First Speleological and Biological Characterization of a Submerged Cave of the Tremiti Archipelago Geomorphosite (Adriatic Sea)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Survey and Sampling
2.3. Physical–Chemical Parameters
2.4. Irradiance
2.5. Water Motion
2.6. Video and Image Acquisition
3. Results
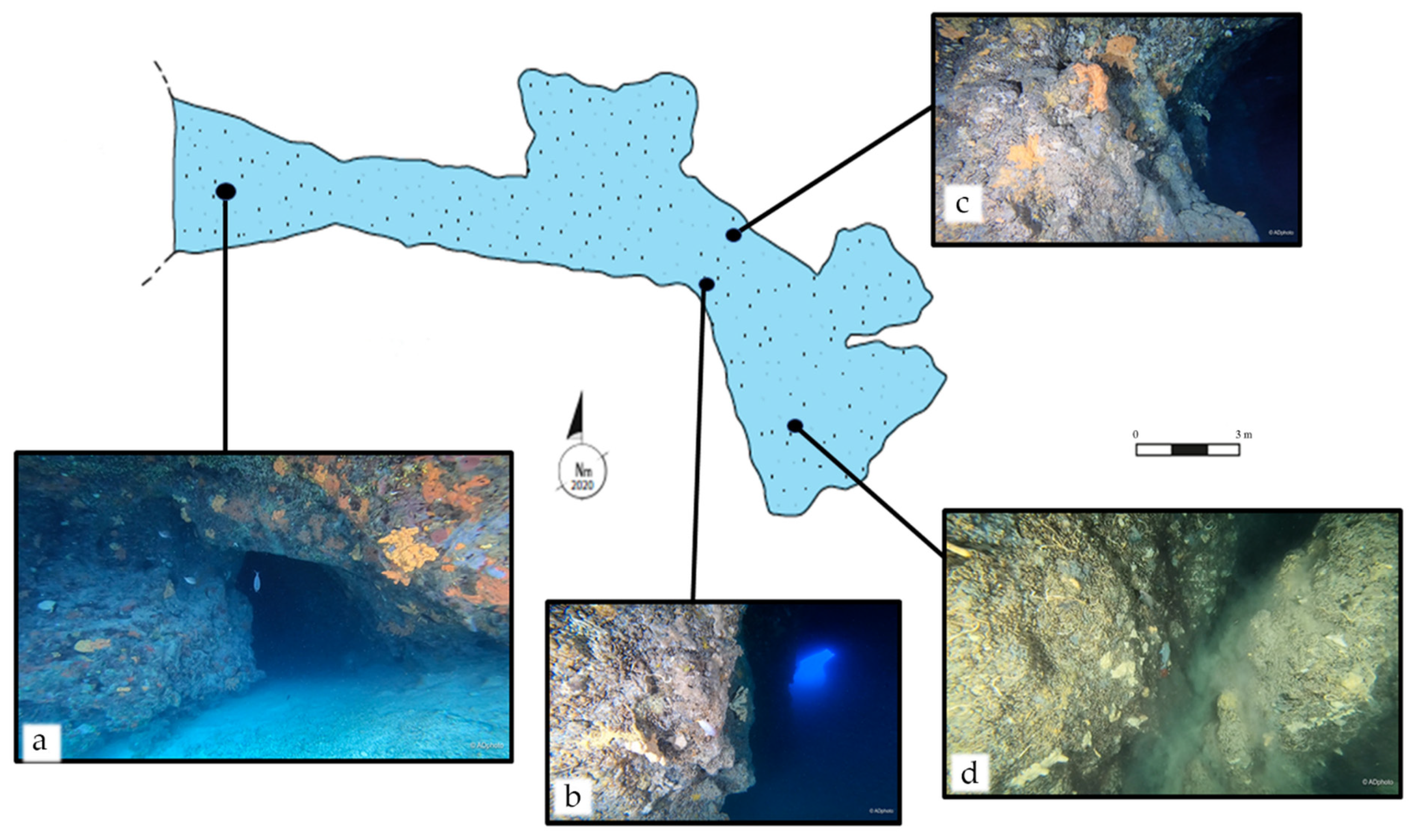
3.1. Morphological Description of the Elle Cave
3.2. Hydrological Parameters
3.3. Biodiversity Pattern of the Benthic Assemblages
4. Discussion
4.1. Proposal of Speleogenetic Processes of the Cave
4.2. Biodiversity Pattern
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onorato, M.; Belmonte, G. Submarine Caves of the Salento Pensinsula: Faunal Aspects. Thalass. Salentina 2017, 39, 47–72. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Bianchi, C.N. Mediterranean Marine Caves: A Synthesis of Current Knowledge. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–87. [Google Scholar] [CrossRef]
- Giakoumi, S.; Sini, M.; Gerovasileiou, V.; Mazor, T.; Beher, J.; Possingham, H.; Abdulla, A.; Cinar, M.E.; Dendrinos, P.; Gucu, A.C.; et al. Ecoregion-Based Conservation Planning in the Mediterranean: Dealing with Large-Scale Heterogeneity. PLoS ONE 2013, 8, e76449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miccadei, E.; Orrù, P.; Piacentini, T.; Mascioli, F.; Puliga, G. Geomorphological map of the Tremiti Islands (Puglia, Southern Adriatic Sea, Italy), scale 1:15,000. J. Maps 2012, 8, 74–87. [Google Scholar] [CrossRef] [Green Version]
- Paglia, G.; Bergamin, L.; Buccolini, M.; Carabella, C.; Cerrone, F.; Chiocci, F.L.; D’Arielli, R.; Esposito, G.; Federico, D.; Mancinelli, V.; et al. A multidisciplinary approach to the study of insular environments: The 1st Summer School on Geomorphology, Ecology, and Marine Biology in the Tremiti Islands (Southern Adriatic Sea, Puglia, Italy). J. Maps 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Balduzzi, A.; Bianch, C.N.; Boero’, F.; Vietti, R.C.; Pansini’, M.; Sara’, M. The Suspension-Feeder Communities of a Mediterranean Sea Cave. Topics in Marine Biology. Sci. Mar. 1989, 53, 387–395. [Google Scholar]
- Onorato, R.; Forti, P.; Belmonte, G.; Poto, M.; Costantini, A. Grotta sottomarina de lu Lampiùne: Novità esplorative e prime indagini ecologiche. Thalass. Salentina 2003, 26, 55–64. [Google Scholar]
- Martí, R.; Uriz, M.J.; Ballesteros, E.; Turon, X. Benthic assemblages in two Mediterranean caves: Species diversity and coverage as a function of abiotic parameters and geographic distance. J. Mar. Biol. Assoc. 2004, 84, 557–572. [Google Scholar] [CrossRef] [Green Version]
