Formation of Gold Alloys during Crustal Differentiation of Convergent Zone Magmas: Constraints from an AU-Rich Websterite in the Stanovoy Suture Zone (Russian Far East)
Abstract
:1. Introduction
2. Geologic Background and Samples
3. Methods
4. Results
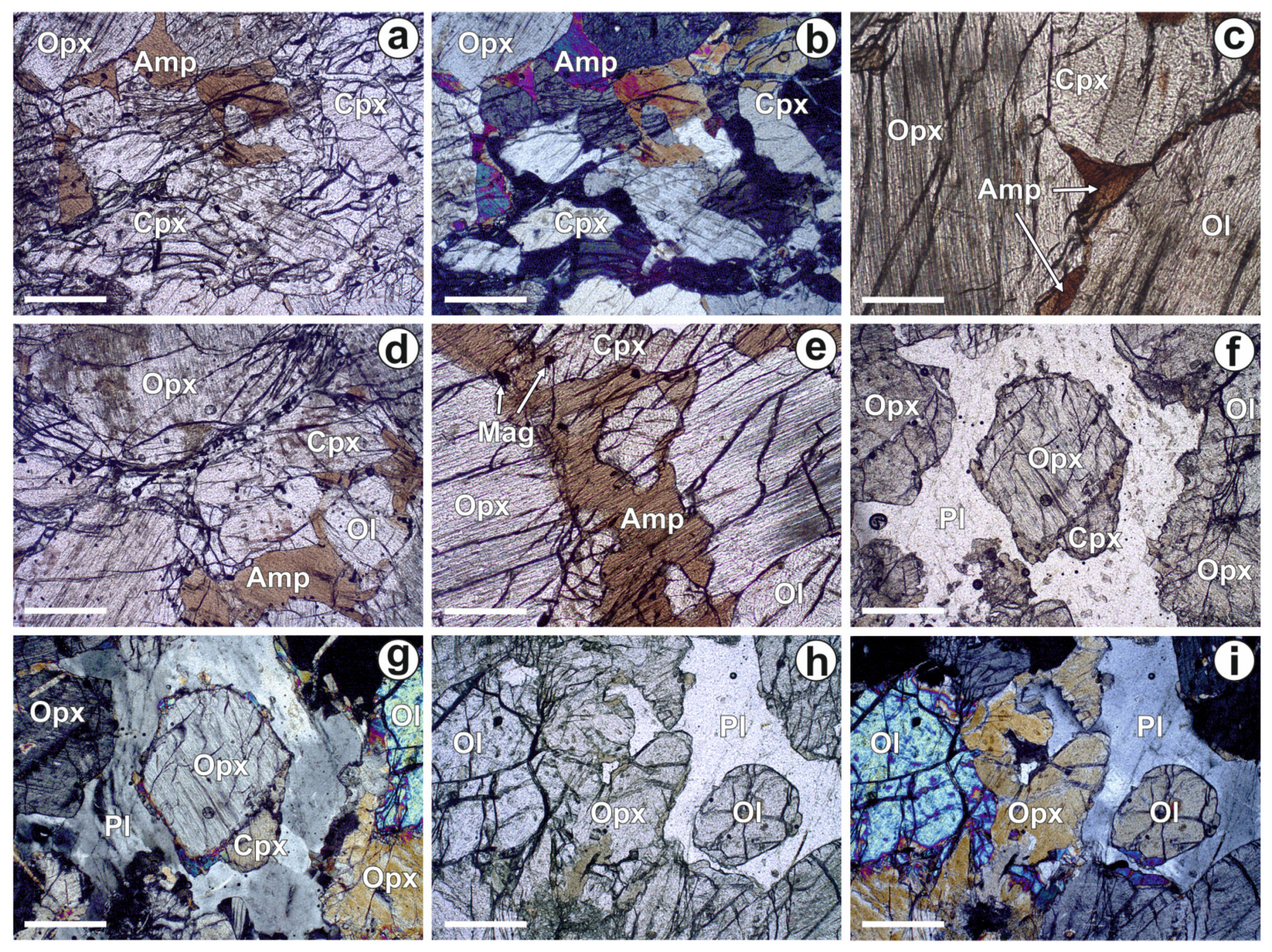
4.1. Petrography of the Ildeus Intrusion
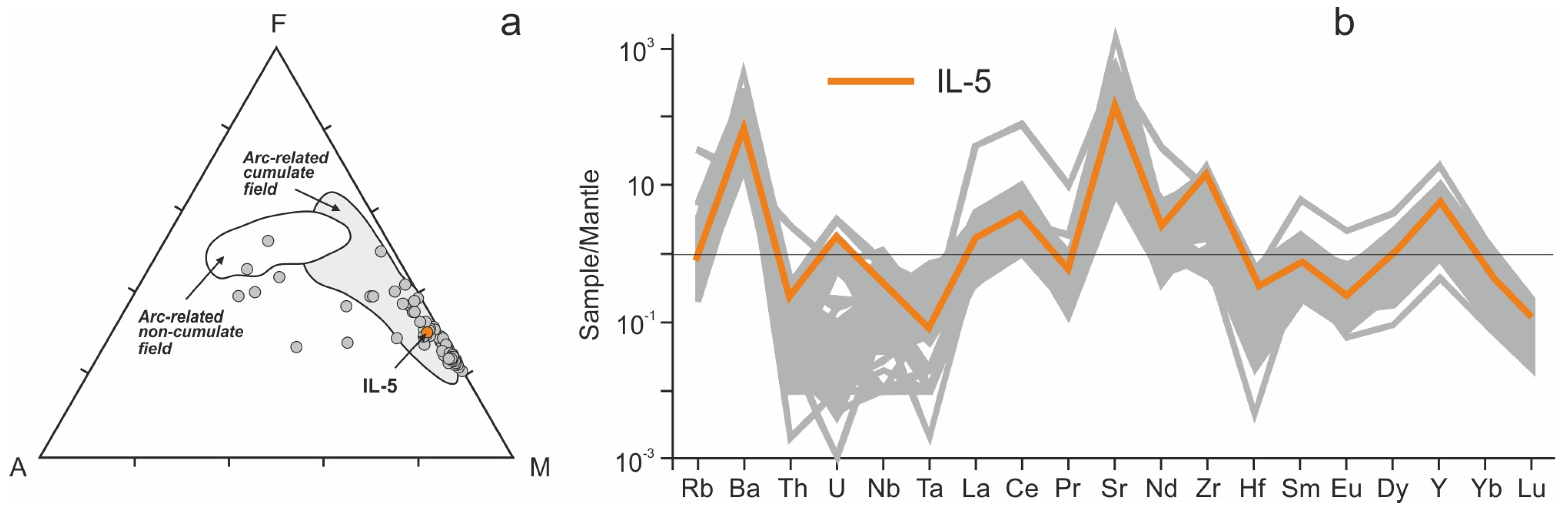
4.2. Geochemistry of the Ildeus Intrusion
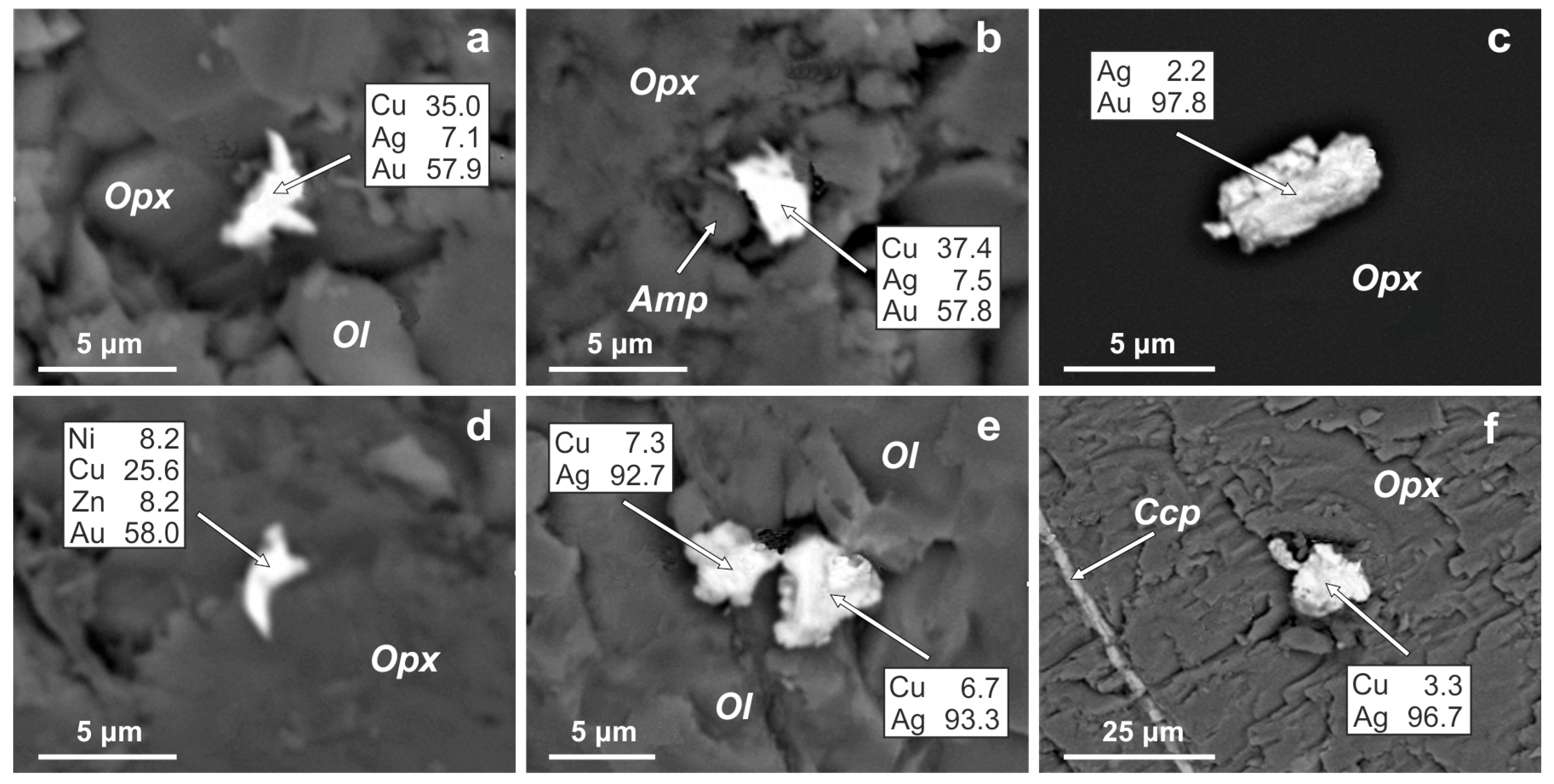
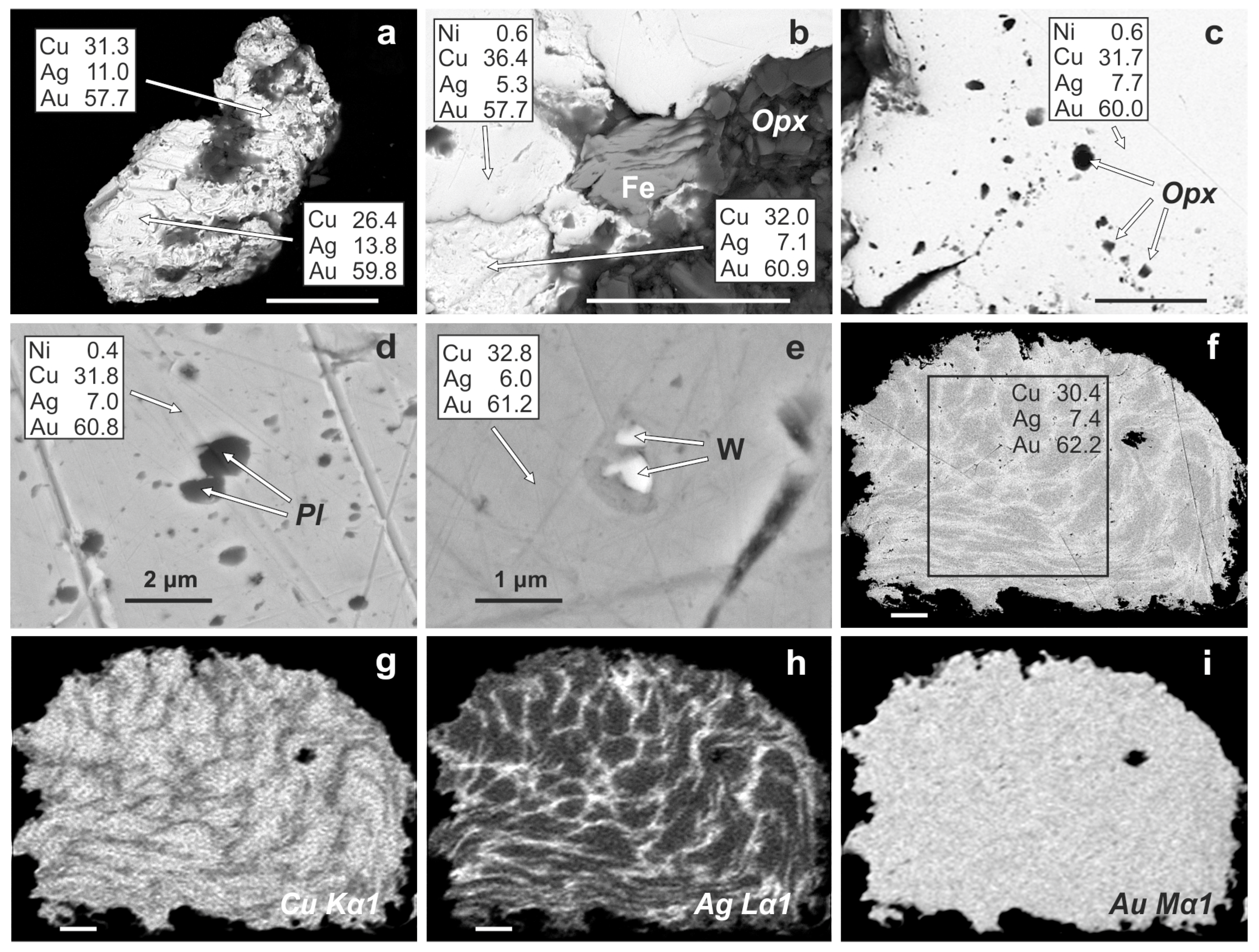
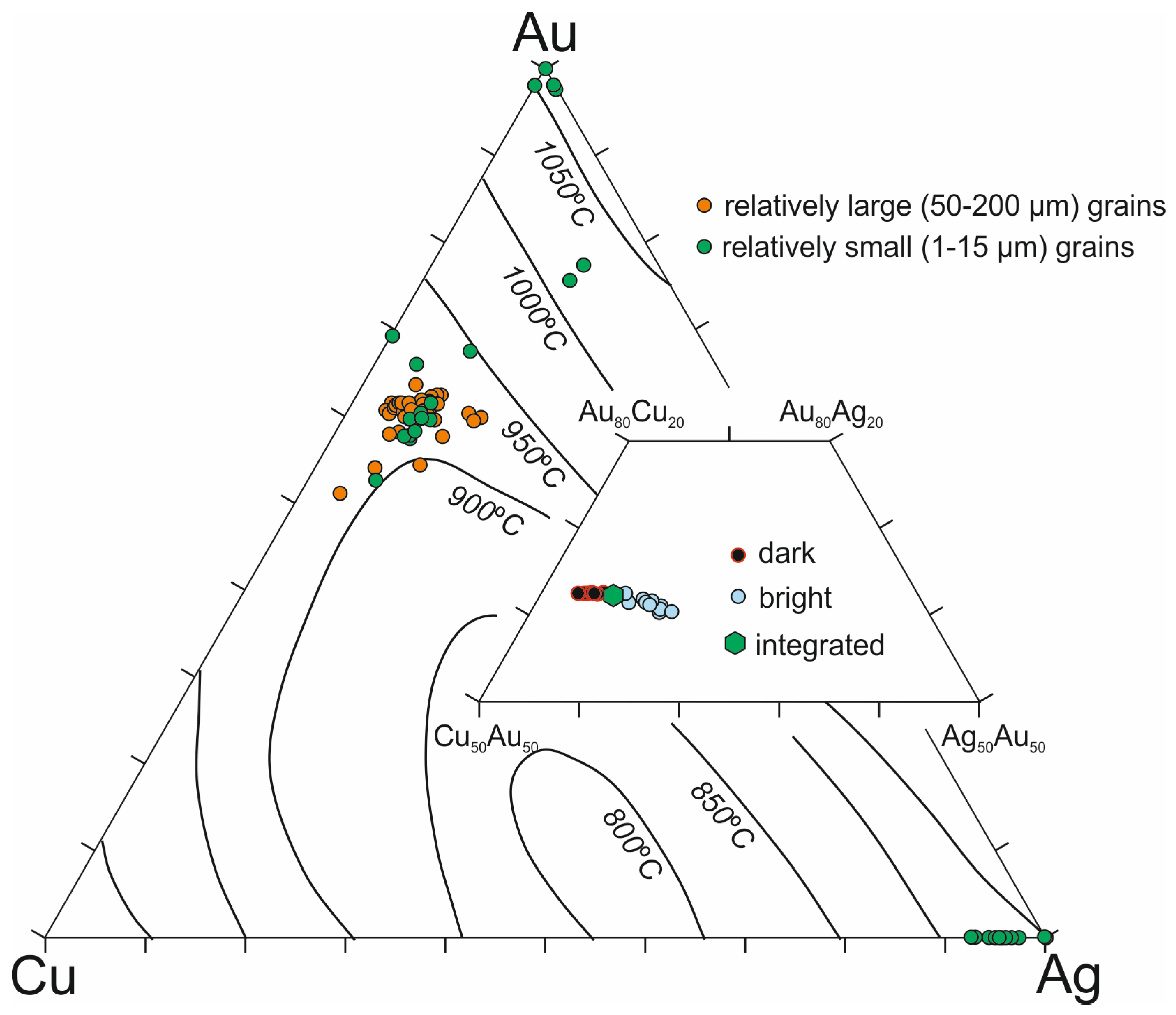
4.3. Gold Alloys in the Ildeus Intrusion
4.4. Heating Experiments on Cu-Ag-Au Alloys
5. Discussion
5.1. Petrological and Geochemical Constraints on the Origin of the Ildeus Intrusion
5.2. Physico-Chemical Conditions of Cu-Ag-Au Alloys Formation
5.3. Geologic Conditions of the Formation of Cu-Ag-Au Alloys in Magmatic Systems
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawkins, F.J. Metal Deposits in Relation to Plate Tectonics; Springer: Berlin, Germany, 1984; 325p. [Google Scholar]
- Sillitoe, R.H. Gold deposits in Western Pacific island arcs: The magmatic connection. In The Geology of Gold Deposits: The Perspective in 1988; Keays, R.R., Ramsay, W.R.H., Groves, D.I., Eds.; The Society of Economic Geology: Littleton, CO, USA, 1989; pp. 274–291. [Google Scholar]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Mungall, J.E. Roasting the mantle: Slab melting and the genesis of major Au and Au-rich Cu deposits. Geology 2002, 30, 915–918. [Google Scholar] [CrossRef]
- Herrington, R.; Maslennikov, V.; Zaykov, V.; Seravkin, I.; Kosarev, A.; Buschmann, B.; Orgeval, J.-J.; Holland, N.; Tesalina, S.; Nimis, P.; et al. 6: Classification of VMS deposits: Lessons from the South Uralides. Ore Geol. Rev. 2005, 27, 203–237. [Google Scholar] [CrossRef]
- Simmons, S.F.; Brown, K.L. Gold in magmatic hydrothermal solutions and the rapid formation of a giant ore deposit. Science 2006, 314, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Sillitoe, R.H. Major gold deposits and belts of the North and South American cordillera: Distribution, tectonomagmatic settings, and metallogenic considerations. Econ. Geol. 2008, 103, 663–687. [Google Scholar] [CrossRef]
- Mercier-Langevin, P.; Hannington, M.D.; Dube, B.; Becu, V. The gold content of volcanogenic massive sulfide deposits. Miner. Depos. 2010, 46, 509–539. [Google Scholar] [CrossRef] [Green Version]
- De Ronde, C.E.J.; Massoth, G.J.; Buttterfield, D.A.; Christenson, B.W.; Ishibashi, J.; Ditchburn, R.G.; Hannington, M.D.; Brathwaite, R.L.; Lupton, J.E.; Kamenetsky, V.S.; et al. Submarine hydrothermal activity and gold-rich mineralization at Brothers Volcano, Kermadec Arc, New Zealand. Mineral. Depos. 2011, 46, 541–584. [Google Scholar] [CrossRef]
- Muntean, J.L.; Cline, J.S.; Simon, A.C.; Longo, A.A. Magmatic-hydrothermal origin of Nevada’s Carlin-type gold deposits. Nat. Geosci. 2011, 4, 122–127. [Google Scholar] [CrossRef]
- Richards, J.P. Magmatic to hydrothermal metal fluxes in convergent and collided margins. Ore Geol. Rev. 2011, 40, 1–26. [Google Scholar] [CrossRef]
- Groves, D.I.; Zhang, L.; Santosh, M. Subduction, mantle metasomatism, and gold: A dynamic and genetic conjunction. Geol. Soc. Amer. Bull. 2020, 132, 1419–1426. [Google Scholar] [CrossRef]
- Sun, W.D.; Arculus, R.J.; Kamenetsky, V.S.; Binns, R.A. Release of gold-bearing fluids in convergent margin magmas prompted by magnetite crystallization. Nature 2004, 431, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, O.; Williams-Jones, A.E.; Stix, J. Sulphide magma as a source of metals in arc-related magmatic hydrothermal ore fluids. Nat. Geosci. 2010, 3, 501–505. [Google Scholar] [CrossRef]
