Evaluating the Existence of Vertebrate Deadfall Communities from the Early Jurassic Posidonienschiefer Formation
Abstract
1. Introduction
1.1. Background
1.2. Previously Documented Associations from the Posidonienschiefer Formation
2. Materials and Methods
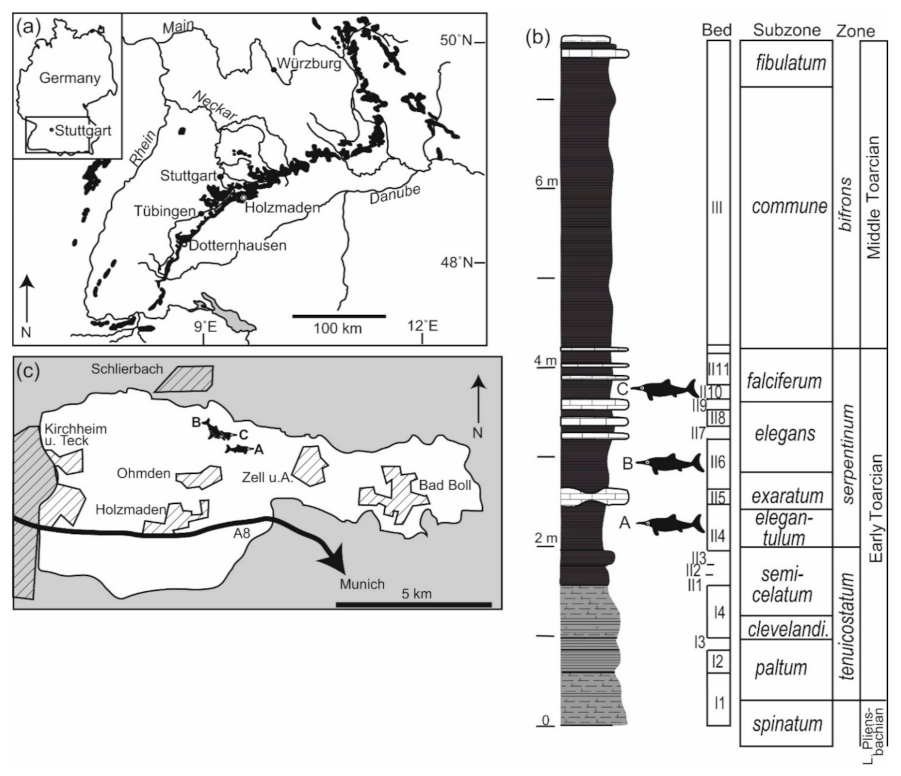
2.1. Geological Context
2.2. Survey and Documentation of Deadfall Assemblages
2.3. Institutional Abbreviations
3. Results
3.1. SMNS 53363, Eurhinosaurus?
3.2. SMNS 80234, Stenopterygius quadriscissus
3.2.1. Taphonomic History
3.2.2. Faunal Association
3.2.3. Interpretation
3.3. SMNS 81841, Stenopterygius quadriscissus
3.3.1. Taphonomic History
3.3.2. Macroinvertebrates
3.3.3. Trace Fossils
3.3.4. Pyritization
3.3.5. Interpretation
3.4. SMNS 81719, Stenopterygius uniter
3.4.1. Taphonomic History
3.4.2. Faunal Association
3.4.3. Trace Fossils
3.4.4. Pyritization
3.4.5. Interpretation
3.5. SMNS 80113, Stenopterygius triscissus
3.5.1. Taphonomic History
3.5.2. Macroinvertebrates
3.5.3. Interpretation
3.6. Chondrites Associations
3.6.1. SMNS 51144, Saurostomus esocinus
3.6.2. SMNS 17500 and MHH 1981/25, Stenopterygius uniter; Holzmaden, εII8
3.7. Crustacean Associations
3.8. Microborings
4. Discussion
4.1. Carcass Disarticulation and Taphonomy
4.2. Invertebrate Associations
4.3. Comparison with Other Jurassic Deadfall Communities
4.4. Jurassic Deadfall Assemblages: Limitations of Recent Analogues
5. Conclusions
- Deadfall community assemblages are indeed exceptionally rare, making up a small fraction of all vertebrate specimens (<3%), but can be identified even in specimens prepared from the underside.
- These communities are found in several horizons within the middle part of the Posidonienschiefer Formation (εII), but overall rarity precludes a detailed understanding of changes in their composition and frequency through the Toarcian.
- Seafloor exposure under oxygenated conditions does not preclude exceptional preservation, including high skeletal articulation, gastric contents, and embryonic remains.
- Articulation and community diversity are unrelated, with the latter being hypothesized to be controlled by duration of benthic oxygenation, and the former related to exposure time on the seafloor prior to burial.
- The macroinvertebrate communities associated with the Posidonienschiefer Formation deadfalls are not especially diverse, and reflect amplification of background diversity rather than a specialist deadfall fauna.
- The mobile scavenger, enrichment opportunist, and reef stages are all detected in the Posidonienschiefer deadfall assemblages; the presence of the sulfophilic stage is equivocal.
