Migration of Salt Ions in Frozen Hydrate-Saturated Sediments: Temperature and Chemistry Constraints
Abstract
:1. Introduction
2. Materials and Methods
- 1.
- Sand samples were saturated with water to the desired moisture content and placed in a pressure cell for saturation with methane hydrate under vacuum.
- 2.
- The hydrate-saturated samples in the pressure cell were frozen and brought to a metastable state by reducing the pressure to 0.1 MPa.
- 3.
- The frozen hydrate-saturated samples were taken out of the pressure cell and measured for water contents, density, and fraction of water converted to hydrate (hydrate coefficient , u.f.).
- 4.
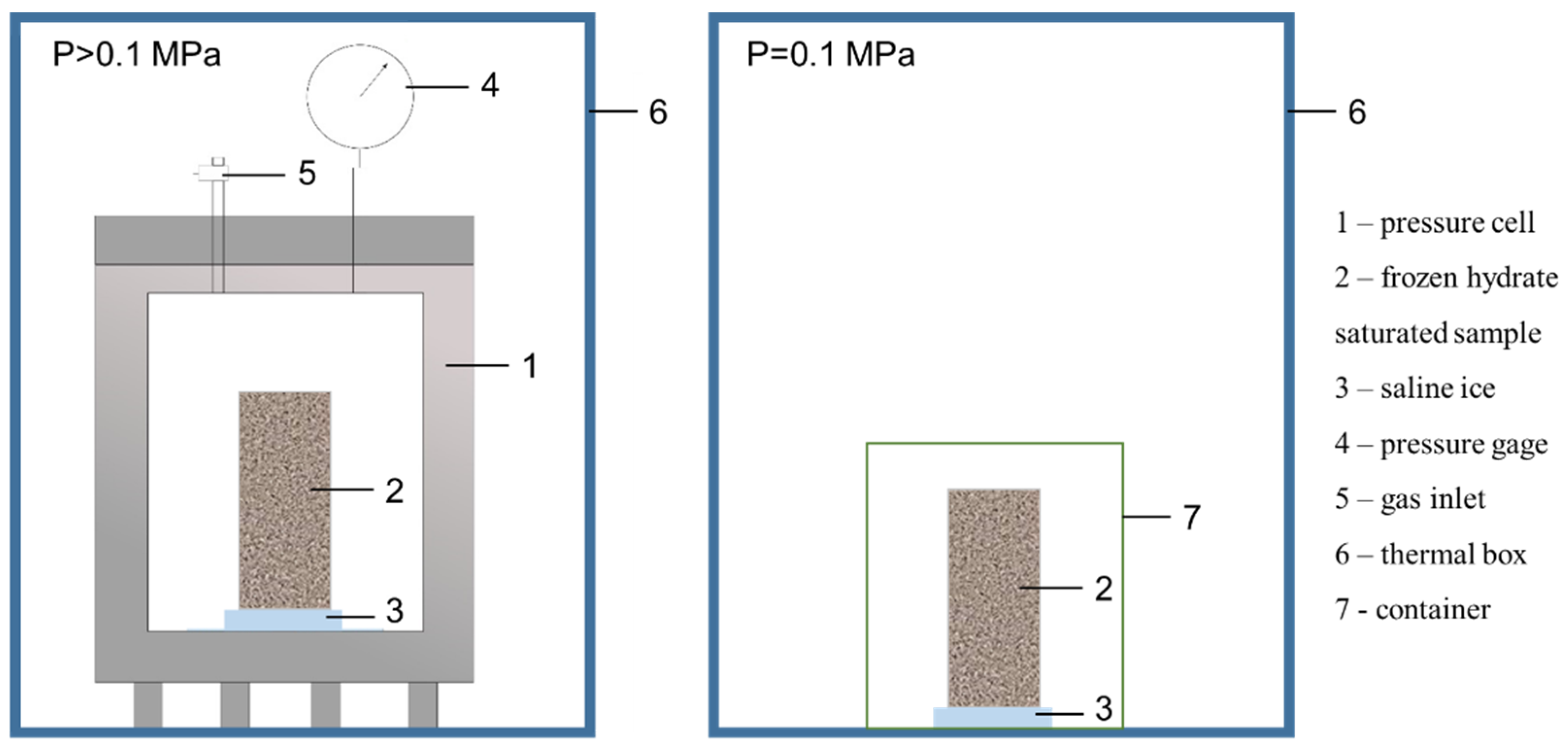
- The samples were juxtaposed against a frozen salt solution (saline ice) at a constant negative temperature and under pressures of 0.1 and 4 MPa, in a tight thermally insulated box. The interaction process was monitored continuously.