- Faresi, L.; Bettoso, N.; Aleffi, F.; Orel, G. Benthic macrofauna of a submarine cave on the Istrian peninsula (Croatia). Ann. Ser. Hist. Nat. 2006, 1, 9–16. [Google Scholar]
- Rosso, A.; Sanfilippo, R.; Taddei Ruggiero, E. Faunas and ecological groups of Serpuloidea, Bryozoa and Brachiopoda from submarine caves in Sicily (Mediterranean Sea). Boll. Della Soc. Paleontol. Ital. 2013, 52, 167–176. [Google Scholar] [CrossRef]
- Sempere-Valverde, J.; Lorenzo, A.S.; Espinosa, F.; Gerovasileiou, V.; Sánchez-Tocino, L.; Navarro-Barranco, C. Taxonomic and morphological descriptors reveal high benthic temporal variability in a Mediterranean marine submerged cave over a decade. Hydrobiologia 2019, 839, 177–194. [Google Scholar] [CrossRef]
- Riedl, R. Biologie der Meereshöhlen; Paul Parey: Hamburg, Germany, 1966; p. 636. [Google Scholar]
- Harmelin, J.G.; Vacelet, J.; Vasseur, P. Les grottes sous-marines obscures: Un milieu extrême et un remarquable biotope refuge. Tethys 1985, 11, 214–229. [Google Scholar]
- Cicogna, F.; Bianchi, C.N.; Ferrari, G.; Forti, P. Grotte Marine: Cinquant‘anni Di Ricerc; Ministero dell’Ambiente e della Tutela del Territorio: Rome, Italy, 2003; p. 505. [Google Scholar]
- Surić, M.; Leončarić, R.; Leončar, N. Submerged caves of Croatia: Distribution, classification and origin. Environ. Earth Sci. 2010, 61, 1473–1480. [Google Scholar] [CrossRef]
- Onorato, R.; Denitto, F.; Belmonte, G. Le grotte marine del Salento: Classificazione, localizzazione e descrizione. Thalass. Salentina 1999, 23, 67–116. [Google Scholar]
- Onorato, R.; Belmonte, G.; Costantini, A. Le grotte sommerse della costa neretina (Salento, S-E Italia). Thalass. Salentina 2006, 29, 39–54. [Google Scholar]
- Gerovasileiou, V.; Chintiroglou, C.; Vafidis, D.; Koutsoubas, D.; Sini, M.; Dailianis, T.; Issaris, Y.; Akritopoulou, E.; Dimarchopoulou, D.; Voutsiadou, E. Census of biodiversity in marine caves of the eastern Mediterranean Sea. Mediterr. Mar. Sci. 2015, 16, 245. [Google Scholar] [CrossRef] [Green Version]
- Bussotti, S.; Terlizzi, A.; Fraschetti, S.; Belmonte, G.; Boero, F. Spatial and temporal variability of sessile benthos in shallow Mediterranean marine caves. Mar. Ecol. Prog. Ser. 2006, 325, 109–119. [Google Scholar] [CrossRef]
- Belmonte, G.; Ingrosso, G.; Poto, M.; Quarta, G.; D’Elia, M.; Onorato, R.; Calcagnile, L. Biogenic stalactites in submarine caves at the Cape of Otranto (S-E Italy): Dating and hypothesis on their formation. Mar. Ecol. Evol. Perspect. 2009, 30, 376–382. [Google Scholar] [CrossRef]