- The published record of Jurassic deadfall communities is sparse, with strong geographical and paleoenvironmental biases. The deadfall communities reported from the Posidonienschiefer Formation complement the published record, but the uneven quality of comparative data masks potential trends in faunal composition and diversity.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, C.R.; Baco, A.R. Ecology of Whale Falls at the Deep-Sea Floor. Oceanogr. Mar.Biol. Annu. Rev. 2003, 41, 311–354. [Google Scholar]
- Smith, C.R. Food for the Deep Sea: Utilization, Dispersal, and Flux of Nekton Falls at the Santa Catalina Basin Floor. Deep.-Sea Res. 1985, 32, 417–442. [Google Scholar] [CrossRef]
- Smith, C.R.; Glover, A.G.; Treude, T.; Higgs, N.D.; Amon, D.J. Whale-Fall Ecosystems: Recent Insights into Ecology, Paleoecology, and Evolution. Annu. Rev. Mar. Sci. 2015, 7, 571–596. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R. Bigger is better: The role of whales as detritus in marine ecosystems. In Whales, Whaling and Ocean Ecosystems, Estes, J.A., DeMaster, D.P., Doak, D.F., Williams, T.M., Brownell, R.L., Eds.; University of California Press: Berkeley, CA, USA, 2006; pp. 286–302. [Google Scholar]
- Glover, A.G.; Källström, B.; Smith, C.R.; Dahlgren, T.G. World-Wide Whale Worms? A New Species of Osedax from the Shallow North Atlantic. Proc. R. Soc. B 2005, 272, 2587–2592. [Google Scholar] [CrossRef]
- Hilario, A.; Cunha, M.R.; Génio, L.; Marçal, A.R.; Ravara, A.; Rodrigues, C.F.; Wiklund, H. First Clues on the Ecology of Whale Falls in the Deep Atlantic Ocean: Results from an Experiment Using Cow Carcasses. Mar. Ecol. 2015, 36, 82–90. [Google Scholar] [CrossRef]
- Lundsten, L.; Schlining, K.L.; Frasier, K.; Johnson, S.B.; Kuhnz, L.A.; Harvey, J.B.J.; Clague, G.; Vrijenhoek, R.C. Time-Series Analysis of Six Whale-Fall Communities in Monterey Canyon, California, USA. Deep.-Sea Res. I 2010, 57, 1573–1584. [Google Scholar] [CrossRef]
- Higgs, N.D.; Gates, A.R.; Jones, D.O.B. Fish Food in the Deep Sea: Revisiting the Role of Large Food-Falls. PLoS ONE 2014, 9, e96016. [Google Scholar] [CrossRef]
- Hogler, J.A. Speculations on the Role of Marine Reptile Deadfalls in Mesozoic Deep-Sea Paleoecology. Palaios 1994, 9, 42–47. [Google Scholar] [CrossRef]
- Martill, D.M.; Cruickshank, A.; Taylor, M. Dispersal via Whale Bones. Nature 1991, 351, 193. [Google Scholar] [CrossRef]
- Danise, S.; Twitchett, R.J.; Matts, K. Ecological Succession of a Jurassic Shallow-Water Ichthyosaur Fall. Nat. Commun. 2014, 5, 4789. [Google Scholar] [CrossRef]
- Danise, S.; Higgs, N.D. Bone-Eating Osedax Worms Lived on Mesozoic Marine Reptile Deadfalls. Biol. Lett. 2015, 11, 20150072. [Google Scholar] [CrossRef] [PubMed]
- Kaim, A.; Kobayashi, Y.; Echizenya, H.; Jenkins, R.G.; Tanabe, K. Chemosynthesis-Based Associations on Cretaceous Plesiosaurid Carcasses. Acta Palaeontol. Polon. 2008, 53, 97–104. [Google Scholar] [CrossRef]
- Paparella, I.; Maxwell, E.E.; Cipriani, A.; Roncacè, S.; Caldwell, M.W. The First Ophthalmosaurid Ichthyosaur from the Upper Jurassic of the Umbrian–Marchean Apennines (Marche, Central Italy). Geol. Mag. 2017, 154, 837–858. [Google Scholar] [CrossRef]
- Serafini, G.; Amalfitano, J.; Cobianchi, M.; Fornaciari, B.; Maxwell, E.E.; Papazzoni, C.A. Evidence of Opportunistic Feeding between Ichthyosaurs and the Oldest Occurrence of the Hexanchid Shark Notidanodon from the Upper Jurassic of Northern Italy. Riv. Ital. Paleontol. Stratigr. 2020, 126, 629–655. [Google Scholar]
- Delsett, L.L.; Novis, L.K.; Roberts, A.J.; Koevoets, M.J.; Hammer, Ø.; Druckenmiller, P.S.; Hurum, J.H. The Slottsmøya Marine Reptile Lagerstätte: Depositional Environments, Taphonomy and Diagenesis. Geol. Soc. Lond. Spec. Publ. 2016, 434, 165–188. [Google Scholar] [CrossRef]
