Evaluating the Effectiveness of Nanotechnology in Environmental Remediation of a Highly Metal-Contaminated Area—Minas Gerais, Brazil
Abstract
:1. Introduction
2. Geographical and Geological Characterization
3. Materials and Methods
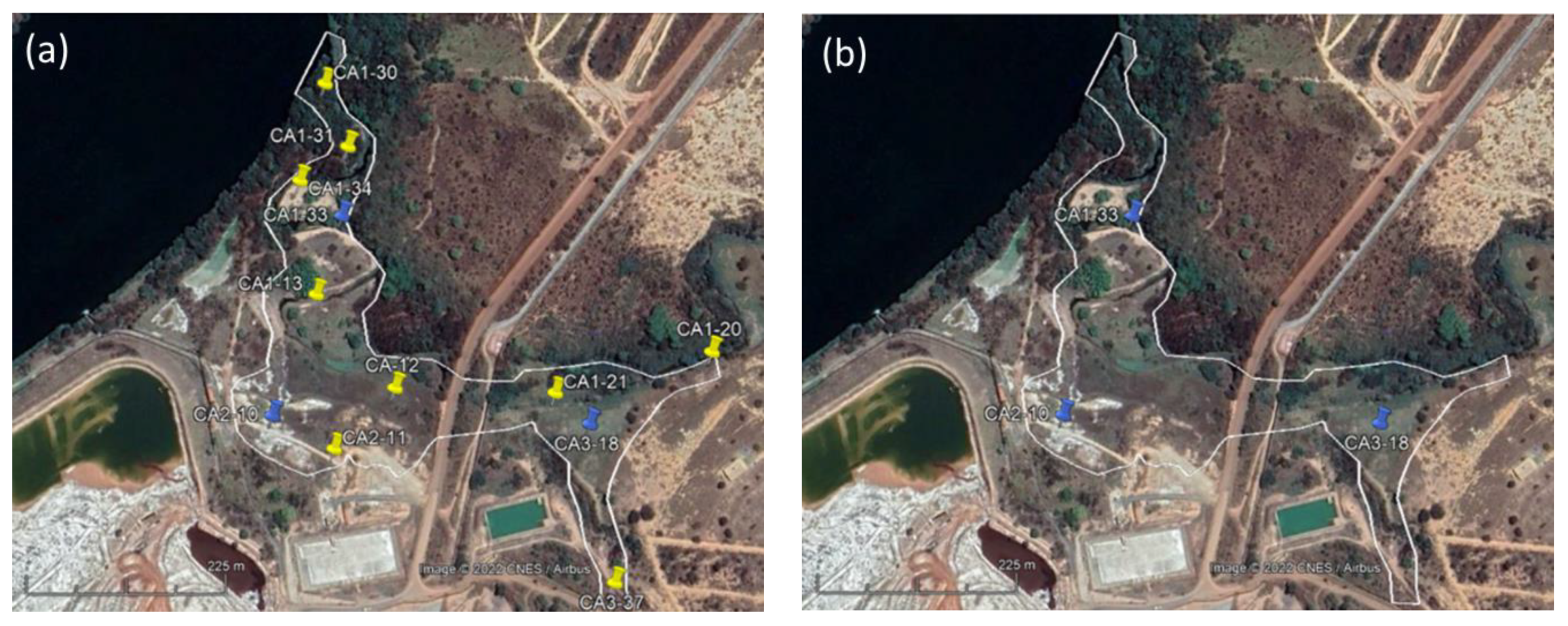
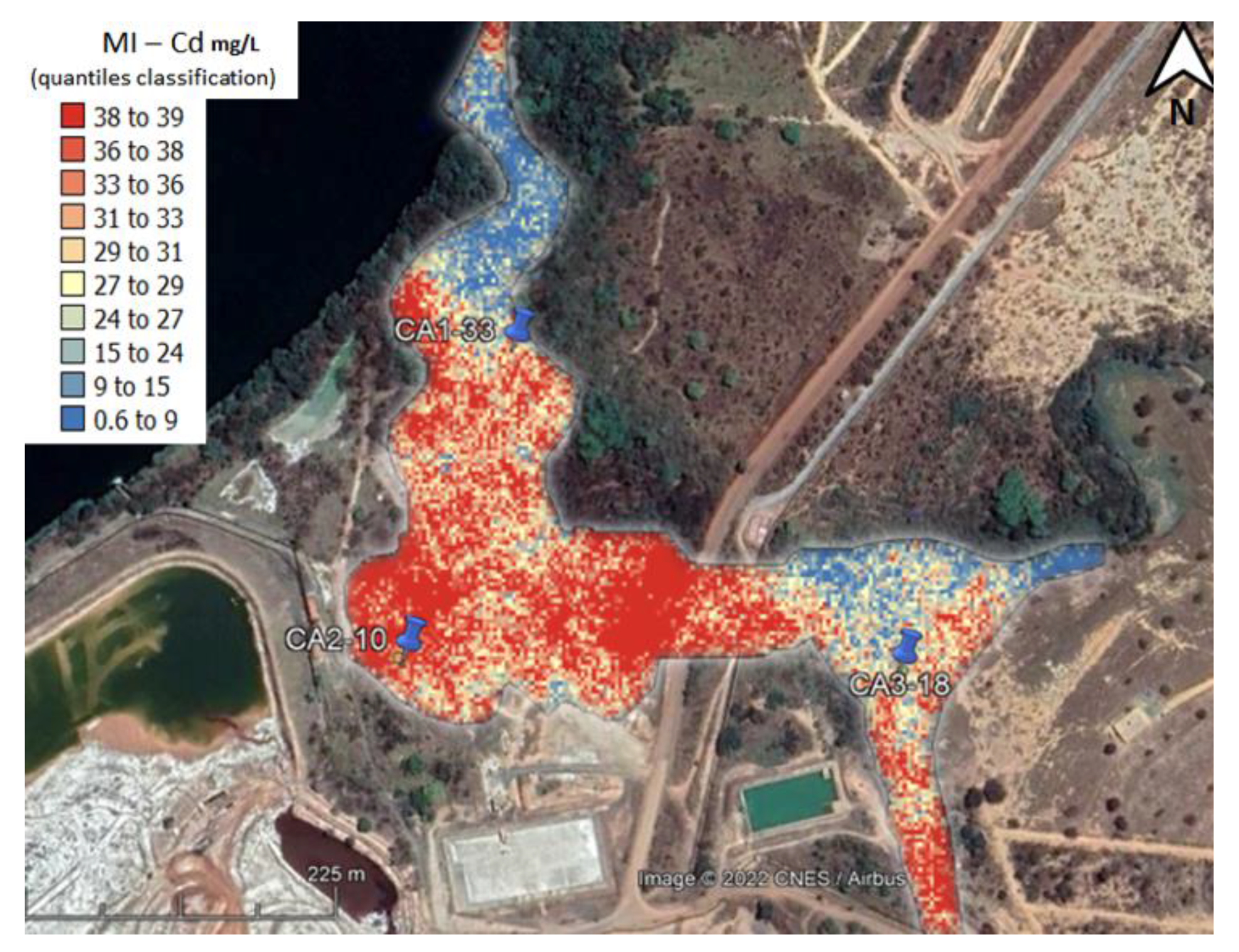
3.1. Samples Selected for This Study
3.2. Solution of Zero-Valent Iron Nanoparticles (nZVI)
3.3. Procedure and Implementation of the Column Laboratory Test
- Blank—Deionized water
- Nanoparticles with 1 g/L concentration
- Nanoparticles with 3 g/L concentration
- Nanoparticles with 7 g/L concentration
- 1st—Before the injection of the solution of nZVI;
- 2nd—After 24 h;
- 3rd—After 48 h;
- 4th—After 72 h;
- 5th—After 1 week;
- 6th—After 2 weeks;
- 7th—After 1 month;
- 8th—After 2 months;
- 9th—After 4 months.