- Jégo, S.; Pichavant, M. Gold solubility in arc magmas: Experimental determination of the effect of sulfur at 1000 °C and 0.4 GPa. Geochim. Cosmochim. Acta 2012, 84, 560–592. [Google Scholar] [CrossRef] [Green Version]
- Zajacz, Z.; Candela, P.; Piccoli, P.M.; Walle, M.; Sanchez-Valle, C. Gold and copper in volatile saturated mafic to intermediate magmas: Solubilities, partitioning, and implications for ore deposit formation. Geochim. Cosmochim. Acta 2012, 91, 140–159. [Google Scholar] [CrossRef]
- Li, J.-L.; Gao, J.; John, T.; Klemd, R.; Su, W. Fluid-mediated metal transport in subduction zones and its link to arc-related giantore deposits: Costraints from a sulfide-bearing HP vein in lawsonite eclogite (Tianshan, China). Geochim. Cosmochim. Acta 2013, 120, 326–362. [Google Scholar] [CrossRef]
- Edmonds, M.; Mather, T.A.; Liu, E.J. A distinct metal fingerprint in arc volcanic emissions. Nat. Geosci. 2018, 11, 790–794. [Google Scholar] [CrossRef] [Green Version]
- Kepezhinskas, P.; Kepezhinskas, N.; Berdnikov, N. Gold, platinum and palladium enrichments in arcs: Role of mantle wedge, arc crust and halogen-rich slab fluids. E3S Web Conf. 2019, 98, 08010. [Google Scholar] [CrossRef] [Green Version]
- Kamenetsky, V.S.; Zelenski, M. Origin of noble-metal nuggets in sulfide-saturated arc magmas: A case study of olivine-hostedsulfide melt inclusions from the Tolbachik volcano (Kamchatka, Russia). Geology 2020, 48, 620–624. [Google Scholar] [CrossRef]
- Gasparrini, C. Gold and Other Precious Metals from Ore to Market; Springer: Berlin, Germany, 1993; 336p. [Google Scholar]
- Kesler, S.E.; Chryssoulis, S.L.; Simon, G. Gold in porphyry copper deposits: Its abundance and fate. Ore Geol. Rev. 2002, 21, 103. [Google Scholar] [CrossRef]
- Pals, D.W.; Spry, P.G. Telluride mineralogy of the low-sulfidation epithermal Emperor gold deposit, Vatukoula, Fiji. Mineral. Petrol. 2003, 79, 285–307. [Google Scholar] [CrossRef]
- Plotinskaya, O.Y.; Kovalenker, V.A.; Seltmann, R.; Stanley, C.J. Te and Se mineralogy of the high-sulfidation Kochbulak andKairagach epithermal gold telluride deposits (Kurama Ridge, Middle Tien Shan, Uzbekistan). Mineral. Petrol. 2006, 87, 187–2006. [Google Scholar] [CrossRef]
- Mavrogenes, J.; Henley, R.W.; Reyes, A.G.; Berger, B. Sulfosalt melts: Evidence of high-temperature vapor transport of metals in the formation of high-sulfidation lode gold deposits. Econ. Geol. 2010, 105, 257–262. [Google Scholar] [CrossRef]
- Tolstykh, N.D.; Vymazalova, A.; Tuhy, M.; Shapovalova, M. Conditions of formation of Au-Se-Te mineralization in the Gaching.ore occurrence (Maletoyvayam ore field), Kamchatka, Russia. Mineral. Mag. 2018, 82, 649–674. [Google Scholar] [CrossRef]
- Palyanova, G.A. Gold and silver minerals in sulfide ore. Geol. Ore Depos. 2020, 62, 383–406. [Google Scholar] [CrossRef]
- Park, J.-W.; Campbell, I.H.; Kim, J.; Moon, J.-W. The role of late sulfide saturation in the formation of a Cu- and Au-rich magma: Insights from the platinum group element geochemistry of Niuatahi-Motutahi lavas, Tonga rear arc. J. Petrol. 2015, 56, 59–81. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Feng, L.; Kiseeva, E.S.; Gao, Z.; Du, Z.; Wang, F.; Shi, L. An essential role for sulfur in sulfide-silicate melt partitioning of gold and magmatic gold transport at subduction settings. Earth Planet. Sci. Lett. 2019, 528, 115850. [Google Scholar] [CrossRef]
- Snoke, A.W.; Quick, J.E.; Bowman, H.R. Bear Mountain igneous complex, Klamath Mountains, California: An ultrabasic to silicic calc-alkaline suite. J. Petrol. 1981, 22, 501–552. [Google Scholar] [CrossRef]