- Dick, D.G. An Ichthyosaur Carcass-Fall Community from the Posidonia Shale (Toarcian) of Germany. Palaios 2015, 30, 353–361. [Google Scholar] [CrossRef]
- Martill, D.M. A Taphonomic and Diagenetic Case Study of a Partially Articulated Ichthyosaur. Palaeontology 1987, 30, 543–555. [Google Scholar]
- Cruickshank, A.R.I. A Juvenile Plesiosaur (Plesiosauria: Reptilia) from the Lower Lias (Hettangian: Lower Jurassic) of Lyme Regis, England: A Pliosauroid-Plesiosauroid Intermediate? Zool. J. Linn. Soc. 1994, 112, 151–178. [Google Scholar] [CrossRef]
- Martill, D.M. Soupy Substrates: A Medium for the Exceptional Preservation of Ichthyosaurs of the Posidonia Shale (Lower Jurassic) of Germany. Kaupia 1993, 2, 77–97. [Google Scholar]
- Seilacher, A. Posidonia Shales (Toarcian, S. Germany)-Stagnant basin model revalidated. In Paleontology, Essential of Historical Geology; Montanaro Gallitelli, E., Ed.; S.T.E.M. Mucchi: Modena, Italy, 1982; pp. 25–55. [Google Scholar]
- Kauffmann, E.G. Ecological Reappraisal of the German Posidonienschiefer (Toarcian) and the stagnant basin model. In Communities of the Past, Gray, J., Boucot, A.J., Berry, W.B.N., Eds.; Hutchinson Ross Publishing: Stroudsburg, PA, USA, 1981; pp. 311–381. [Google Scholar]
- Pardo-Pérez, J.; Kear, B.P.; Mallison, H.; Gómez, M.; Moroni, M.; Maxwell, E.E. Pathological Survey on Temnodontosaurus from the Early Jurassic of Southern Germany. PLoS ONE 2018, 13, e0204951. [Google Scholar]
- Martill, D.M. The Stratigraphic Distribution and Preservation of Fossil Vertebrates in the Oxford Clay of England. Mercian Geol. 1986, 10, 161–186. [Google Scholar]
- Reolid, M.; Santos, A.; Mayoral, E. Grazing Activity as Taphonomic Record of Necrobiotic Interaction: A Case Study of a Sea Turtle Carapace from the Upper Jurassic of the Prebetic (South Spain). Rev. Mex. Cienc. Geol. 2015, 32, 21–28. [Google Scholar]
- Wahl, W.R. Taphonomy of a Nose Dive: Bone and Tooth Displacement and Mineral Accretion in an Ichthyosaur Skull. Paludicola 2009, 7, 107–116. [Google Scholar]
- Grange, D.R.; Benton, M.J. Kimmeridgian Metriorhynchid Crocodiles from England. Palaeontology 1996, 39, 497–514. [Google Scholar]
- Wilkinson, L.E.; Young, M.T.; Benton, M.J. A New Metriorhynchid Crocodilian (Mesoeucrocodylia: Thalattosuchia) from the Kimmeridgian (Upper Jurassic) of Wiltshire, UK. Palaeontology 2008, 51, 1307–1333. [Google Scholar] [CrossRef]
- Palmer, C.P. The Kimmeridge Fauna Associated with the Portland Plesiosaur. Proc. Dorset Nat. Hist. Archaeol. Soc. 1987, 109, 109–112. [Google Scholar]
- Gale, A.; Smith, A.B.; Thuy, B. Echinoderms. In Fossils of the Kimmeridge Clay Formation Volume 1, Introduction, Geology and Invertebrate Palaeontology; Palaeontological Association Filed Guide to Fossils: Number 16; Martill, D.M., Etches, S., Eds.; The Palaeontological Association: London, UK, 2020; p. 327. [Google Scholar]
- Leuzinger, L.; Cuny, G.; Popov, E.; Billon-Bruyat, J.-P. A New Chondrichthyan Fauna from the Late Jurassic of the Swiss Jura (Kimmeridgian) Dominated by Hybodonts, Chimaeroids and Guitarfishes. Pap. Palaeontol. 2017, 3, 471–511. [Google Scholar] [CrossRef]
- Meyer, C.A. Amazing Graze–Grazing Traces of Sea Urchins on Turtles–An Example from the Late Jurassic of Switzerland. Ann. Naturhist. Mus. Wien. Ser. A 2011, 113, 555–565. [Google Scholar]
- Röhl, H.-J.; Schmid-Röhl, A.; Oschmann, W.; Frimmel, A.; Schwark, L. Erratum to “The Posidonia Shale (Lower Toarcian) of SW-Germany: An Oxygen-Depleted Ecosystem Controlled by Sea Level and Palaeoclimate”. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 165, 27–52. [Google Scholar] [CrossRef]
