Itrongay pegmatite (Madagascar): Evidence from in situ 40Ar/39Ar dating of gem-quality alkali feldspar and U Pb dating of protogenetic apatite inclusions. Chem. Geol. 2020, 556, 119841. [Google Scholar] [CrossRef]
- Eadington, P.J.; Nashar, B. Evidence for the magmatic origin of quartz-topaz rocks from the New England batholith, Australia. Contrib. Miner. Pet. 1978, 67, 433–438. [Google Scholar] [CrossRef]
- Granovskaya, N.V.; Kobzareva, J.S. An importance of thermobarochemistry for sedimentary basins katagenesis study; the Bashkirskij Meganticlinorium as example. In Proceedings of the XIII International conference on thermobarogeochemistry and IVth APIFIS Symposium, Moscow, Russia, 22–25 September 2008; Volume 2, pp. 214–217. [Google Scholar]
- Prokofiev, V.; Kamenetsky, V.S.; Selektor, S.L.; Rodemann, T.; Kovalenker, V.A.; Vatsadze, S.Z. First direct evidence for natural occurrence of colloidal silica in chalcedony-hosted vacuoles and implications for ore-forming processes. Geology 2017, 45, 71–74. [Google Scholar] [CrossRef]
- Khakimov, A.K. Genetic types of inclusions of agate amygdales and veins of the Idzhebanskii deposit in Armenia. In Mineralogical Thermometry and Barometry. Volume II. New Methods and Results of Study of Parameters of Ore Formation; Ermakov, N.P., Sobolev, V.S., Smirnov, V.I., Khitarov, N.I., Kuznetsov, V.A., Dolgov, Y.A., Eds.; Nauka: Moscow, USSR, 1968; pp. 230–236. [Google Scholar]
- Eugster, H.P.; Jones, B.F. Gels Composed of Sodium-Aluminum Silicate, Lake Magadi, Kenya. Science 1968, 161, 160–163. [Google Scholar] [CrossRef]
- White, D.E.; Brannock, W.; Murata, K. Silica in hot-spring waters. Geochim. Cosmochim. Acta 1956, 10, 27–59. [Google Scholar] [CrossRef]
- Renaut, R.W.; Owen, R.B.; Lowenstein, T.K.; De Cort, G.; Mcnulty, E.; Scott, J.J.; Mbuthia, A. The role of hydrothermal fluids in sedimentation in saline alkaline lakes: Evidence from Nasikie Engida, Kenya Rift Valley. Sedimentology 2020, 68, 108–134. [Google Scholar] [CrossRef]
- Spezia, G. Sopra un deposito di quarzo e di silice gelatinosa trovato nel traforo del Sempione. Atti R. Accad. Sci. Torino 1899, 34, 705–713. [Google Scholar]
- Markl, G.; Bäuerle, J.; Grujic, D. Metamorphic evolution of Pan-African granulite facies metapelites from Southern Madagascar. Precambrian Res. 2000, 102, 47–68. [Google Scholar] [CrossRef]
- Tucker, R.D.; Roig, J.Y.; Moine, B.; Delor, C.; Peters, S.G. A geological synthesis of the Precambrian shield in Madagascar. J. Afr. Earth Sci. 2014, 94, 9–30. [Google Scholar] [CrossRef]
- De Wit, M.J.; Bowring, S.A.; Ashwal, L.D.; Randrianasolo, L.G.; Morel, V.P.I.; Rambeloson, R.A. Age and tectonic evolution of Neoproterozoic ductile shear zones in southwestern Madagascar, with implications for Gondwana studies. Tectonics 2001, 20, 1–45. [Google Scholar] [CrossRef]
- Collins, A.S.; Kinny, P.D.; Razakamanana, T. Depositional age, provenance and metamorphic age of metasedimentary rocks from southern Madagascar. Gondwana Res. 2012, 21, 353–361. [Google Scholar] [CrossRef]
- Martelat, J.-E.; Lardeaux, J.-M.; Nicollet, C.; Rakotondrazafy, R. Strain pattern and late Precambrian deformation history in southern Madagascar. Precambrian Res. 2000, 102, 1–20. [Google Scholar] [CrossRef]
- Collins, A.S. Madagascar and the amalgamation of Central Gondwana. Gondwana Res. 2006, 9, 3–16. [Google Scholar] [CrossRef]