- Belmonte, G.; Costantini, A.; Sorrentino, F.; Licchelli, A.; Poto, M.; Onorato, R. Le grotte sommerse dell’Area Marina Protetta “Porto Cesareo”. Thalass. Salentina 2010–2011, 33, 15–38. [Google Scholar]
- Russo, R.; Valente, S.; Colangelo, G.; Belmonte, G. Meiofauna distribution on hard substrata in a submarine cave. J. Mar. Biol. Assoc. 2015, 95, 1555–1564. [Google Scholar] [CrossRef]
- Miccadei, E.; Mascioli, F.; Piacentini, T. Quaternary geomorphological evolution of the Tremiti Islands (Puglia, Italy). Quat. Int. 2011, 233, 3–15. [Google Scholar] [CrossRef]
- Caldara, M.; Palmentola, G. Lineamenti Geomorfologici del Gargano Con Particolare Riferimento al Carsismo. Bonifica 1993, 8, 43e52. [Google Scholar]
- Muus, B.J. A Field Method for Measuring “Exposure” by Means of Plaster Balls: A Preliminary Account. Sarsia 1968, 34, 61–68. [Google Scholar] [CrossRef]
- Gambi, M.C.; Buia, M.C.; Casola, E.; Scardi, M. Estimates of Water Movement in Posidonia Oceanica Beds: A First Approach. In Second International Workshop on Posidonia Oceanica Beds (1985); Boudouresque, C.F., Meinesz, A., Fresi, E., Gravez, V., Eds.; GIS Posidonie: Marseille, France, 1989; Volume 2, pp. 101–112. [Google Scholar]
- Cattaneo, R.; Pastorino, M.V. Popolamenti Algali e Fauna Bentonica Nelle Cavità Naturali Della Regione Litorale Mediterranea. Rass. Speleol. Ital. 1974, 12, 272–281. [Google Scholar]
- Bögli, A. Endokarst and Karst Hydrology. In Karst Hydrology and Physical Speleology; Boegli, A., Ed.; Springer: Berlin, Germany, 1980; pp. 73–76. [Google Scholar] [CrossRef]
- de Waele, J.; Piccini, L. Speleogenesi e Morfologia Dei Sistemi Carsici in Rocce Carbonatiche. In Proceedings of the Atti del 45° Corso CNSS-SSI di III Livello di Geo-Morfologia Carsica, Grottaglie, Italy, 2–3 February 2008; pp. 23–74. [Google Scholar]
- Bayari, C.S. Development of Coastal and Submarine Caves in the South-Western Part of Turkey. In Marine Caves of the Eastern Mediterranean Sea. Biodiversity, Threats and Conservation; Öztürk, B., Ed.; TUDAV: İstanbul, Turkey, 2019; Volume 53, pp. 41–68. [Google Scholar]
- Mylroie, J.E.; Carew, J.L. Speleogenesis in Coastal and Oceanic Setting. In Speleogenesis; Evolution of Karst Aquifers; Klimchouk, A.V., Ford, D.C., Palmer, A.N., Dreybrodt, W., Eds.; National Speleological Society of America: Huntsville, AL, USA, 2000; pp. 226–233. [Google Scholar]