4. Results
4.1. Geochemical Characterization of the Samples Used for Testing nZVI at a Laboratory Scale, and Evaluation of the Contamination Degree of the Industrial Area
4.2. Data Obtained in the Laboratory-Scale Test of Injection of Nanoparticles of Zero-Valent Iron (nZVI)
4.2.1. General Remarks
4.2.2. Variation of the Physic-Chemical Parameters (pH, Redox Potential, Conductivity)
- pH:
- Redox Potential:
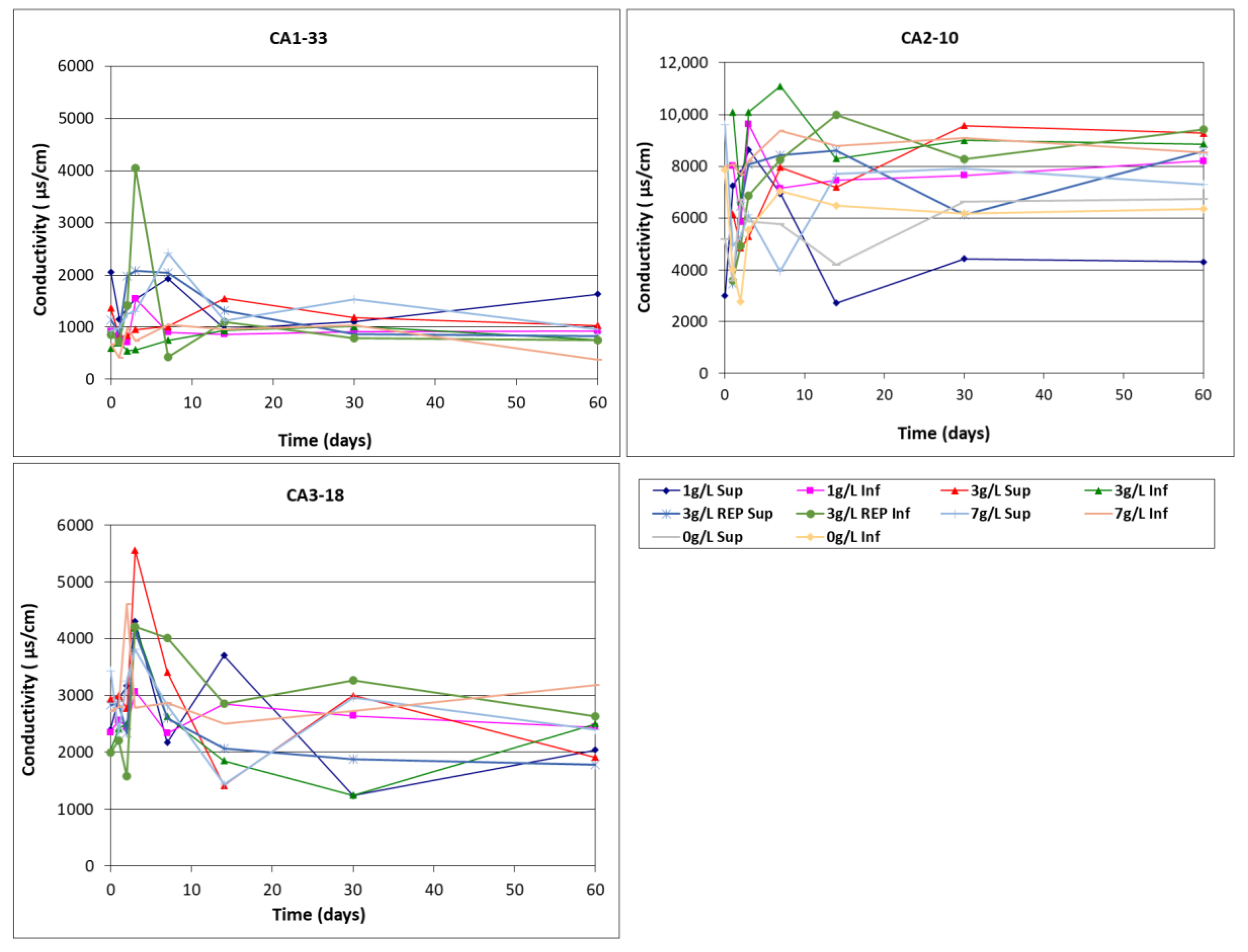
- Conductivity
4.2.3. Variation of the Immobilization Rate of the Target Contaminants
- pH Effect
- Effect of Suspension Concentration and Injection Time of nZVI
- (1)
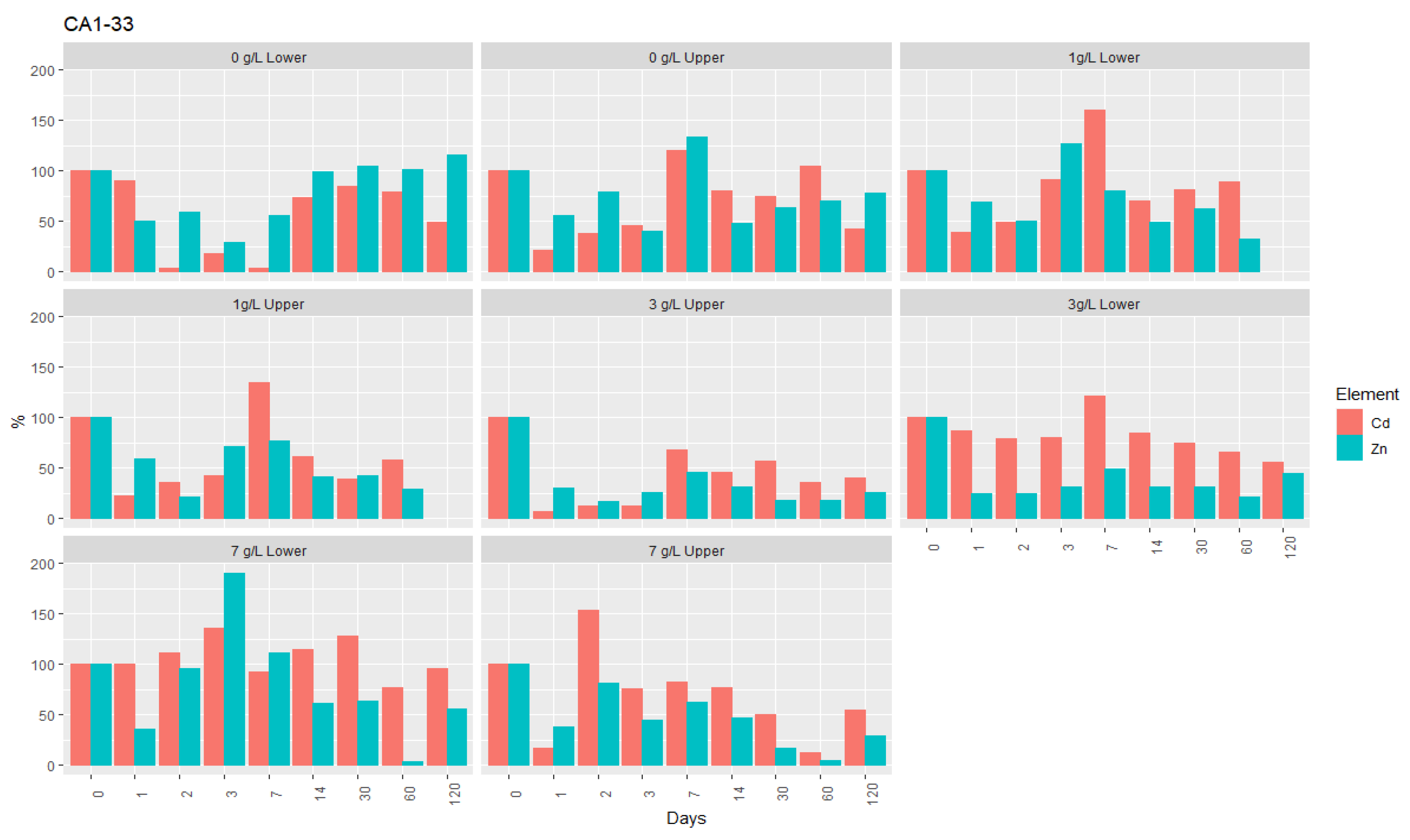
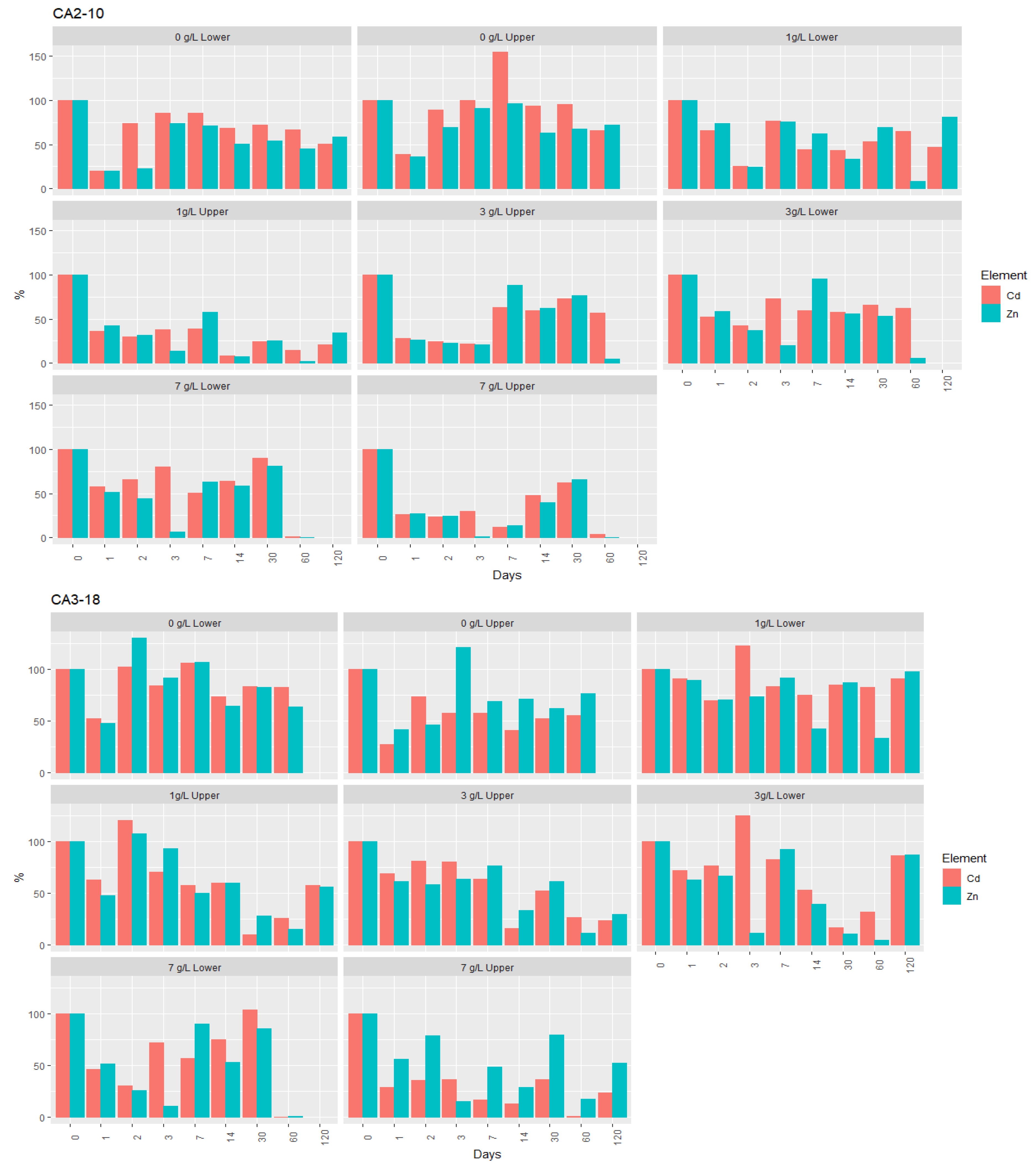