- Urlichs, M.; Wild, R.; Ziegler, B. Der Posidonien-Schiefer und Seine Fossilien. Stuttgarter Beitr. Naturk. Ser. C 1994, 36, 1–95. [Google Scholar]
- Riegraf, W.; Werner, G.; Lörcher, F. Der Posidonienschiefer. Biostratigraphie, Fauna und Fazies des südwestdeutschen Untertoarciums (Lias ε); Ferdinand Enke: Stuttgart, Germany, 1984; p. 195. [Google Scholar]
- Einsele, G.; Mosebach, R. Zur Petrographie, Fossilerhaltung und Entstehung des Posidonienschiefers im Schwäbischen Jura. N. Jb. Geol. Paläontol. Abh. 1955, 101, 319–430. [Google Scholar]
- Hauff, B. Untersuchung der Fossilfundstätten von Holzmaden im Posidonienschiefer des Oberen Lias Württembergs. Palaeontographica 1921, 64, 1–42. [Google Scholar]
- Wild, R. Holzmaden. In Palaeobiology: A Synthesis; Briggs, D.E., Crowther, P.R., Eds.; Blackwell Scientific Publications: Oxford, UK, 1990; pp. 282–285. [Google Scholar]
- Teichert, S.; Nützel, A. Early Jurassic Anoxia Triggered the Evolution of the Oldest Holoplanktonic Gastropod Coelodiscus minutus by Means of Heterochrony. Acta Palaeontol. Pol. 2015, 60, 269–276. [Google Scholar] [CrossRef]
- Seilacher, A. Die Holzmadener Posidonienschiefer Entstehung der Fossillagerstätte und eines Erdölmuttergesteins. In Klassische Fundstellen der Paläontologie; Weidert, W.K., Ed.; Goldschneck-Verlag: Korb, Germany, 1990; Volume 2, pp. 107–131. [Google Scholar]
- Mángano, M.G.; Buatois, L.A.; West, R.R.; Maples, C.G. Ichnology of a Pennsylvanian Equatorial Tidal Flat—The Stull Shale Member at Waverly, Eastern Kansas. Bull. Kans. Geol. Surv. 2002, 245, 1–133. [Google Scholar]
- Seilacher, A. Trace Fossil Analysis; Springer: Berlin, Germany, 2007; p. 226. [Google Scholar]
- Brenner, K.; Seilacher, A. New Aspects about the Origin of the Toarcian Posidonia Shales. N. Jb. Geol. Paläontol. Abh. 1978, 157, 11–18. [Google Scholar]
- Kauffmann, E.G. Benthic Environments and Paleoecology of the Posidonienschiefer (Toarcian). N. Jb. Geol. Paläontol. Abh. 1978, 157, 18–36. [Google Scholar]
- Schmid-Röhl, A.; Röhl, H.-J. Overgrowth on Ammonite Conchs: Environmental Implications for the lower Toarcian Posidonia Shale. Palaeontology 2003, 46, 339–352. [Google Scholar] [CrossRef]
- Röhl, H.-J.; Schmid-Röhl, A. Lower Toarcian (Upper Liassic) Black Shales of the Central European Basin: A Sequence Stratigraphic Case Study from the SW German Posidonia Shale. SEPM Spec. Publ. 2005, 82, 165–189. [Google Scholar]
- Beardmore, S.R.; Furrer, H. Evidence of a Preservational Gradient in the Skeletal Taphonomy of Ichthyopterygia (Reptilia) from Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 443, 131–144. [Google Scholar] [CrossRef]
- Mallison, H.; Wings, O. Photogrammetry in Paleontology, a Practical Guide. J. Paleontol. Tech. 2014, 12, 1–31. [Google Scholar]
- Mujal, E.; Marchetti, L.; Schoch, R.R.; Fortuny, J. Upper Paleozoic to Lower Mesozoic Tetrapod Ichnology Revisited: Photogrammetry and Relative Depth Pattern Inferences on Functional Prevalence of Autopodia. Front. Earth Sci. 2020, 8, 248. [Google Scholar] [CrossRef]
- Motani, R. Estimating Body Mass from Silhouettes: Teting the Assumption of Elliptical Body Cross-Sections. Paleobiology 2001, 27, 735–750. [Google Scholar] [CrossRef]
- Reisdorf, A.G.; Bux, R.; Wyler, D.; Benecke, M.; Klug, C.; Maisch, M.W.; Fornaro, P.; Wetzel, A. Float, Explode or Sink: Postmortem Fate of Lung-Breathing Marine Vertebrates. Palaeobiodivers. Palaeoenviron. 2012, 92, 67–81. [Google Scholar] [CrossRef]
- Reisdorf, A.G. No Joke Movement: Mehr über den Hauensteiner Ichthyosaurier und rezente marine Lungenatmer. Textnoten zur Physiologie, Pathologie und Taphonomie; weiterführende Literatur. Mitt. Naturk. Ges. Kanton Soloth. 2007, 40, 23–49. [Google Scholar]