- Sanfilippo, R.; Rosso, A.; Guido, A.; Mastandrea, A.; Russo, F.; Ryding, R.; Taddei, R.E. Metazooan/Microbial Biostalactites from Modern Submarine Caves in the Mediterranean Sea. Mar. Ecol. 2015, 36, 1277–1293. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Rosso, A.; Guido, A.; Gerovasileiou, V. Serpulid communities from two marine caves in the Aegean Sea, eastern Mediterranean. J. Mar. Biol. Assoc. 2017, 97, 1059–1068. [Google Scholar] [CrossRef] [Green Version]
- Guido, A.; Jimenez, C.; Achilleos, K.; Rosso, A.; Sanfilippo, R.; Hadjioannou, L.; Petrou, A.; Russo, F.; Mastandrea, A. Cryptic serpulid-microbialite bioconstructions in the Kakoskali submarine cave (Cyprus, Eastern Mediterranean). Facies 2017, 63, 21. [Google Scholar] [CrossRef]
- Guido, A.; Rosso, A.; Sanfilippo, R.; Russo, F.; Mastandrea, A. Microbial Biomineralization in Biotic Crusts from a Pleistocene Marine Cave (NW Sicily, Italy). Geomicrobiol. J. 2017, 34, 864–872. [Google Scholar] [CrossRef]
- Rosso, A.; Sanfilippo, R.; Guido, A.; Gerovasileiou, V.; Ruggiero, E.T.; Belmonte, G. Colonisers of the dark: Biostalactite-associated metazoans from “lu Lampiùne” submarine cave (Apulia, Mediterranean Sea). Mar. Ecol. 2021, 42, e12634. [Google Scholar] [CrossRef]
- Gili, J.M.; Riera, T.; Zabala, M. Physical and biological gradients in a submarine cave on the Western Mediterranean coast (north-east Spain). Mar. Biol. 1986, 90, 291–297. [Google Scholar] [CrossRef]
- Bibiloni, M.A.; Uriz, M.; Gili, J. Sponge Communities in Three Submarine Caves of the Balearic Islands (Western Mediterranean): Adaptations and Faunistic Composition. Mar. Ecol. 1989, 10, 317–334. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Abbiati, M.; Airoldi, L.; Benedetti-Cecchi, L.; Cappelletti, A.; Gemelli, F.; Colantoni, P.; Dando, P.R.; Morri, C.; Niccolai, I.; et al. Hydrology and water budget of a submarine cave with sulphidic springs: The “Grotta Azzurra” of Capo Palinuro (Southern Italy). In Proceedings of the Atti 12° Congresso dell’Associazione Italiana di Oceanologia e Limnologia, Isola di Vulcano, Italy, 18–21 September 1998; Volume 2, pp. 285–301. [Google Scholar]
- Harmelin, J.G. Bryozoaires des grottes sous-marines obscures de la région marseillaise. Faunistique et ecologie. Téthys 1969, 1, 793–806. [Google Scholar]
- Gili, J.M.; Ballesteros, E. Structure of Cnidarian Populations in Mediterranean Sublittoral Benthic Communities as a Result of Adaptations to Different Environmental Conditions. Oecología Aquática 1991, 10, 243–254. [Google Scholar]
- Bianchi, C.N.; Morri, C. Southern Species in the Ligurian Sea (Northern Mediterranean): New Records and a Review. Boll. Ist. Mus. Biol. Univ. Genova 1994, 58–59, 181–197. [Google Scholar]
- Sarà, M. La fauna di Poriferi delle grotte delle isole Tremiti. Studio ecologico e sistematico. Arch. Zool. Ital. 1961, 46, 1–59. [Google Scholar]