- Burns, L.E. The Border Ranges ultramafic and mafic complex, southcentral Alaska: Cumulate fractionates of island-arc volcanics. Can. J. Earth Sci. 1985, 22, 1020–1038. [Google Scholar] [CrossRef]
- Kepezhinskas, P.K.; Reuber, I.; Tanaka, H.; Miyashita, S. Zoned calc-alkaline plutons in Northeastern Kamchatka, Russia: Implications for the crustal growth in magmatic arcs. Mineral. Petrol. 1993, 49, 147–174. [Google Scholar] [CrossRef]
- Candela, P.A. A review of shallow, ore-related granites: Textures, volatiles, and ore metals. J. Petrol. 1997, 38, 1619–1633. [Google Scholar] [CrossRef]
- Olson, N.H.; Dilles, J.H.; Kent, A.J.R.; Lang, J.R. Geochemistry of the Cretaceous Kaskanak Batholith and genesis of the Pebble porphyry Cu-Au-Mo deposit, Southwest Alaska. Amer. Mineral. 2017, 102, 1597–1621. [Google Scholar] [CrossRef]
- Audetat, A. The metal content of magmatic-hydrothermal fluids and its relationship to mineralization potential. Econ. Geol. 2019, 114, 1033–1056. [Google Scholar] [CrossRef]
- Kepezhinskas, P.K.; Kepezhinskas, N.P.; Berdnikov, N.V.; Krutikova, V.O. Native metals and intermetallic compounds in subduction-related ultramafic rocks from the Stanovoy mobile belt (Russian Far East): Implications for redox heterogeneity in subduction zones. Ore Geol. Rev. 2020, 127, 103800. [Google Scholar] [CrossRef]
- Muntean, J.L.; Einaudi, M.T. Porphyry gold deposits of the Refugio district, Maricunga Belt, Northern Chile. Econ. Geol. 2000, 95, 1445–1472. [Google Scholar] [CrossRef] [Green Version]
- Arif, J.; Baker, T. Gold paragenesis and chemistry at Batu Hijau, Indonesia: Implications for gold-rich porphyry copper deposits. Mineral. Depos. 2004, 39, 523–535. [Google Scholar] [CrossRef]
- Crespo, J.; Reich, M.; Barra, F.; Verdugo, J.J.; Martinez, C. Critical metal particles in copper sulfides from the supergiant Rio Blanco porphyry Cu-Mo deposit, Chile. Minerals 2018, 8, 519. [Google Scholar] [CrossRef] [Green Version]
- Chudnenko, K.V.; Palyanova, G.A. Thermodynamic properties of solid solutions in the Ag-Au-Cu system. Russ. Geol. Geophys. 2014, 55, 349–360. [Google Scholar] [CrossRef]
- Palyanova, G.; Karmanov, N.; Savva, N. Sulfidation of native gold. Amer. Mineral. 2014, 99, 1095–1103. [Google Scholar] [CrossRef]
- Palyanova, G.A.; Savva, N.E.; Zhuravkova, T.V.; Kolova, E.E. Gold and silver minerals in low-sulfidation ores of the Julietta deposit (northeastern Russia). Russ. Geol. Geophys. 2016, 57, 1171–1190. [Google Scholar] [CrossRef]
- Kepezhinskas, K.B. Structural-metamorphic evolution of late Proterozoic ophiolites and Precambrian basement in the Central Asian foldbelt of Mongolia. Precambrian Res. 1986, 33, 209–223. [Google Scholar] [CrossRef]
- Kepezhinskas, P.K.; Kepezhinskas, K.B.; Puchtel, I.S. Lower Paleozoic oceanic crust in Mongolian Caledonides: Sm-Nd and trace element data. Geophys. Res. Lett. 1991, 18, 1301–1304. [Google Scholar] [CrossRef]
- Jahn, B.M. The Central Asian Orogenic Belt and growth of the continental crust in the Phanerozoic. Geol. Soc. Lond. Spec. Publ. 2004, 226, 73–100. [Google Scholar] [CrossRef]
- Windley, B.F.; Alexeiev, D.; Xiao, W.; Kröner, A.; Badarch, G. Tectonic models for accretion of the Central Asian Orogenic Belt. J. Geol. Soc. Lond. 2007, 164, 31–47. [Google Scholar] [CrossRef] [Green Version]
- Velikoslavinsky, S.D.; Kotov, A.B.; Kovach, V.P.; Tolmacheva, E.V.; Sorokin, A.A.; Sal’nikova, E.B.; Larin, A.M.; Zagornaya, N.Y.; Wang, K.L.; Chung, S.-L. Age and tectonic position of the Stanovoi metamorphic complex in the eastern part of the Central Asian foldbelt. Geotectonics 2017, 51, 341–352. [Google Scholar] [CrossRef]