- Hofmann, J. Einbettung und Zerfall der Ichthyosaurier im Lias von Holzmaden. Meyniana 1958, 6, 10–55. [Google Scholar]
- Pardo-Pérez, J.; Kear, B.P.; Maxwell, E.E. Palaeoepidemiology in Extinct Vertebrate Populations: Factors Influencing Skeletal Health in Jurassic Marine Reptiles. R. Soc. Open Sci. 2019, 6, 190264. [Google Scholar] [CrossRef]
- Baldanza, A.; Bizzarri, R.; Famiani, F.; Garassino, A.; Pasini, G.; Cherin, M.; Rosatini, F. The Early Pleistocene Whale-Fall Community of Bargiano (Umbria, Central Italy): Paleoecological Insights from Benthic Foraminifera and Brachyuran Crabs. Palaeontol. Electron. 2018, 21, 1–27. [Google Scholar] [CrossRef]
- Jagt, J.W.M.; Deckers, M.M.L.; De Leebeeck, M.; Donovan, S.K.; Nieuwenhuis, E. Episkeletozoans and Bioerosional Ichnotaxa on Isolated Bones of Late Cretaceous Mosasaurs and Cheloniid Turtles from the Maastricht Area, the Netherlands. Geologos 2020, 26, 39–49. [Google Scholar] [CrossRef]
- Wada, H.; Naganuma, T.; Fujioka, K.; Kitazato, H.; Kawamura, K.; Akazawa, Y. The Discovery of the Torishima Whale Bone Animal Community and Its Meaning. J. Deep.-Sea Res. 1994, 10, 37–47. [Google Scholar]
- Massare, J.A.; Wahl, W.R.; Ross, M.; Connely, M.V. Palaeoecology of the Marine Reptiles of the Redwater Shale Member of the Sundance Formation (Jurassic) of central Wyoming, USA. Geol. Mag. 2014, 151, 167–182. [Google Scholar] [CrossRef]
- Belaústegui, Z.; de Gibert, J.M.; Domnèch, R.; Muñez, F.; Martinell, J. Clavate Borings in a Miocene Cetacean Skeleton from Tarragona (NE Spain) and the Fossil Record of Marine Bone Bioerosion. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 323–325, 68–74. [Google Scholar] [CrossRef]
- Kelly, S.R.; Bromley, R.G. Ichnological Nomenclature of Clavate Borings. Palaeontology 1984, 27, 793–807. [Google Scholar]
- Bertling, M.; Braddy, S.J.; Bromley, R.G.; Demathieu, G.R.; Genise, J.; Mikuláš, R.; Nielsen, J.K.; Nielsen, K.S.S.; Rindsberg, A.K.; Schlirf, M.; et al. Names for Tracefossils: A Uniform Approach. Lethaia 2006, 39, 265–286. [Google Scholar] [CrossRef]
- Amalfitano, J.; Giusberti, L.; Fornaciari, E.; Dalla Vecchia, F.M.; Luciani, V.; Kriwet, J.; Carnevale, G. Large Deadfalls of the ‘Ginsu’ Shark Cretoxyrhina mantelli (Agassiz, 1835) (Neoselachii, Lamniformes) from the Upper Cretaceous of Northeastern Italy. Cretac. Res. 2019, 98, 250–275. [Google Scholar] [CrossRef]
- Serrano-Brañas, C.I.; Espinosa-Chávez, B.; Maccracken, S.A. Gastrochaenolites Leymerie in Dinosaur Bones from the Upper Cretaceous of Coahuila, North-Central Mexico: Taphonomic Implications for Isolated Bone Fragments. Cretac. Res. 2018, 92, 18–25. [Google Scholar] [CrossRef]
- Boreske, J.R.; Goldberg, L.; Cameron, B. A Reworked Cetacean with Clam Borings: Miocene of North Carolina. J. Paleontol. 1972, 46, 130–139. [Google Scholar]
- Bromley, R.G. Bioerosion: Eating rocks for fun and profit. In Trace Fossils; Maples, C.G., West, R.R., Eds.; Paleontological Society: Knoxville, TN, USA, 1992; pp. 121–129. [Google Scholar]
- Höpner, S.; Bertling, M. Holes in Bones: Ichnotaxonomy of Bone Borings. Ichnos 2017, 24, 259–282. [Google Scholar] [CrossRef]
- MacLeod, K.; Hoppe, K. Evidence that Inoceramid Bivalves Were Benthic and Harbored Chemosynthetic Symbionts. Geology 1992, 20, 117–120. [Google Scholar] [CrossRef]
- Esperante, R.; Muñiz Guinea, F.; Nick, K.E. Taphonomy of a Mysticeti Whale in the Lower Pliocene Huelva Sands Formation (Southern Spain). Geol. Acta 2009, 7, 489–505. [Google Scholar]
- Cooper, S.L.; Maxwell, E.E. Revision of the Pachycormid Fish Saurostomus Esocinus AGASSIZ from the Early Jurassic (Toarcian) of Europe, with New Insight into the Origins of Suspension Feeding in Pachycormidae. Pap. Palaeontol. in review.