- Corriero, G.; Liaci, L.S.; Pronzato, R. Didiscus spinoxeatus, a new species of Porifera (Demospongiae) from the Mediterranean Sea. Ophelia 1997, 47, 63–70. [Google Scholar] [CrossRef]
- Corriero, G.; Liaci, L.S.; Ruggiero, D.; Pansini, M. The Sponge Community of a Semi-Submerged Mediterranean Cave. Mar. Ecol. 2000, 21, 85–96. [Google Scholar] [CrossRef]
- Bell, J.J. The Sponge Community in a Semi-Submerged Temperate Sea Cave: Density, Diversity and Richness. Mar. Ecol. 2002, 23, 297–311. [Google Scholar] [CrossRef]
- Cinelli, F.; Fresi, E.; Mazzella, L.; Pansini, M.; Pronzato, R.; Svoboda, A. Distribution of Benthic Phyto- and Zoo-Coenoses along a Light Gradient in a Superficial Marine Cave. In Biology of Benthic Organisms; Keegan, B.F., O’Cèidigh, P., Boaden, P.J.S., Eds.; Pergamon Press: London, UK, 1977; pp. 173–183. [Google Scholar]
- Pansini, M.; Pronzato, R.; Fresi, E.; Cinelli, F.; Mazzella, L.; Ponticelli, M.P. Evoluzione Delle Biocenosi Bentoniche Di Substrato Duro Lungo Un Gradiente Di Luce in Una Grotta Marina Superficiale: Poriferi. In Proceedings of the Atti IX Congresso S. I. B. M., Lacco Ameno, Italy, 19–22 May 1977; pp. 315–330. [Google Scholar]
- Gerovasileiou, V.; Voultsiadou, E. Sponge diversity gradients in marine caves of the eastern Mediterranean. J. Mar. Biol. Assoc. 2015, 96, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Manconi, R.; Ledda, F.D.; Serusi, A.; Corso, G.; Stocchino, G.A. Sponges of marine caves: Notes on the status of the Mediterranean palaeoendemicPetrobiona massiliana(Porifera: Calcarea: Lithonida) with new records from Sardinia. Ital. J. Zool. 2009, 76, 306–315. [Google Scholar] [CrossRef]
- Vacelet, J.; Lévi, C. Un Cas de Survivance, En Méditerranée, Du Groupe d’éponges Fossiles Des Pharétronides. C. R. Hebd. Séances L’académie Des Sci. 1958, 246, 318–320. [Google Scholar]
- Vacelet, J.; Boury-Esnault, N.; Harmelin, J.-G. Hexactinellid cave, a unique deep-sea habitat in the scuba zone. Deep Sea Res. Part I Oceanogr. Res. Pap. 1994, 41, 965–973. [Google Scholar] [CrossRef]
- Vacelet, J.; Boury-Esnault, N. A New Species of Carnivorous Sponge (Demospongiae, Cladorhizidae) from a Mediterranean Cave. Recent Advances in Sponge Biodiversity Inventory and Documentation. Bull. L’institut R. Sci. Nat. Belg. Biol. 1996, 66, 109–116. [Google Scholar]
- Perez, T.; Vacelet, J.; Bitar, G.; Zibrowius, H. Two new lithistids (Porifera: Demospongiae) from a shallow eastern Mediterranean cave (Lebanon). J. Mar. Biol. Assoc. 1999, 84, 15–24. [Google Scholar] [CrossRef]