- 5.
- The samples were placed back into the pressure cell where the methane pressure increased to 4 MPa for the given temperature conditions; then, the system was placed into a thermal box (Figure 1).
3. Results
3.1. Effect of Temperature
3.2. Effect of Salt Concentration
3.3. Effect of Solution Chemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherskiy, N.V.; Groysman, A.G.; Nikitina, L.M.; Tserev, V.P. First Experimental Determination of Heats of Decomposition of Natural-Gas Hydrates. Dokl. Acad. Sci. USSR Earth Sci. Sect. 1984, 265, 163–167. (In Russian) [Google Scholar]
- Chuvilin, E.M.; Yakushev, V.S.; Perlova, E.V. Gas and Possible Gas Hydrates in the Permafrost of Bovanenkovo Gas Field, Yamal Peninsula, West Siberia. Polarforschung 2000, 68, 215–219. [Google Scholar]
- Yakushev, V.S. Natural Gas and Gas Hydrates in the Permafrost; Gazprom VNIIGAZ: Moscow, Russia, 2009; p. 192. (In Russian) [Google Scholar]
- Max, M. (Ed.) Natural Gas Hydrate in Oceanic and Permafrost Environments; Kluwer Academic Publishers: Washington, DC, USA, 2000; p. 419. ISBN 978-1-4020-1362-1. [Google Scholar] [CrossRef]
- Delisle, G. Temporal Variability of Subsea Permafrost and Gas Hydrate Occurrences as Function of Climate Change in the Laptev Sea, Siberia. Polarforschung 2000, 68, 221–225. [Google Scholar]
- Wang, J.; Lau, H.C. Thickness of Gas Hydrate Stability Zone in Permafrost and Marine Gas Hydrate Deposits: Analysis and Implications. Fuel 2020, 282, 118784. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007; p. 752. ISBN 9780429129148. [Google Scholar] [CrossRef]
- Nimblett, J.N.; Shipp, R.C.; Strijbos, F. Gas Hydrate as a Drilling Hazard: Examples from Global Deepwater Settings. In Proceedings of the Annual Offshore Technology Conference, Houston, TX, USA, 2–5 May 2005; pp. 1429–1435. [Google Scholar] [CrossRef]
- Chuvilin, E.; Ekimova, V.; Davletshina, D.; Sokolova, N.; Bukhanov, B. Evidence of Gas Emissions from Permafrost in the Russian Arctic. Geosciences 2020, 10, 383. [Google Scholar] [CrossRef]
- Chuvilin, E.; Davletshina, D. Formation and Accumulation of Pore Methane Hydrates in Permafrost: Experimental Modeling. Geosciences 2018, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Chuvilin, E.M.; Ebinuma, T.; Kamata, Y.; Uchida, T.; Takeya, S.; Nagao, J.; Narita, H. Effects of Temperature Cycling on the Phase Transition of Water in Gas-Saturated Sediments. Can. J. Phys. 2003, 81, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Chuvilin, E.M.; Yakushev, V.S.; Perlova, E.V. Experimental Study of Gas Hydrate Formation in Porous Media. In Advances in Cold-Region Thermal Engineering and Sciences; Springer: Berlin/Heidelberg, Germany, 1999; pp. 431–440. [Google Scholar] [CrossRef]
- Koh, C.A.; Sloan, E.D. Natural Gas Hydrates: Recent Advances and Challenges in Energy and Environmental Applications. AIChE J. 2007, 53, 1636–1643. [Google Scholar] [CrossRef]
- Kuhs, W.F.; Genov, G.; Staykova, D.K.; Hansen, T. Ice Perfection and Onset of Anomalous Preservation of Gas Hydrates. Phys. Chem. Chem. Phys. 2004, 6, 4917–4920. [Google Scholar] [CrossRef]