- Anderson, K.L.; Druckenmiller, P.S.; Erickson, G.M.; Maxwell, E.E. Skeletal microstructure of Stenopterygius quadriscissus (Reptilia, Ichthyosauria) from the Posidonienschiefer (Posidonia Shale, Lower Jurassic) of Germany. Palaeontology 2019, 62, 433–449. [Google Scholar] [CrossRef]
- Sinha, S.; Muscente, A.D.; Schiffbauer, J.D.; Williams, M.; Schweigert, G.; Martindale, R.C. Global Controls on Phosphatization of Fossils during the Toarcian Oceanic Anoxic Event. Sci. Rep. 2021, 11, 24087. [Google Scholar] [CrossRef] [PubMed]
- Danise, S.; Dominici, S. A Record of Fossil Shallow-Water Whale Falls from Italy. Lethaia 2014, 47, 229–243. [Google Scholar] [CrossRef]
- Bosio, G.; Collareta, A.; Di Celma, C.; Lambert, O.; Marx, F.G.; de Muizon, C.; Gioncada, A.; Gariboldi, K.; Malinverno, E.; Varas Malca, R.; et al. Taphonomy of Marine Vertebrates of the Pisco Formation (Miocene, Peru): Insights into the Origin of an Outstanding Fossil-Lagerstätte. PLoS ONE 2021, 16, e0254395. [Google Scholar] [CrossRef] [PubMed]
- van Loon, A.J. Ichthyosaur Embryos Outside the Mother Body: Not due to Carcass Explosion but to Carcass Implosion. Palaeobiodivers. Palaeoenviron. 2013, 93, 103–109. [Google Scholar] [CrossRef][Green Version]
- Smith, K.T.; Wuttke, M. From Tree to Shining Sea: Taphonomy of the Arboreal Lizard Geiseltaliellus maarius from Messel, Germany. Palaeobiodivers. Palaeoenviron. 2012, 92, 45–65. [Google Scholar] [CrossRef]
- Lindgren, J.; Sjövall, P.; Thiel, V.; Yheng, W.; Ito, S.; Wakamatsu, K.; Hauff, R.; Kear, B.P.; Engdahl, A.; Alwmark, C.; et al. Soft-Tissue Evidence for Homeothermy and Crypsis in a Jurassic Ichthyosaur. Nature 2018, 564, 359–365. [Google Scholar] [CrossRef]
- Keller, T. “Weichteil-Erhaltung” bei Großen Vertebraten (Ichthyosauriern) des Posidonienschiefers Holzmadens (Oberer Lias, Mesozoikum Süddeutschlands). Kaupia Darmstädter Beitr. Naturk. 1992, 1, 23–62. [Google Scholar]
- Schwark, L.; Frimmel, A. Chemostratigraphy of the Posidonia Black Shale, SW-Germany II. Assessment of Extent and Persistence of Photic-Zone Anoxia Using Aryl Isoprenoid Distributions. Chem. Geol. 2004, 206, 231–248. [Google Scholar] [CrossRef]
- Etter, W.; Tang, C.M. Posidonia shale: Germany’s Jurassic marine park. In Exceptional Fossil Preservation: A Unique View on the Evolution of Marine Life; Bottjer, D.J., Etter, W., Hagadorn, J.W., Tang, C.M., Eds.; Columbia University Press: New York, NY, USA, 2002; pp. 265–292. [Google Scholar]
- Seilacher, A.; Reif, W.-E.; Westphal, F. Sedimentological, Ecological and Temporal Patterns of Fossil Lagerstätten. Phil. Trans. R. Soc. Lond. Ser. B 1985, 311, 5–23. [Google Scholar]
- Riegraf, W. Mikrofauna, Biostratigraphie und Fazies im Unteren Toarcium Südwestdeutschlands und Vergleiche mit Benachbarten Gebieten. Tübinger Mikropaläontol. Mitt. 1985, 3, 1–232. [Google Scholar]
- Bardack, D. First Fossil Hagfish (Myxinoidea): A Record from the Pennsylvanian of Illinois. Science 1991, 254, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Houssaye, A.; Sander, P.M.; Klein, N. Adaptive Patterns in Aquatic Amniote Bone Microanatomy—More Complex than Previously Thought. Integr. Comp. Biol. 2016, 56, 1349–1369. [Google Scholar] [CrossRef] [PubMed]
- Higgs, N.D.; Little, C.T.S.; Glover, A.G. Bones as Biofuel: A Review of Whale Bone Composition with Implications for Deep-Sea Biology and Palaeoanthropology. Proc. R. Soc. B 2011, 278, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Shadwick, R.E.; Goldbogen, J.A.; Pyenson, N.D.; Whale, J.C.A. Structure and Function in the Lunge Feeding Apparatus: Mechanical Properties of the Fin Whale Mandible. Anat. Rec. 2017, 300, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Braby, C.E.; Rouse, G.W.; Jouhnson, S.B.; Jones, W.J.; Vrijenhoek, R.C. Bathymetric and Temporal Variation among Osedax Boneworms and Associated Megafauna on Whale-Falls in Monterey Bay, California. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 1773–1791. [Google Scholar] [CrossRef]
- Dominici, S.; Danise, S.; Cau, S.; Freschi, A. The Awkward Rcord of Fossil Whales. Earth-Sci. Rev. 2020, 205, 103057. [Google Scholar] [CrossRef]