- Bakran-Petricioli, T.; Vacelet, J.; Zibrowius, H.; Petricioli, D.; Chevaldonné, P.; Raa, T. New data on the distribution of the ‘deep-sea’ sponges Asbestopluma hypogea and Oopsacas minuta in the Mediterranean Sea: New Distribution Data on Mediterranean ‘Deep-Sea’ Sponges. Mar. Ecol. 2007, 28, 10–23. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Voultsiadou, E. Marine Caves of the Mediterranean Sea: A Sponge Biodiversity Reservoir within a Biodiversity Hotspot. PLoS ONE 2012, 7, e39873. [Google Scholar] [CrossRef] [PubMed]
- Cinar, M.E.; Evcen, A. Macrozoobenthic Invertebrates in Three Submarine Caves of the Aegean Sea: Preliminary Results. In Marine Caves of the Eastern Mediterranean Sea. Biodiversity, Threats and Conservation; Öztürk, B., Ed.; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2019; pp. 69–83. [Google Scholar]
- Grenier, M.; Ruiz, C.; Fourt, M.; Santonja, M.; Dubois, M.; Klautau, M.; Vacelet, J.; Boury-Esnault, N.; Pérez, T. Sponge inventory of the French Mediterranean waters, with an emphasis on cave-dwelling species. Zootaxa 2018, 4466, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.N.; Cattaneo-Vietti, R.; Cinelli, F.; Morri, C.; Pansini, M. Lo Studio Biologico Delle Grotte Sottomarine Del Mediterraneo: Conoscenze Attuali e Prospettive. Boll. Mus. Ist. Biol. Univ. Genova 1996, 60–61, 41–69. [Google Scholar]





| (a) | |||||
| Zone | Date | Mean (±SD) | Average % | ||
| 1 | 23-25 August | 5.041 (±0.039) | 39.7 | ||
| 2 | 3-5 September | 4.288 (±0.116) | 33.6 | ||
| 3 | 14-16 September | 3.933 (±0.059) | 31.0 | ||
| (b) | |||||
| Sum of Sqrs | df | Mean Square | F | p | |
| Between groups | 1.914 | 2 | 0.957 | 262.6 | <0.001 |
| Within groups | 0.022 | 6 | 0.004 | ||
| Total | 1.936 | 8 | |||
| (a) | |||||
| Zone | Date | Mean (±SD) | |||
| 1 | 23–25 August | 8.13 (±0.01) | |||
| 2 | 3–5 September | 8.23 (±0.01) | |||
| 3 | 14–16 September | 8.06 (±0.02) | |||
| (b) | |||||
| Sum of Sqrs | df | Mean Square | F | p | |
| Between groups | 0.659 | 2 | 0.3294 | 1760 | <0.001 |
| Within groups | 0.025 | 138 | 0.0002 | ||
| Error | 0.017 | 92 | 0.0002 | ||
| Between hours | 0.008 | 46 | 0.0002 | ||
| Total | 0.684 | 140 | |||
| (a) | |||||
| Zone | Date | mg/L | % Saturation | ||
| 1 | 23–25 August | 8.5 (±0.4) | 100.1 (±3.5) | ||
| 2 | 3–5 September | 7.74 (±0.73) | 92.5 (±7.4) | ||
| 3 | 14–16 September | 7.36 (±0.61) | 88.5 (±7.2) | ||
| (b) | |||||
| Sum of Sqrs | df | Mean Square | F | p | |
| Between groups | 31.95 | 2 | 15.97 | 47.15 | <0.001 |
| Within groups | 48.58 | 138 | 0.35 | ||