- Niels, J.; Schenk, V. The ultrahigh temperature granulites of southern Madagascar in a polymetamorphic context: Implications for the amalgamation of the Gondwana supercontinent. Eur. J. Miner. 2011, 23, 127–156. [Google Scholar] [CrossRef] [Green Version]
- Horton, F.; Hacker, B.; Kylander-Clark, A.; Holder, R.; Jöns, N. Focused radiogenic heating of middle crust caused ultrahigh temperatures in southern Madagascar. Tectonics 2016, 35, 293–314. [Google Scholar] [CrossRef] [Green Version]
- Horton, F.; Holder, R.M.; Swindle, C.R. An extensive record of orogenesis recorded in a Madagascar granulite. J. Metamorph. Geol. 2021, jmg.12628. [Google Scholar] [CrossRef]
- Holder, R.M.; Hacker, B.R.; Horton, F.; Rakotondrazafy, A.F.M. Ultrahigh-temperature osumilite gneisses in southern Madagascar record combined heat advection and high rates of radiogenic heat production in a long-lived high-T orogen. J. Metamorph. Geol. 2018, 36, 855–880. [Google Scholar] [CrossRef]
- Holder, R.M.; Hacker, B.R. Fluid-driven resetting of titanite following ultrahigh-temperature metamorphism in southern Madagascar. Chem. Geol. 2019, 504, 38–52. [Google Scholar] [CrossRef]
- Emmel, B.; Jöns, N.; Kröner, A.; Jacobs, J.; Wartho, J.-A.; Schenk, V.; Razakamanana, T.; Austegard, A. From Closure of the Mozambique Ocean to Gondwana Breakup: New Evidence from Geochronological Data of the Vohibory Terrane, Southwest Madagascar. J. Geol. 2008, 116, 21–38. [Google Scholar] [CrossRef]
- Emmel, B.; Jacobs, J.; Razakamanana, T. Titanite and apatite fission track analyses on basement rocks of central-southern Madagascar: Constraints on exhumation and denudation rates along the eastern rift shoulder of the Morondava basin. J. Afr. Earth Sci. 2004, 38, 343–361. [Google Scholar] [CrossRef]
- Montel, J.-M.; Razafimahatratra, D.; de Parseval, P.; Poitrasson, F.; Moine, B.; Seydoux-Guillaume, A.-M.; Pik, R.; Arnaud, N.; Gibert, F. The giant monazite crystals from Manangotry (Madagascar). Chem. Geol. 2018, 484, 36–50. [Google Scholar] [CrossRef]
- Seward, D.; Grujic, D.; Schreurs, G. An insight into the breakup of Gondwana: Identifying events through low-temperature thermochronology from the basement rocks of Madagascar. Tectonics 2004, 23. [Google Scholar] [CrossRef] [Green Version]
- Emmel, B.; Boger, S.; Jacobs, J.; Daszinnies, M. Maturity of central Madagascar’s landscape—Low-temperature thermochronological constraints. Gondwana Res. 2012, 21, 704–713. [Google Scholar] [CrossRef]
- Arnaud, N.O.; Kelley, S.P. Argon behaviour in gem-quality orthoclase from Madagascar: Experiments and some consequences for 40Ar39Ar geochronology. Geochim. Cosmochim. Acta 1997, 61, 3227–3255. [Google Scholar] [CrossRef]
- Nägler, T.; Villa, I.M. In pursuit of the 40K branching ratios: K-Ca and 39Ar–40Ar dating of gem silicates. Chem. Geol. 2000, 169, 5–16. [Google Scholar] [CrossRef]
- Flude, S.; Halton, A.M.; Kelley, S.P.; Sherlock, S.C.; Schwanethal, J.; Wilkinson, C.M. Observation of centimetre-scale argon diffusion in alkali feldspars: Implications for 40Ar/39Ar thermochronology. Geol. Soc. Lond. Spéc. Publ. 2014, 378, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Lafuente, B.; Downs, R.S.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Curtis, N.J.; Gascooke, J.R.; Johnston, M.R.; Pring, A. A Review of the Classification of Opal with Reference to Recent New Localities. Minerals 2019, 9, 299. [Google Scholar] [CrossRef] [Green Version]