- Chuvilin, E.; Bukhanov, B.; Davletshina, D.; Grebenkin, S.; Istomin, V. Dissociation and Self-Preservation of Gas Hydrates in Permafrost. Geosciences 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Takeya, S.; Ebinuma, T.; Uchida, T.; Nagao, J.; Narita, H. Self-Preservation Effect and Dissociation Rates of CH4 Hydrate. J. Cryst. Growth 2002, 237, 379–382. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Guryeva, O.M. Experimental Study of Self-Preservation Effect of Gas Hydrates in Frozen Sediments. In Proceedings of the 9th International Conference on Permafrost, Fairbanks, AK, USA, 29 June–3 July 2008; Volume 28. [Google Scholar]
- Buffett, B.A.; Zatsepina, O.Y. Metastability of Gas Hydrate. Geophys. Res. Lett. 1999, 26, 2981–2984. [Google Scholar] [CrossRef]
- Istomin, V.A.; Yakushev, V.S.; Makhonina, N.A.; Kwon, V.G.; Chuvilin, E.M. Self-Preservation Phenomenon of Gas Hydrate. Gas Ind. 2006, 36–46. (In Russian) [Google Scholar]
- Stern, L.A.; Circone, S.; Kirby, S.H.; Durham, W.B. Temperature, Pressure, and Compositional Effects on Anomalous or “Self” Preservation of Gas Hydrates. Can. J. Phys. 2003, 81, 271–283. [Google Scholar] [CrossRef]
- Saw, V.K.; Das, B.B.; Ahmad, I.; Mandal, A.; Laik, S. Influence of Electrolytes on Methane Hydrate Formation and Dissociation. Energy Sources Part A Recovery Util. Environ. Eff. 2014, 36, 1659–1669. [Google Scholar] [CrossRef]
- Najibi, H.; Mohammadi, A.H.; Tohidi, B. Estimating the Hydrate Safety Margin in the Presence of Salt and/or Organic Inhibitor Using Freezing Point Depression Data of Aqueous Solutions. Ind. Eng. Chem. Res. 2006, 45, 4441–4446. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, W.; Liu, Y.; Xie, Y.; Chen, X. The Influence of NaCl Ions on Hydrate Structure and Thermodynamic Equilibrium Conditions of Gas Hydrates. Fluid Phase Equilibria 2012, 325, 6–10. [Google Scholar] [CrossRef]
- Kamath, V.A.; Mutalik, P.N.; Sira, J.H.; Patil, S.L. Experimental Study of Brine Injection Depressurization of Gas Hydrates Dissociation of Gas Hydrates. SPE Form. Eval. 1991, 6, 477–484. [Google Scholar] [CrossRef]
- Masoudi, R.; Tohidi, B. On Modelling Gas Hydrate Inhibition by Salts and Organic Inhibitors. J. Pet. Sci. Eng. 2010, 74, 132–137. [Google Scholar] [CrossRef]
- Wu, G.; Ji, H.; Tian, L.; Chen, D. Effects of Salt Ions on the Methane Hydrate Formation and Dissociation in the Clay Pore Water and Bulk Water. Energy Fuels 2018, 32, 12486–12494. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Lu, Y.; Kuang, Y.; Ling, Z.; Yang, L.; Dong, H.; Yang, S.; Zhao, J.; Song, Y. Molecular Dynamics Simulation and In-Situ MRI Observation of Organic Exclusion during CO2 Hydrate Growth. Chem. Phys. Lett. 2021, 764, 138287. [Google Scholar] [CrossRef]
- Chuvilin, E.; Ekimova, V.; Davletshina, D.; Bukhanov, B.; Krivokhat, E.; Shilenkov, V. Temperature Variation during Salt Migration in Frozen Hydrate-Bearing Sediments: Experimental Modeling. Geosciences 2022, 12, 261. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Lv, X.; Ge, K.; Jiang, Z.; Li, Y. Experimental Measurement and Thermodynamic Modeling of Methane Hydrate Phase Equilibria in the Presence of Chloride Salts. Chem. Eng. J. 2020, 395, 125126. [Google Scholar] [CrossRef]