- Johnson, S.B.; Warén, A.; Lee, R.W.; Kano, Y.; Kaim, A.; Davis, A.; Strong, E.E.; Vrijenhoek, R.C. Rubyspira, New Genus and Two New Species of Bone-Eating Deep-Sea Snails with Ancient Habits. Biol. Bull. 2010, 219, 166–177. [Google Scholar] [CrossRef]
- Talevi, M.; Fernández, M.S. Unexpected Skeletal Histology of an Ichthyosaur from the Middle Jurassic of Patagonia: Implications for Evolution of Bone Microstructure among Secondary Qquatic Tetrapods. Naturwissenschaften 2012, 99, 241–244. [Google Scholar] [CrossRef]








| Specimen | Taxon | Associated Fauna | Fall Stage | Age | Formation | Deposit Environment | Reference |
|---|---|---|---|---|---|---|---|
| OUM J.28585 | Eurycleidus arcuatus (Plesiosauria) | Ostreids Serpulids | Reef | Hettangian–Sinemurian | Lower Lias (Blue Lias or Charmouth Mudstone), UK | Near shore/offshore basin, periodical bottom oxygenation | [19] |
| PMU | Stenopterygius sp. | Chondrites burrows | Enrichment-opportunistic/ Sulfophilic? | Toarcian | Posidonienschiefer Fm., Germany | Epicontinental anoxic basin | [22] |
| SMNS 81841 | Stenopterygius quadriscissus (Ichthyosauria) | Dactylioceras sp. Harpoceras elegans Ammonite aptychi Sinosura brodiei | Enrichment-opportunistic | Early Toarcian | Posidonienschiefer Fm., Germany | Epicontinental anoxic basin | [17]; starred taxa (asterisk) were found to be absent following reappraisal |
| Parainoceramya dubia Oxytoma inequivalvis Propeamussium pumilus Bositra buchi * Crinoids | Reef | ||||||
| SMNS 80234 | Stenopterygius quadriscissus (Ichthyosauria) | Harpoceras sp. Ammonite aptychi Sinosura brodiei | Enrichment-opportunistic | Early Toarcian | Posidonienschiefer Fm., Germany | Epicontinental anoxic basin | [17] |
| SMNS 53363 | Eurhinosaurus sp. (Ichthyosauria) | Liostrea sp. | Reef | Early Toarcian | Posidonienschiefer Fm., Germany | Epicontinental anoxic basin | [20] |
| UMO no. 14 | Temnodontosaurus sp. (Ichthyosauria) | Shark bite traces | Mobile scavenger | Late Toarcian | Jurensismergel Fm., Germany | Epicontinental basin | [23] |
| Encrusting bivalve | Reef | ||||||
| SMNS 52112 | Stenopterygius sp. (Ichthyosauria) | Toarctocera subpunctata Leioceras opalinum | Enrichment-opportunistic? | Early Aalenian | Opalinuston Fm., Germany | Epicontinental basin | [17] |
| ? | “large reptile skeletons” | Hybodus obtusus teeth | Mobile scavenger | Middle Callovian | Oxford Clay, UK | Deep shelf, periodical bottom oxygenation | [24] |
| BCM 1983/1008 | Ophthalmosaurus sp. (Ichthyosauria) | Ostreids Serpulids | Reef | Middle Callovian | Lower Oxford Clay, UK | Deep shelf, periodical bottom oxygenation | [18] |
| RGCH-62-52 | Hispaniachelys prebetica (Testudines) | Gnathichnus pentax (echinoid graze trace) | Enrichment-opportunistic | Late Oxfordian | Riogazas-Chorro section, Spain | Epipelagic | [25] |
| BRSMG Ce16719 | Ophthalmosaurus sp. (Ichthyosauria) | Fish bite traces | Mobile scavenger | Late Oxfordian | Ringstead Clay Member (Sandsfoot Fm.), UK | Shallow water offshore, slow sedimentation, low energy | [11] |
| Gnathichnus pentax Corbulomima suprajurensis cf. Isocyprina sp. Modiolus bipartitus Dicroloma trifida Rhabdocidaris sp | Enrichment-opportunistic | ||||||
| Microborings (microbial) | ?Sulfophilic | ||||||
| Serpula sulcata cf. Placunopsis radiata Nanogyra nana Ostreids Mytilidae indet. Atreta sp. Camptonectes auritus | Reef | ||||||
| UW 24816 | Ophthalmosaurus natans (Ichthyosauria) | Episkeletal borings | Enrichment-opportunistic? Reef? | Oxfordian | Sundance Fm. (Redwater Shale Member), USA | Shallow inland sea | [26] |
| MCSNV V7101 | Ophthalmo-sauridae (Ichthyosauria) | Notidanodon sp. teeth Ichthyosaur tooth | Mobile scavenger | Early Kimmeridgian | Rosso Ammonitico Veronese Fm., Italy | Epi-mesopelagic deposit, slow sedimentation rates, well-oxygenated waters | [15] |
| Ammonite aptychi (Laevaptychus, Lamellaptychus) Rhyncholites (cf. Gonatocheilus) | Enrichment-opportunistic? | ||||||
| BRSMG Ce17365 | Dakosaurus carpenteri (Thalattosuchia) | Cytheracean ostracods Foraminifera | Enrichment-opportunistic? | Middle Kimmeridgian | Kimmeridge Clay, UK | Restricted epipelagic basin | [27] |