- Velikoslavinsky, S.D.; Kotov, A.B.; Salnikova, E.B.; Sorokin, A.A.; Larin, A.M.; Yakovleva, S.Z.; Kovach, V.P.; Tolmacheva, E.V.; Anisimova, I.V.; Plotkina, Y.V. Metabasalts of the Bryanta sequence of the Stanovoi complex of the Dzhugdzhur-Stanovoi superterrane, Central Asian fold belt: Age and geodynamic environment of formation. Petrology 2012, 20, 240–254. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, H. Determination of platinum-group elements and gold in geological samples with ICP-MS using sodium peroxide fusion and tellurium co-precipitation. J. Anal. Atom. Spectrom. 2000, 15, 747–751. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; Berdnikov, N.; Kepezhinskas, N.; Konovalova, N. Metals in Avachinsky peridotite xenoliths with implications for redox heterogeneity and metal enrichment in the Kamchatka mantle wedge. Lithos 2022, 127, 103800. [Google Scholar] [CrossRef]
- Woodhead, J.; Eggins, S.; Gamble, J. High field strength and transition element systematics in island arc and back-arc basin basalts: Evidence for multi-phase melt extraction and a depleted mantle wedge. Earth Planet. Sci. Lett. 1993, 114, 491–504. [Google Scholar] [CrossRef]
- Kepezhinskas, P.; McDermott, F.; Defant, M.; Hochstaedter, A.; Drummond, M.S.; Hawkesworth, C.J.; Koloskov, A.; Maury, R.C.; Bellon, H. Trace element and Sr-Nd-Pb isotopic constraints on a three-component model of Kamchatka arc petrogenesis. Geochim. Cosmochim. Acta 1997, 61, 577–600. [Google Scholar] [CrossRef]
- Drummond, M.S.; Defant, M.J.; Kepezhinskas, P. The petrogenesis of slab derived trondhjemite-tonalite-dacite/adakite magmas. Trans. R. Soc. Edinb. Earth Sci. 1996, 87, 205–216. [Google Scholar]
- Kepezhinskas, P.; Berdnikov, N.; Kepezhinskas, N.; Konovalova, N. Adakites, high-Nb basalts and copper-gold deposits in magmatic arcs and collisional orogens: An overview. Geosciences 2022, 12, 29. [Google Scholar] [CrossRef]
- Kepezhinskas, P.K.; Defant, M.J.; Drummond, M.S. Progressive enrichment of island arc mantle by melt-peridotite interaction inferred from Kamchatka xenoliths. Geochim. Cosmochim. Acta 1996, 60, 1217–1229. [Google Scholar] [CrossRef]
- Kepezhinskas, N.; Kamenov, G.D.; Foster, D.A.; Kepezhinskas, P. Petrology and geochemistry of alkaline basalts and gabbroic xenoliths from Utila Island (Bay Islands, Honduras): Insights into back-arc processes in the Central American volcanic arc. Lithos 2020, 352–353, 105306. [Google Scholar] [CrossRef]
- Beard, J.S. Characteristic mineralogy of arc-related cumulate gabbros: Implications for the tectonic setting of gabbroic plutons and for andesite genesis. Geology 1986, 14, 848–851. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Vincent, E.A.; Crocket, J.H. Studies in the geochemistry of some basic and ultrabasic rocks and some meteorites. Geochim. Cosmochim. Acta 1960, 18, 143–148. [Google Scholar] [CrossRef]
- Oshin, I.O.; Crocket, J.H. Noble metals in Thetford Mines Ophiolite, Quebec, Canada. Part I: Distribution of gold, iridium, platinum and palladium in the ultramafic and gabbroic rocks. Econ. Geol. 1982, 77, 1556–1570. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distributions of the elements in some units of the Earth’s crust. Geol. Soc. Amer. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Chou, C.L.; Shaw, D.M.; Crocket, J.H. Siderophile trace elements in the earth’s oceanic crust and upper mantle. J. Geophys. Res. 1983, 88, A517–A518. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Wise, J. Gold Recovery, Properties and Applications; D. Van Nostrand Company: Taylorville, IL, USA, 1964; 167p. [Google Scholar]