- Zatsepina, O.Y.; Buffett, B.A. Thermodynamic Conditions for the Stability of Gas Hydrate in the Seafloor. J. Geophys. Res. Solid Earth 1998, 103, 24127–24139. [Google Scholar] [CrossRef]
- Dholabhai, P.D.; Englezos, P.; Kalogerakis, N.; Bishnoi, P.R. Equilibrium Conditions for Methane Hydrate Formation in Aqueous Mixed Electrolyte Solutions. Can. J. Chem. Eng. 1991, 69, 800–805. [Google Scholar] [CrossRef]
- Dickens, G.R.; Quinby-Hunt, M.S. Methane Hydrate Stability in Seawater. Geophys. Res. Lett. 1994, 21, 2115–2118. [Google Scholar] [CrossRef]
- Cha, M.; Hu, Y.; Sum, A.K. Methane Hydrate Phase Equilibria for Systems Containing NaCl, KCl, and NH4Cl. Fluid Phase Equilibria 2016, 413, 2–9. [Google Scholar] [CrossRef]
- Alekseev, S. Cryogenesis of Groundwater and Rocks (on the Example of the Daldino-Alakitsky Region of Western Yakutia); SRC OIGGM SO: Novosibirsk, Russia, 2000; p. 119. (In Russian) [Google Scholar]
- Chuvilin, E.M.; Ershov, E.D.; Smirnova, O.G. Ionic Migration in Frozen Soils and Ice. In Proceedings of the 7th International Permafrost Conference, Yellowknife, NT, Canada, 23–27 June 1998; pp. 167–171. [Google Scholar]
- Ershov, E.D.; She, Z.S.; Lebedenko, Y.; Chuvilin, E.M.; Kryuchkov, K.Y. Mass Transfer and Deformation Processes in Frozen Rocks Interacting with Aqueous Salt Solutions. In Engineering-Geological Study and Evaluation of Frozen, Freezing and Thawing Soils (IGK-92). Materials of the III Scientific and Technical Workshop; Vedeneev VNIIG: St Petersburg, Russia, 1993; pp. 67–77. (In Russian) [Google Scholar]
- Ershov, E.D.; Chuvilin, E.N.; Smirnova, O.G. Mobility of Ions of Chemical Elements in Ice and Frozen Rocks. Dokl. Akad. Nauk 1999, 367, 796–798. (In Russian) [Google Scholar]
- Borisov, V.; Alekseev, S. Factors of Interaction of Brines with Ice (Frozen Rock) at a Negative Temperature. In Fundamental Problems of Water and Water Resources at the Turn of the III Millennium; NTL: Tomsk, Russia, 2000; pp. 584–589. (In Russian) [Google Scholar]
- Gaidaenko, E.P. Solubility of Ice in Frozen Soils under the Influence of Saline Solutions. In Problems of Engineering Glaciology; Nauka: Novosibirsk, Russia, 1986; pp. 32–36. (In Russian) [Google Scholar]
- Ershov, E.D. (Ed.) Fundamentals of Geocryology. Part 1. Physical and Chemical Foundations of Geocryology; MSU: Moscow, Russia, 1995; p. 368. ISBN 5-211-02464-8. (In Russian) [Google Scholar]
- Chuvilin, E.M.; Davletshina, D.A.; Lupachik, M.V. Hydrate Formation in Frozen and Thawing Methane-Saturated Sediments. Earth’s Cryosphere 2019, 23, 44–52. [Google Scholar] [CrossRef]
- Chuvilin, E.; Ekimova, V.; Bukhanov, B.; Grebenkin, S.; Shakhova, N.; Semiletov, I. Role of Salt Migration in Destabilization of Intra Permafrost Hydrates in the Arctic Shelf: Experimental Modeling. Geosciences 2019, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chen, Z.; Liu, J.; Sun, Z.; Wang, X.; Zhang, J. A Molecular Dynamic Study on the Dissociation Mechanism of SI Methane Hydrate in Inorganic Salt Aqueous Solutions. J. Mol. Graph. Model. 2017, 75, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Li, X.; Negahban, S. Phase Boundary of Gas Hydrates in Single and Mixed Electrolyte Solutions: Using a Novel Unified Equation of State. J. Mol. Liq. 2022, 345, 117825. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Kozlova, E.V.; Skolotneva, T.S. Experimental Simulation of Frozen Hydrate-Containing Sediments Formation. In Proceedings of the Fifth International Conference on Gas Hydrates, Trondheim, Norway, 13–16 June 2005; pp. 1561–1567. [Google Scholar]