- Ostrooumov, M.; Fritsch, E.; Lasnier, B.; Lefrant, S. Spectres Raman des opales: Aspect diagnostique et aide à la classification. Eur. J. Miner. 1999, 11, 899–908. [Google Scholar] [CrossRef]
- Cicconi, M.R.; Neuville, D.R. Natural Glasses. In Springer Handbook of Glass; Musgraves, J.D., Hu, J., Calvez, L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 771–812. ISBN 978-3-319-93726-7. [Google Scholar]
- Kingma, K.J.; Hemley, R.J. Raman Spectroscopic Study of Microcrystalline Silica. Am. Mineral. 1994, 79, 269–273. [Google Scholar]
- Touret, J. The Significance of Fluid Inclusions in Metamorphic Rocks. In Thermodynamics in Geology; Fraser, D.G., Ed.; D. Reidel Publishing Company: Dordrecht, Germany, 1977; pp. 203–227. [Google Scholar]
- Touret, J. Faciès Granulite et Fluides Carboniques. Ann. Soc. Géol. Belg. Publ. Spéc. Géol. Domaines Cristal. Centen. Soc. Géol. Belg. 1974, 267–287. [Google Scholar]
- Sirbescu, M.-L.C.; Nabelek, P.I. Crustal melts below 400 °C. Geology 2003, 31, 685. [Google Scholar] [CrossRef]
- Sirbescu, M.-L.C.; Nabelek, P.I. Crystallization conditions and evolution of magmatic fluids in the Harney Peak Granite and associated pegmatites, Black Hills, South Dakota—Evidence from fluid inclusions. Geochim. Cosmochim. Acta 2003, 67, 2443–2465. [Google Scholar] [CrossRef]
- Henisch, H.K. Crystal Growth in Gels. Helv. Phys. Acta 1968, 41, 888–897. [Google Scholar] [CrossRef]
- Henisch, H.K. Crystals in Gels and Liesegang Rings: In Vitro Veritas; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1988; ISBN 978-0-521-34503-3. [Google Scholar]
- Zagorsky, V. Mineralogy of pockets of the Malkhan tourmaline deposit (Transbaikalia): Feldspars of the Sosedka vein. Russ. Geol. Geophys. 2012, 53, 522–534. [Google Scholar] [CrossRef]
- Zagorsky, V.Y. Sosedka pegmatite body at the Malkhan deposit of gem tourmaline, Transbaikalia: Composition, inner structure, and petrogenesis. Petrology 2015, 23, 68–92. [Google Scholar] [CrossRef]
- Talantsev, A.S. Chamber Pegmatites of the Urals; Nedra: Moscow, Russia, 1988. [Google Scholar]
- Hulsbosch, N.; Boiron, M.-C.; Thomas, R.; van Daele, J.; Dewaele, S.; Muchez, P. Evaluation of the petrogenetic significance of melt inclusions in pegmatitic schorl-dravite from graphic tourmaline-quartz assemblages: Application of LA-ICP-QMS analyses and volume ratio calculations. Geochim. Cosmochim. Acta 2019, 244, 308–335. [Google Scholar] [CrossRef]
- Morteani, G.; Kostitsyn, Y.A.; Gilg, H.A.; Preinfalk, C.; Razakamanana, T. Geochemistry of phlogopite, diopside, calcite, anhydrite and apatite pegmatites and syenites of southern Madagascar: Evidence for crustal silicocarbonatitic (CSC) melt formation in a Panafrican collisional tectonic setting. Acta Diabetol. 2012, 102, 627–645. [Google Scholar] [CrossRef]
- Martin, R.F.; Randrianandraisana, A.; Boulvais, P. Ampandrandava and similar phlogopite deposits in southern Madagascar: Derivation from a silicocarbonatitic melt of crustal origin. J. Afr. Earth Sci. 2014, 94, 111–118. [Google Scholar] [CrossRef]
- Smith, S.R.; Kelley, S.P.; Tindle, A.G.; Breaks, F.W. Compositional controls on 40Ar/39Ar ages of zoned mica from a rare-element pegmatite. Contrib. Miner. Pet. 2005, 149, 613–626. [Google Scholar] [CrossRef]