- Berdnikov, N.; Nevstruev, V.; Kepezhinskas, P.; Astapov, I.; Konovalova, N. Gold in mineralized volcanic systems from theLesser Khingan Range (Russian Far East): Textural types, composition and possible origins. Geosciences 2021, 11, 103. [Google Scholar] [CrossRef]
- Raub, E.Z. Die Legierungen des Goldes mit Chrom, Molybdan und Wolfram. Int. J. Mater. Res. 1960, 51, 290–291. [Google Scholar] [CrossRef]
- Lyakishev, N.P. Phase Diagrams for Binary Metallic Systems; Mashinostroenie: Moscow, Russia, 1997; 1024p. (In Russian) [Google Scholar]
- Petrunin, I.E.; Grzhimal’skii, L.L. Interaction of tungsten with copper, manganese, silver, and tin. Metal Sci. Heat Treat. 1969, 11, 24–26. [Google Scholar] [CrossRef]
- Kelley, K.A.; Cottrell, E. Water and the oxidation state of subduction zone magmas. Science 2009, 325, 605–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, M.C.S.; Brooker, R.A.; Fraser, D.G.; Burgisser, A.; Mangan, M.T.; McCammon, C. Coupled interactions between volatile activity and Fe oxidation state during arc crustal processes. J. Petrol. 2015, 56, 795–814. [Google Scholar] [CrossRef] [Green Version]
- Lazar, C. Using silica activity to model redox-dependent fluid compositions in serpentinites from 100 to 700 °C and from 1 to 20 kbar. J. Petrol. 2020, 61, egaa101. [Google Scholar] [CrossRef]
- Evans, K.A.; Frost, B.R. Deserpentinization in subduction zones as a source of oxidation in arcs: A reality check. J. Petrol. 2021, 62, egab016. [Google Scholar] [CrossRef]
- Tang, M.; Erdman, M.; Eldridge, G.; Lee, C.-T.A. The redox “filter” beneath magmatic orogens and the formation of continental crust. Sci. Adv. 2018, 4, eaar4444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tollan, P.; Hermann, J. Arc magmas oxidized by water dissociation and hydrogen incorporation in orthopyroxene. Nat. Geosci. 2019, 12, 667–671. [Google Scholar] [CrossRef]
- Berdnikov, N.V.; Nevstruev, V.G.; Kepezhinskas, P.K.; Krutikova, V.O.; Konovalova, N.S.; Astapov, I.A. Silicate, Fe-oxide, and Au-Cu-Ag micropsherules in ores and pyroclastic rocks of the Kostenga iron deposit, in the Far East of Russia. J. Russ. Pac. Geol. 2021, 15, 236–251. [Google Scholar] [CrossRef]
- Rapson, W.S. The metallurgy of the coloured carat gold alloys. Gold Bull. 1990, 23, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Townley, B.K.; Herail, G.; Maksaev, V.; Palacios, C.; de Parseval, P.; Sepuldeva, F.; Orellana, R.; Rivas, P.; Ulloa, C. Gold grain morphology and composition as an exploration tool: Application to gold exploration in covered areas. Geochem. Explor. Env. Anal. 2003, 3, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Chapman, R.J.; Mortensen, J.K.; Crawford, E.; LeBarge, W. Microchemical studies of placer and lode gold in Bonanza and Eldorado creeks, Klondike District, Yukon, Canada: Evidence for a small, gold-rich, orogenic hydrothermal system. Econ. Geol. 2010, 105, 1393–1410. [Google Scholar] [CrossRef]
- Chapman, R.J.; Mortensen, J.K.; LeBarge, W.P. Styles of lode gold mineralization contributing to the placers of the Indian River and Black Hills Creek, Yukon Territory, Canada as deduced from microchemical characterization of placer gold grains. Miner. Depos. 2011, 46, 881–903. [Google Scholar] [CrossRef]
- Oen, I.; Kieft, C. Nickeline with pyrrhotite and cubanite exsolutions, Ni-Co-rich loellingite and an Au-Cu-alloy in Cr-Ni-ores from Beni-Bousera, Morocco. Neues Jahrb. Miner. Monatsh. 1974, 115, 1–8. [Google Scholar]