- Yang, L.; Falenty, A.; Chaouachi, M.; Haberthür, D.; Kuhs, W.F. Synchrotron X-ray Computed Microtomography Study on Gas Hydrate Decomposition in a Sedimentary Matrix. Geochem. Geophys. Geosyst. 2016, 17, 3717–3732. [Google Scholar] [CrossRef] [Green Version]
- Sergeeva, D.; Istomin, V.; Chuvilin, E.; Bukhanov, B.; Sokolova, N. Influence of Hydrate-Forming Gas Pressure on Equilibrium Pore Water Content in Soils. Energies 2021, 14, 1841. [Google Scholar] [CrossRef]
- Lebedenko, Y.P. Cryogenic Migration of Ions and Bound Moisture in Ice-Saturated Frozen Rocks. Eng. Geol. 1989, 4, 21–30. (In Russian) [Google Scholar]
- Benediktova, N.A. Physical and Chemical Processes in Frozen Rocks during Their Interaction with Saline Solutions; Moscow State University: Moscow, Russia, 1992. (In Russian) [Google Scholar]

















| Sample | Particle Size Distribution, % | Mineralogy * | |||||
|---|---|---|---|---|---|---|---|
| 1–0.5 | 0.5–0.25 | 0.25–0.1 | 0.1–0.05 | 0.05–0.001 | <0.001 | ||
| Fine Sand | >0.1 | 1.5 | 80.4 | 17.3 | 0.8 | >0.1 | >98% quartz |
| Soil Type | Anions, mg-EQ/100 g | Cations, mg-EQ/100 g | TDS, % | |||||
|---|---|---|---|---|---|---|---|---|
| pH | HCO3− | Cl− | SO42− | Ca2+ | Mg2+ | Na+ + K+ | ||
| Sand | 7.1 | 0.075 | 0.025 | 0.06 | 0.025 | - | 0.135 | 0.01 |
| Salt | mg-EQ/100 g | |
|---|---|---|
| MgCl2 | 3.23 | 0.08 |
| NaCl | 4.89 | 0.11 |
| CaCl2 | 3.29 | 0.13 |
| KCl | 3.90 | 0.15 |
| Na2SO4 | 3.50 | 0.16 |
| Stages | Gas pressure | ||
| P ≥ Peq | P < Peq | ||
| Initial state (Cin = 0%) |  |  |  |
| Ccrhyd ≥ C > Cin |  |  | |
| Ccrhyd < C ≥ Ccr ice |  |  | |
| Ccrhyd < C > Ccr ice |  |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuvilin, E.; Ekimova, V.; Davletshina, D.; Bukhanov, B.; Krivokhat, E.; Shilenkov, V. Migration of Salt Ions in Frozen Hydrate-Saturated Sediments: Temperature and Chemistry Constraints. Geosciences 2022, 12, 276. https://doi.org/10.3390/geosciences12070276
Chuvilin E, Ekimova V, Davletshina D, Bukhanov B, Krivokhat E, Shilenkov V. Migration of Salt Ions in Frozen Hydrate-Saturated Sediments: Temperature and Chemistry Constraints. Geosciences. 2022; 12(7):276. https://doi.org/10.3390/geosciences12070276
Chicago/Turabian StyleChuvilin, Evgeny, Valentina Ekimova, Dinara Davletshina, Boris Bukhanov, Ekaterina Krivokhat, and Vladimir Shilenkov. 2022. "Migration of Salt Ions in Frozen Hydrate-Saturated Sediments: Temperature and Chemistry Constraints" Geosciences 12, no. 7: 276. https://doi.org/10.3390/geosciences12070276
APA StyleChuvilin, E., Ekimova, V., Davletshina, D., Bukhanov, B., Krivokhat, E., & Shilenkov, V. (2022). Migration of Salt Ions in Frozen Hydrate-Saturated Sediments: Temperature and Chemistry Constraints. Geosciences, 12(7), 276. https://doi.org/10.3390/geosciences12070276