- Schaltegger, U.; Ulianov, A.; Müntener, O.; Ovtcharova, M.; Peytcheva, I.; Vonlanthen, P.; Vennemann, T.; Antognini, M.; Girlanda, F. Megacrystic zircon with planar fractures in miaskite-type nepheline pegmatites formed at high pressures in the lower crust (Ivrea Zone, southern Alps, Switzerland). Am. Miner. 2015, 100, 83–94. [Google Scholar] [CrossRef]
- Bergemann, C.; Gnos, E.; Berger, A.; Whitehouse, M.; Mullis, J.; Wehrens, P.; Pettke, T.; Janots, E. Th-Pb ion probe dating of zoned hydrothermal monazite and its implications for repeated shear zone activity: An example from the Central Alps, Switzerland. Tectonics 2017, 36, 671–689. [Google Scholar] [CrossRef]
- Ricchi, E.; Bergemann, C.A.; Gnos, E.; Berger, A.; Rubatto, D.; Whitehouse, M.J. Constraining deformation phases in the Aar Massif and the Gotthard Nappe (Switzerland) using Th-Pb crystallization ages of fissure monazite-(Ce). Lithos 2019, 342–343, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Fulignati, P.; Kamenetsky, V.S.; Marianelli, P.; Sbrana, A.; Meffre, S. First insights on the metallogenic signature of magmatic fluids exsolved from the active magma chamber of Vesuvius (AD 79 “Pompei” eruption). J. Volcanol. Geotherm. Res. 2011, 200, 223–233. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B. Magmatic fluids immiscible with silicate melts: Examples from inclusions in phenocrysts and glasses, and implications for magma evolution and metal transport. Geofluids 2010, 10, 293–311. [Google Scholar] [CrossRef]
- Fiedrich, A.M.; Heinrich, C.A.; Bachmann, O. Evolution from magmatic to hydrothermal activity beneath the Cerro Escorial volcano (NW Argentina) as sampled by erupted quartz and brines. Lithos 2020, 374–375, 105706. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Naumov, V.B.; Davidson, P.; van Achterbergh, E.; Ryan, C.G. Immiscibility between silicate magmas and aqueous fluids: A melt inclusion pursuit into the magmatic-hydrothermal transition in the Omsukchan Granite (NE Russia). Chem. Geol. 2004, 210, 73–90. [Google Scholar] [CrossRef]
- Ferrero, S.; Wunder, B.; Ziemann, M.A.; Wälle, M.; O’Brien, P.J. Carbonatitic and granitic melts produced under conditions of primary immiscibility during anatexis in the lower crust. Earth Planet. Sci. Lett. 2016, 454, 121–131. [Google Scholar] [CrossRef]
- Lowenstern, J.B.; Van Hinsberg, V.; Berlo, K.; Liesegang, M.; Iacovino, K.; Bindeman, I.N.; Wright, H.M. Opal-A in Glassy Pumice, Acid Alteration, and the 1817 Phreatomagmatic Eruption at Kawah Ijen (Java), Indonesia. Front. Earth Sci. 2018, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Saunders, J.A. Silica and gold textures in bonanza ores of the Sleeper Deposit, Humboldt County, Nevada; evidence for colloids and implications for epithermal ore-forming processes. Econ. Geol. 1994, 89, 628–638. [Google Scholar] [CrossRef]
- Martin, E.; Gaillou, E. Insight on gem opal formation in volcanic ash deposits from a supereruption: A case study through oxygen and hydrogen isotopic composition of opals from Lake Tecopa, California, USA. Am. Miner. 2018, 103, 803–811. [Google Scholar] [CrossRef]
- Ivanova, D.A.; Shcherbakov, V.D.; Plechov, P.Y.; Nekrylov, N.A.; Davydova, V.O.; Turova, M.A.; Stepanov, O.V. Cristobalite in extrusive rocks of Bezymianny volcano. New Data Miner. 2018, 52, 51–59. [Google Scholar] [CrossRef]