- Kepezhinskas, P.; Defant, M.J.; Widom, E. Abundance and distribution of PGE and Au in the island-arc mantle: Implications for sub-arc metasomatism. Lithos 2002, 60, 113–128. [Google Scholar] [CrossRef]
- Widom, E.; Kepezhinskas, P.; Defant, M. The nature of metasomatism in the sub-arc mantle wedge: Evidence from Re-Os isotopes in Kamchatka peridotite xenoliths. Chem. Geol. 2003, 196, 283–306. [Google Scholar] [CrossRef]
- Saunders, J.E.; Pearson, N.J.; O’Reilly, S.Y.; Griffin, W.L. Gold in the mantle: A global assessment of abundance and redistribution processes. Lithos 2018, 322, 376–391. [Google Scholar] [CrossRef]
- Anoshin, G.N.; Kepezhinskas, V.V. Petrochemical features related to gold distribution for the Cenozoic volcanic rocks of the Kuril-Kamchatka province. Geochem. Int. 1972, 9, 618–629. [Google Scholar]
- Kutyrev, A.; Zelenski, M.; Nekrylov, N.; Savelyev, D.; Kontonikas-Charos, A.; Kamenetsky, V.S. Noble metals in arc basaltic magmas worldwide: A case study of modern and pre-historic lavas of the Tolbachik volcano, Kamchatka. Front. Earth Sci. 2021, 9, 791465. [Google Scholar] [CrossRef]
- Pitcairn, I.K.; Craw, D.; Teagle, D.A.H. Metabasalts as sources of metals in orogenic gold deposits. Mineral. Depos. 2014, 50, 373–390. [Google Scholar] [CrossRef]
- Tomkins, A.G.; Rebryna, K.C.; Weinberg, R.F.; Schaefer, B.F. Magmatic sulfide formation by reduction of oxidized arc basalt. J. Petrol. 2012, 53, 1537–1567. [Google Scholar] [CrossRef]
- Botcharnikov, R.; Linnen, R.; Wilke, M.; Holtz, F.; Jugo, P.; Berndt, J. High gold concentrations in sulphide-bearing magma under oxidizing conditions. Nat. Geosci. 2011, 4. [Google Scholar] [CrossRef]
- Luhr, J.F. Experimental phase relations of water- and sulfur-saturated arc magmas and the 1982 eruptions of El Chichón volcano. J. Petrol. 1990, 31, 1071–1114. [Google Scholar] [CrossRef]
- Baker, L.L.; Rutherford, M.J. Crystallization of anhydrite-bearing magmas. Earth Environ. Sci. Trans. R. Soc. Edinb. 1996, 87, 243–250. [Google Scholar] [CrossRef]
- Richards, J.P. Porphyry copper deposit formation in arcs: What are the odds? Geosphere 2021. [Google Scholar] [CrossRef]
- Matjuschkin, V.; Blundy, J.D.; Brooker, R.A. The effect of pressure on sulphur speciation in mid- to deep-crustal arc magmas and implications for the formation of porphyry copper deposits. Contrib. Mineral. Petrol. 2016, 171. [Google Scholar] [CrossRef] [Green Version]




| Sample | 001H | 007H | 008H | 036H | 069F | 070F | 074F | 037F | 040F |
|---|---|---|---|---|---|---|---|---|---|
| Rock Type | Pl-D | Pl-D | D | D | Pl-D | Pl-D | Pl-D | Whr | Whr |
| SiO2 (wt.%) | 40.15 | 39.36 | 38.49 | 48.55 | 41.39 | 42.18 | 44.70 | 45.47 | 45.82 |
| TiO2 | 0.11 | 0.15 | 0.16 | 0.23 | 0.16 | 0.20 | 0.27 | 0.59 | 0.57 |
| Al2O3 | 5.07 | 4.75 | 1.32 | 2.83 | 4.74 | 4.50 | 5.08 | 5.52 | 6.17 |
| Fe2O3 | 12.12 | 11.38 | 12.40 | 12.84 | 12.55 | 12.93 | 11.80 | 10.59 | 11.83 |
| MnO | 0.16 | 0.17 | 0.18 | 0.18 | 0.18 | 0.23 | 0.23 | 0.18 | 0.19 |
| MgO | 32.29 | 34.38 | 35.17 | 32.84 | 31.72 | 31.16 | 31.52 | 24.25 | 23.61 |
| CaO | 2.85 | 3.19 | 0.52 | 1.94 | 3.54 | 3.14 | 2.34 | 11.20 | 8.91 |
| Na2O | 0.40 | 1.00 | 0.06 | 0.25 | 0.60 | 0.42 | 0.25 | 0.89 | 0.66 |
| K2O | 0.05 | 0.05 | 0.02 | 0.02 | 0.05 | 0.04 | 0.03 | 0.06 | 0.05 |
| P2O5 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.02 | 0.00 | 0.00 |
| LOI | 6.76 | 5.54 | 11.70 | 0.24 | 5.02 | 5.14 | 3.78 | 1.24 | 2.18 |
| Total | 99.99 | 99.99 | 100.05 | 100.00 | 99.98 | 99.99 | 100.00 | 100.00 | 100.00 |