| Error | 31.17 | 92 | 0.34 | ||
| Between subjects | 17.42 | 46 | 0.38 | ||
| Total | 80.53 | 140 | |||
| (a) | |||||
| Zone | Date | Mean (±SD) | |||
| 1 | 23–25 August | 23.6 (±0.8) | |||
| 2 | 3–5 September | 24.4 (±1.0) | |||
| 3 | 14–16 September | 24.6 (±0.2) | |||
| (b) | |||||
| Sum of Sqrs | df | Mean Square | F | p | |
| Between groups | 29.64 | 2 | 14.82 | 31.2 | <0.001 |
| Within groups | 78.93 | 138 | 0.57 | ||
| Error | 43.71 | 92 | 0.48 | ||
| Between hours | 35.23 | 46 | 0.77 | ||
| Total | 108.57 | 140 | |||
| (c) | |||||
| ST1 | ST2 | ST3 | |||
| ST1 | p < 0.001 | p < 0.001 | |||
| ST2 | 8.35 | N.S. | |||
| ST3 | 10.6 | 2.26 | |||
| ES-Wall | ES-Ceiling | IS-Wall | IS-Ceiling | BS-Wall | BS-Ceiling | |
|---|---|---|---|---|---|---|
| RHODOPHYTA | ||||||
| Encrusting Coralline Algae | x | x | ||||
| CHLOROPHYTA | ||||||
| Chlorophyta ind. spp. | x | |||||
| Palmophyllum crassum (Naccari Rabenhorst, 1868) | x | |||||
| PORIFERA | ||||||
| Petrobiona massiliana (Vacelet and Lévi, 1958) | x | x | x | x | x | |
| cf. Pseudocorticium jarrei (Boury–Esnault, Muricy, Gallissian and Vacelet, 1995) | x | x | x | |||
| Oscarella spp. | x | |||||
| Erylus discophorus (Schmidt, 1862) | x | x | ||||
| Dercitus plicatus (Schmidt, 1868) | x | |||||
| Cliona schmidtii (Ridley, 1881) | x | |||||
| Spirastrella spp. | x | x | x | x | x | |
| Diplastrella bistellata (Schmidt, 1862) | x | x | x | |||
| Pseudosuberites sulphureus (Bowerbank, 1866) | x | x | x | |||
| Terpios gelatinosus (Bowerbank, 1866) | x | x | x | |||
| Timea sp. | x | |||||
| Chondrosia reniformis (Nardo, 1847) | x | x | ||||
| Antho incostans (Topsent, 1925) | x | |||||
| Phorbas tenacior (Topsent, 1925) | x | |||||
| cf. Merlia normani (Kirkpatrick, 1908) | x | |||||
| Axinella damicornis (Esper, 1794) | x | |||||
| Didiscus sp. | x | |||||
| Acanthella acuta (Schmidt, 1862) | x | x | ||||
| Dictyonella sp. | x | |||||
| Agelas oroides (Schmidt, 1864) | x | x | x | x | ||
| Dendroxea cf. lenis (Topsent, 1892) | x | x | x | x | x | x |
| Haliclona (Soestella) mucosa (Griessinger, 1971) | x | x | ||||
| Petrosia (Petrosia) ficiformis (Poiret, 1789) | x | x | x | x | ||
| Ircinia oros (Schmidt, 1864) | x | |||||
| Ircinia variabilis (Schmidt, 1862) | x | |||||
| Cacospongia mollior (Schmidt, 1862) | x | |||||
| Fasciospongia cavernosa (Schmidt, 1862) | x | |||||
| Spongia sp. 1 | x | |||||
| Spongia sp. 2 | x | |||||
| Hexadella cf. pruvoti (Topsent, 1896) | x | x | x | |||
| Demospongiae 1 | x | |||||
| Demospongiae 2 | x | |||||
| Demospongiae 3 | x | x | ||||
| Demospongiae 4 | x | x | x | |||