| Ostreid: Nanogyra? Serpulids Bryozoan: Stomatopora? | Reef | ||||||
| BRSMG Cd7203 | Dakosaurus carpenteri (Thalattosuchia) | Ostreid: Nanogyra? | Reef | Middle Kimmeridgian | Kimmeridge Clay, UK | Restricted epipelagic basin | [28] |
| NHMUK PV R 10062 | Colymbosaurus sp. | Balanocrinus sp. | Reef | Middle Kimmeridgian | Kimmeridge Clay, UK | Restricted epipelagic basin | [29] in [30] |
| MJSN SCR010-497 | Ischyodus quenstedti (Holocephali) | Sabellid tubes of Glomerula gordialis | Reef | Late Kimmeridgian | Reuchenette Fm., Switzerland | Shallow-water platform, lagoonal | [31] |
| NMS 8490 NMS 8545 | Plesiochelys sp. (Testudines) | Gnathichnus pentax (echinoid graze trace) | Enrichment-opportunistic | Late Kimmeridgian | Reuchenette Fm., Switzerland | Shallow-water platform, lagoonal | [32] |
| MSVG 39617 | Gengasaurus nicosiai (Ichthyosauria) | Notidanodon teeth | Mobile scavenger | Late Kimmeridgian–earliest Tithonian | Calcari Diasprigni Fm., Italy | Epi-mesopelagic deposit | [14] |
| PMO 222.663 | Plesiosauria indet. | Belemnites | Mobile scavenger | Tithonian | Slottsmøya Member, Spitsbergen | Open marine shelf, slightly dysoxic | [16] |
| Buchia sp. | Reef | ||||||
| SVB 1450 | Spitrasaurus larseni (Plesiosauria) | Ichthyosaur tooth | Mobile scavenger | Tithonian | Slottsmøya Member, Spitsbergen | Open marine shelf, slightly dysoxic | [16] |
| PMO 212.662 | Plesiosauria indet. | Buchia sp. | Reef? | Tithonian | Slottsmøya Member, Spitsbergen | Open marine shelf, slightly dysoxic | [16] |
| PMO 222.670 | Ichthyosauria indet. | Serpulids Buchia sp. | Reef | Tithonian | Slottsmøya Member, Spitsbergen | Open marine shelf, slightly dysoxic with periodical bottom oxygenation | [16] |
| PMO 219.718 | Spitrasaurus wensaasi (Plesiosauria) | Ophiuroids | Enrichment-opportunistic | Tithonian | Slottsmøya Member, Spitsbergen | Open marine shelf, slightly dysoxic | [16] |
| PMO 214.135 | Pliosaurus funkei (Plesiosauria) | Ophiuroids | Enrichment-opportunistic | Tithonian | Slottsmøya Member, Spitsbergen | Open marine shelf, slightly dysoxic | [16] |
| Specimen | Locality | Bed | Taxa/Abundance | Stage |
|---|---|---|---|---|
| SMNS 53363 | Zell u. A. | εII3 | Liostrea, rare | Reef |
| SMNS 80234 | Ohmden | εII4 | Crustacea, rare Sinosura brodiei, rare Diademopsis crinifera, rare Reophax, abundant | Mobile scavenger, enrichment opportunist |
| SMNS 81841 | Schlierbach | εII6 | Ophiuroidea, abundant Echinoidea, rare Crinoidea, rare Serpulidae, rare Oxytoma inaequivalvis (B), abundant Propeamussium pumilus (B), abundant Eopecten strionatis (B), rare Plagiostoma sp. (B), rare Parainoceramya dubia (B), abundant Liostrea (C), abundant Meleagrinella sp. (B), rare “Cucullaea” muensteri (B), rare | Enrichment opportunist, reef |
| SMNS 81719 | Ohmden | εII10 | Propeamussium pumilus (B) Plagiostoma sp. (B), rare Parainoceramya dubia (B) Liostrea (C) | Enrichment opportunist, reef |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxwell, E.E.; Cooper, S.L.A.; Mujal, E.; Miedema, F.; Serafini, G.; Schweigert, G. Evaluating the Existence of Vertebrate Deadfall Communities from the Early Jurassic Posidonienschiefer Formation. Geosciences 2022, 12, 158. https://doi.org/10.3390/geosciences12040158
Maxwell EE, Cooper SLA, Mujal E, Miedema F, Serafini G, Schweigert G. Evaluating the Existence of Vertebrate Deadfall Communities from the Early Jurassic Posidonienschiefer Formation. Geosciences. 2022; 12(4):158. https://doi.org/10.3390/geosciences12040158
Chicago/Turabian StyleMaxwell, Erin E., Samuel L. A. Cooper, Eudald Mujal, Feiko Miedema, Giovanni Serafini, and Günter Schweigert. 2022. "Evaluating the Existence of Vertebrate Deadfall Communities from the Early Jurassic Posidonienschiefer Formation" Geosciences 12, no. 4: 158. https://doi.org/10.3390/geosciences12040158
APA StyleMaxwell, E. E., Cooper, S. L. A., Mujal, E., Miedema, F., Serafini, G., & Schweigert, G. (2022). Evaluating the Existence of Vertebrate Deadfall Communities from the Early Jurassic Posidonienschiefer Formation. Geosciences, 12(4), 158. https://doi.org/10.3390/geosciences12040158