- Horwell, C.J.; Williamson, B.J.; Llewellin, E.W.; Damby, D.E.; Le Blond, J.S. The nature and formation of cristobalite at the Soufrière Hills volcano, Montserrat: Implications for the petrology and stability of silicic lava domes. Bull. Volcanol. 2013, 75, 696. [Google Scholar] [CrossRef] [Green Version]
- Popov, D.V.; Brovchenko, V.D.; Nekrylov, N.A.; Plechov, P.Y.; Spikings, R.A.; Tyutyunnik, O.A.; Krigman, L.V.; Anosova, M.O.; Kostitsyn, Y.A.; Soloviev, A.V. Removing a mask of alteration: Geochemistry and age of the Karadag volcanic sequence in SE Crimea. Lithos 2019, 324–325, 371–384. [Google Scholar] [CrossRef]
- André, L.; Abraham, K.; Hofmann, A.; Monin, L.; Kleinhanns, I.C.; Foley, S. Early continental crust generated by reworking of basalts variably silicified by seawater. Nat. Geosci. 2019, 12, 769–773. [Google Scholar] [CrossRef]
- Collins, W.J.; Murphy, J.B.; Johnson, T.E.; Huang, H.-Q. Critical role of water in the formation of continental crust. Nat. Geosci. 2020, 13, 331–338. [Google Scholar] [CrossRef]
- Clay, P.L.; O’Driscoll, B.; Gertisser, R.; Busemann, H.; Sherlock, S.C.; Kelley, S.P. Textural characterization, major and volatile element quantification and Ar–Ar systematics of spherulites in the Rocche Rosse obsidian flow, Lipari, Aeolian Islands: A temperature continuum growth model. Contrib. Miner. Pet. 2013, 165, 373–395. [Google Scholar] [CrossRef]
- Breitkreuz, C. Spherulites and lithophysae—200 years of investigation on high-temperature crystallization domains in silica-rich volcanic rocks. Bull. Volcanol. 2013, 75, 705. [Google Scholar] [CrossRef]
- Seaman, S.J. Microtexture development during rapid cooling in three rhyolitic lava flows. Am. Miner. 2013, 98, 304–318. [Google Scholar] [CrossRef]
- Bullock, L.A.; Gertisser, R.; O’Driscoll, B. Spherulite formation in obsidian lavas in the Aeolian Islands, Italy. Period. Mineral. 2017, 86. [Google Scholar] [CrossRef]
- Lofgren, G. Experimentally Produced Devitrification Textures in Natural Rhyolitic Glass. GSA Bull. 1971, 82, 111. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, D.V.; Spikings, R.A.; Razakamanana, T. Inclusions of Amorphous and Crystalline SiO2 in Minerals from Itrongay (Madagascar) and Other Evidence for the Natural Occurrence of Hydrosilicate Fluids. Geosciences 2022, 12, 28. https://doi.org/10.3390/geosciences12010028
Popov DV, Spikings RA, Razakamanana T. Inclusions of Amorphous and Crystalline SiO2 in Minerals from Itrongay (Madagascar) and Other Evidence for the Natural Occurrence of Hydrosilicate Fluids. Geosciences. 2022; 12(1):28. https://doi.org/10.3390/geosciences12010028
Chicago/Turabian StylePopov, Daniil V., Richard A. Spikings, and Théodore Razakamanana. 2022. "Inclusions of Amorphous and Crystalline SiO2 in Minerals from Itrongay (Madagascar) and Other Evidence for the Natural Occurrence of Hydrosilicate Fluids" Geosciences 12, no. 1: 28. https://doi.org/10.3390/geosciences12010028
APA StylePopov, D. V., Spikings, R. A., & Razakamanana, T. (2022). Inclusions of Amorphous and Crystalline SiO2 in Minerals from Itrongay (Madagascar) and Other Evidence for the Natural Occurrence of Hydrosilicate Fluids. Geosciences, 12(1), 28. https://doi.org/10.3390/geosciences12010028