| Li (ppm) | 2.09 | 2.09 | 1.13 | 1.80 | 1.72 | 1.17 | 1.13 | 1.18 | 2.40 |
| Sc | 9.21 | 8.74 | 9.91 | 17.96 | 11.18 | 14.14 | 21.41 | 44.36 | 42.12 |
| V | 48.88 | 37.00 | 42.69 | 67.99 | 44.36 | 64.86 | 83.16 | 135.07 | 159.27 |
| Cr | 2406 | 672.4 | 980.9 | 511.6 | 854.5 | 2496 | 2358 | 2675 | 1950 |
| Co | 107.18 | 93.4 | 134.2 | 81.45 | 83.03 | 89.56 | 72.73 | 44.66 | 42.00 |
| Ni | 1489 | 1393 | 1899 | 1291 | 850.98 | 898.4 | 731.12 | 393.1 | 386.0 |
| Cu | 80.01 | 21.16 | 50.58 | 42.42 | 49.67 | 39.74 | 14.07 | 11.10 | 99.47 |
| Zn | 76.57 | 13.13 | 36.78 | 75.31 | 19.29 | 49.83 | 53.95 | 49.10 | 52.62 |
| Cs | 0.08 | 0.01 | 0.03 | 0.08 | 0.11 | 0.10 | 0.21 | 0.07 | 0.05 |
| Rb | 0.65 | 0.29 | 0.46 | 1.22 | 1.11 | 1.25 | 1.25 | 0.65 | 0.69 |
| Ba | 47.16 | 73.30 | 198.1 | 26.03 | 84.31 | 87.85 | 57.65 | 37.60 | 16.63 |
| Sr | 85.36 | 397.8 | 32.15 | 10.95 | 237.5 | 103.75 | 26.20 | 70.48 | 25.53 |
| Zr | 3.04 | 5.15 | 6.38 | 4.27 | 3.27 | 3.10 | 2.61 | 11.16 | 3.77 |
| Y | 3.60 | 3.02 | 4.36 | 2.34 | 1.86 | 2.67 | 2.90 | 10.32 | 9.12 |
| Nb | 1.22 | 0.41 | 0.19 | 0.06 | 0.028 | 0.037 | <0.001 | 0.28 | 0.24 |
| Ta | 1.47 | 0.54 | 0.14 | 0.075 | 0.093 | 0.065 | 0.023 | 0.42 | 0.32 |
| Hf | 0.12 | 0.16 | 0.20 | 0.15 | 0.12 | 0.13 | 0.13 | 0.61 | 0.31 |
| Th | 0.32 | 0.12 | 0.26 | 0.15 | 0.036 | 0.092 | 0.05 | 0.10 | 0.19 |
| U | 0.068 | 0.085 | 0.22 | <0.001 | 0.013 | 0.043 | 0.01 | <0.001 | <0.001 |
| La | 3.36 | 2.46 | 4.15 | 0.68 | 0.68 | 0.84 | 0.38 | 1.41 | 0.50 |
| Ce | 7.97 | 5.61 | 10.37 | 1.57 | 1.54 | 1.78 | 1.04 | 5.29 | 2.11 |
| Pr | 0.60 | 0.39 | 0.76 | 0.19 | 0.19 | 0.26 | 0.20 | 1.05 | 0.49 |
| Nd | 3.44 | 2.69 | 3.91 | 0.85 | 0.96 | 1.21 | 0.83 | 5.96 | 3.02 |
| Sm | 0.71 | 0.59 | 0.85 | 0.24 | 0.27 | 0.34 | 0.27 | 1.90 | 1.15 |
| Eu | 0.24 | 0.27 | 0.22 | 0.08 | 0.16 | 0.14 | 0.10 | 0.57 | 0.40 |
| Gd | 0.85 | 0.73 | 1.08 | 0.33 | 0.36 | 0.46 | 0.41 | 2.35 | 1.63 |
| Tb | 0.13 | 0.11 | 0.16 | 0.06 | 0.061 | 0.082 | 0.078 | 0.38 | 0.29 |
| Dy | 0.69 | 0.57 | 0.85 | 0.40 | 0.37 | 0.50 | 0.52 | 2.18 | 1.80 |
| Ho | 0.15 | 0.12 | 0.18 | 0.10 | 0.081 | 0.12 | 0.13 | 0.45 | 0.40 |
| Er | 0.40 | 0.33 | 0.49 | 0.30 | 0.23 | 0.34 | 0.38 | 1.19 | 1.11 |
| Tm | 0.059 | 0.048 | 0.071 | 0.05 | 0.038 | 0.053 | 0.063 | 0.17 | 0.17 |
| Yb | 0.36 | 0.30 | 0.44 | 0.35 | 0.12 | 0.13 | 0.13 | 1.00 | 1.06 |
| Lu | 0.060 | 0.048 | 0.072 | 0.06 | 0.093 | 0.065 | 0.023 | 0.15 | 0.17 |
| Sample | IL-5 | 018H | 028F | 044H | 051S | 063F | 013B | 058F | 060F |
| Rock Type | Web | Web | Web | Web | Web | Web | Pyr | Pyr | Pyr |
| SiO2 (wt.%) | 48.07 | 50.15 | 51.00 | 46.65 | 47.01 | 48.42 | 46.54 | 47.05 | 46.55 |
| TiO2 | 0.49 | 0.49 | 0.58 | 0.31 | 0.31 | 0.65 | 1.07 | 0.46 | 0.30 |
| Al2O3 | 5.50 | 6.45 | 6.61 | 5.45 | 7.04 | 4.22 | 7.23 | 12.21 | 14.48 |
| Fe2O3 | 12.59 | 12.91 | 13.73 | 12.96 | 11.98 | 1.66 | 14.85 | 10.73 | 7.87 |
| MnO | 0.21 | 0.22 | 0.23 | 0.22 | 0.21 | 0.27 | 0.21 | 0.19 | 0.13 |
| MgO | 27.72 | 22.03 | 21.39 | 28.27 | 25.90 | 24.58 | 19.29 | 14.07 | 16.68 |
| CaO | 2.72 | 5.87 | 4.12 | 3.90 | 5.07 | 7.41 | 4.21 | 10.52 | 9.30 |
| Na2O | 1.17 | 1.16 | 1.59 | 0.88 | 1.33 | 0.56 | 1.51 | 2.60 | 2.62 |
| K2O | 0.09 | 0.07 | 0.16 | 0.10 | 0.09 | 0.06 | 2.51 | 0.15 | 0.15 |
| P2O5 | 0.04 | 0.02 | 0.05 | 0.02 | 0.04 | 0.01 | 0.72 | 0.01 | 0.01 |
| LOI | 0.76 | 0.64 | 0.54 | 1.24 | 1.02 | 2.16 | 1.86 | 2.00 | 1.92 |
| Total | 99.36 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Li (ppm) | 1.41 | 3.35 | 3.56 | 1.79 | 1.82 | 2.09 | 9.62 | 5.53 | 3.56 |
| Sc | 24.52 | 39.08 | 22.00 | 22.32 | 20.49 | 49.55 | 13.38 | 29.59 | 18.13 |