| Demospongiae 5 | x | |||||
| Demospongiae 6 | x | |||||
| Demospongiae 7 | x | x | x | x | ||
| Demospongiae 8 | x | |||||
| Demospongiae 9 | x | x | ||||
| CNIDARIA | ||||||
| Hydrozoa ind. | x | x | ||||
| Madracis pharensis (Heller, 1868) | x | x | x | |||
| Hoplangia durotrix (Gosse, 1860) | x | x | x | |||
| Leptopsammia pruvoti (Lacaze–Duthiers, 1897) | x | x | x | |||
| Scleractinia ind. | x | |||||
| ANNELIDA | ||||||
| Sabellidae ind. spp. | x | |||||
| Placostegus crystallinus (Zibrowius, 1968) | x | |||||
| Protula tubularia (Montagu, 1803) | x | x | x | x | x | |
| Vermiliopsis infundibulum (Philippi, 1844) | x | |||||
| Vermiliopsis labiata (O. G. Costa, 1861) | x | x | ||||
| Spiraserpula massiliensis (Zibrowius, 1968) | x | x | x | |||
| Serpulinae gen. sp1 | x | x | ||||
| Serpulinae gen. spp. * | x | x | x | x | x | x |
| Serpulinae gen. spp. ** | x | x | x | x | x | |
| Spirorbinae gen. spp. | x | x | x | x | x | x |
| MOLLUSCA | ||||||
| Rocellaria dubia (Pennant, 1777) | x | |||||
| BRACHIOPODA | ||||||
| Novocrania anomala (O. F. Müller, 1776) | x | x | ||||
| Articulata gen. sp. | x | x | ||||
| BRYOZOA | ||||||
| Bryozoa ind. *** | x | |||||
| Bryozoa ind. **** | x | x | x | |||
| Ascophorina gen. spp. | x | x | ||||
| CHORDATA | ||||||
| Halocynthia papillosa (Linnaeus, 1767) | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardone, F.; Mazzetti, M.; Sorci, A.; Cesaretti, A.; Cimmaruta, R.; Gravina, M.F. First Speleological and Biological Characterization of a Submerged Cave of the Tremiti Archipelago Geomorphosite (Adriatic Sea). Geosciences 2022, 12, 213. https://doi.org/10.3390/geosciences12050213
Cardone F, Mazzetti M, Sorci A, Cesaretti A, Cimmaruta R, Gravina MF. First Speleological and Biological Characterization of a Submerged Cave of the Tremiti Archipelago Geomorphosite (Adriatic Sea). Geosciences. 2022; 12(5):213. https://doi.org/10.3390/geosciences12050213
Chicago/Turabian StyleCardone, Frine, Martina Mazzetti, Adelmo Sorci, Andrea Cesaretti, Roberta Cimmaruta, and Maria Flavia Gravina. 2022. "First Speleological and Biological Characterization of a Submerged Cave of the Tremiti Archipelago Geomorphosite (Adriatic Sea)" Geosciences 12, no. 5: 213. https://doi.org/10.3390/geosciences12050213
APA StyleCardone, F., Mazzetti, M., Sorci, A., Cesaretti, A., Cimmaruta, R., & Gravina, M. F. (2022). First Speleological and Biological Characterization of a Submerged Cave of the Tremiti Archipelago Geomorphosite (Adriatic Sea). Geosciences, 12(5), 213. https://doi.org/10.3390/geosciences12050213