| V | 103.39 | 138.77 | 100.84 | 81.18 | 74.51 | 180.71 | 106.00 | 114.48 | 76.34 |
| Cr | 1741 | 1673 | 1429 | 1782 | 1630 | 1732 | 483.33 | 910.28 | 709.90 |
| Co | 74.25 | 60.35 | 51.49 | 70.93 | 58.09 | 53.20 | 78.44 | 43.31 | 35.28 |
| Ni | 733.76 | 330.91 | 392.00 | 596.99 | 565.32 | 325.22 | 329.14 | 290.91 | 292.39 |
| Cu | 104.33 | 77.74 | 9.06 | 75.13 | 66.82 | 37.83 | 11.38 | 28.69 | 16.79 |
| Zn | 48.74 | 47.06 | 69.19 | 36.63 | 33.82 | 59.15 | 155.15 | 39.51 | 36 |
| Cs | 0.08 | 0.09 | 0.25 | 0.06 | 0.15 | 0.07 | 0.61 | 0.09 | 0.11 |
| Rb | 0.88 | 0.69 | 2.41 | 0.93 | 1.67 | 1.54 | 31.47 | 1.11 | 1.17 |
| Ba | 66.81 | 83.42 | 116.62 | 79.23 | 59.40 | 18.89 | 1521.9 | 74.22 | 93.46 |
| Sr | 150.26 | 135.89 | 189.67 | 99.79 | 174.49 | 27.49 | 311.42 | 520.51 | 625.65 |
| Zr | 14.39 | 9.41 | 5.85 | 6.60 | 7.40 | 6.87 | 13.88 | 3.55 | 3.65 |
| Y | 5.77 | 6.14 | 4.35 | 3.60 | 3.36 | 10.65 | 16.73 | 6.98 | 3.38 |
| Nb | 0.47 | 0.22 | 0.33 | 0.13 | 0.58 | 0.093 | 1.91 | 0.36 | 0.37 |
| Ta | 0.09 | 0.73 | 0.21 | 0.097 | 0.40 | 0.11 | 0.57 | 0.48 | 0.41 |
| Hf | 0.41 | 0.34 | 0.25 | 0.24 | 0.25 | 0.41 | 0.73 | 0.27 | 0.21 |
| Th | 0.25 | 0.085 | 0.24 | 0.059 | 0.22 | 0.13 | 3.37 | 0.19 | 0.11 |
| U | 1.82 | 0.006 | <0.001 | <0.001 | 0.01 | 0.041 | 0.71 | 0.047 | 0.064 |
| La | 1.77 | 1.12 | 1.64 | 0.73 | 1.14 | 1.21 | 36.09 | 1.45 | 1.28 |
| Ce | 4.39 | 2.91 | 3.69 | 1.92 | 2.57 | 4.18 | 75.60 | 3.88 | 2.97 |
| Pr | 0.59 | 0.13 | 0.50 | 0.26 | 0.34 | 0.73 | 8.94 | 0.51 | 0.38 |
| Nd | 2.76 | 2.10 | 2.20 | 1.32 | 1.53 | 4.13 | 33.07 | 2.77 | 1.87 |
| Sm | 0.75 | 0.68 | 0.57 | 0.39 | 0.41 | 1.41 | 5.53 | 0.92 | 0.49 |
| Eu | 0.28 | 0.30 | 0.27 | 0.18 | 0.19 | 0.49 | 1.80 | 0.49 | 0.38 |
| Gd | 1.01 | 0.98 | 0.75 | 0.57 | 0.57 | 2.00 | 6.02 | 1.32 | 0.67 |
| Tb | 0.16 | 0.17 | 0.13 | 0.11 | 0.10 | 0.35 | 0.72 | 0.23 | 0.11 |
| Dy | 1.02 | 1.07 | 0.78 | 0.66 | 0.62 | 2.10 | 3.34 | 1.40 | 0.67 |
| Ho | 0.22 | 0.24 | 0.18 | 0.15 | 0.14 | 0.46 | 0.64 | 0.31 | 0.15 |
| Er | 0.67 | 0.68 | 0.53 | 0.45 | 0.42 | 1.24 | 1.74 | 0.84 | 0.41 |
| Tm | 0.10 | 0.11 | 0.09 | 0.072 | 0.068 | 0.18 | 0.23 | 0.13 | 0.062 |
| Yb | 0.67 | 0.69 | 0.56 | 0.45 | 0.44 | 1.13 | 1.40 | 0.76 | 0.39 |
| Lu | 0.11 | 0.11 | 0.092 | 0.12 | 0.08 | 0.17 | 0.21 | 0.12 | 0.063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berdnikov, N.; Kepezhinskas, P.; Konovalova, N.; Kepezhinskas, N. Formation of Gold Alloys during Crustal Differentiation of Convergent Zone Magmas: Constraints from an AU-Rich Websterite in the Stanovoy Suture Zone (Russian Far East). Geosciences 2022, 12, 126. https://doi.org/10.3390/geosciences12030126
Berdnikov N, Kepezhinskas P, Konovalova N, Kepezhinskas N. Formation of Gold Alloys during Crustal Differentiation of Convergent Zone Magmas: Constraints from an AU-Rich Websterite in the Stanovoy Suture Zone (Russian Far East). Geosciences. 2022; 12(3):126. https://doi.org/10.3390/geosciences12030126
Chicago/Turabian StyleBerdnikov, Nikolai, Pavel Kepezhinskas, Natalia Konovalova, and Nikita Kepezhinskas. 2022. "Formation of Gold Alloys during Crustal Differentiation of Convergent Zone Magmas: Constraints from an AU-Rich Websterite in the Stanovoy Suture Zone (Russian Far East)" Geosciences 12, no. 3: 126. https://doi.org/10.3390/geosciences12030126
APA StyleBerdnikov, N., Kepezhinskas, P., Konovalova, N., & Kepezhinskas, N. (2022). Formation of Gold Alloys during Crustal Differentiation of Convergent Zone Magmas: Constraints from an AU-Rich Websterite in the Stanovoy Suture Zone (Russian Far East). Geosciences, 12(3), 126. https://doi.org/10.3390/geosciences12030126