- Regarding the reaction with nZVI, there is high similarity between zinc and cadmium (Figure 6); however, after 60 days, the average removal of Zn was higher than for Cd. The removal of both elements in the interstitial water of the samples over the 120 days of testing can be summarized as follows:
- (i)
- After 60 days, the removal rate of Zn varied between 80% and 100%, and for Cd between 50% and 80%, with the last element corresponding to levels ranging from 0.6 mg/L to 3 mg/L. For Zn, for the higher nZVI concentrations, the average removal was approximately 99% (7 g/L) and 95% (3 g/L), with the corresponding concentrations ranging from 0.5 and 2.0 mg/L. Although with a significant reduction in relation to the initial contents, the highest removal rates of both elements from the soluble phase of the samples were not enough to keep their concentrations below the values tabulated for groundwater by the Legislation for the State of Minas Gerais, which is 1.05 mg/L for Zn and 0.005 mg/L for Cd [53].
- (ii)
- After 120 days, an increase of the levels of these two elements in the interstitial water of the samples was observed, particularly significant in CA2-10 for Zn (removal rates between 2% and 48%), which is the sample with the highest contamination index regarding these elements, the highest sulphate content, and the lowest permeability. However, when comparing the initial values before the injection of nZVI with the final values obtained in a composite sample, a marked decrease in both elements is perceptible in all the samples (Table 4).
- (2)
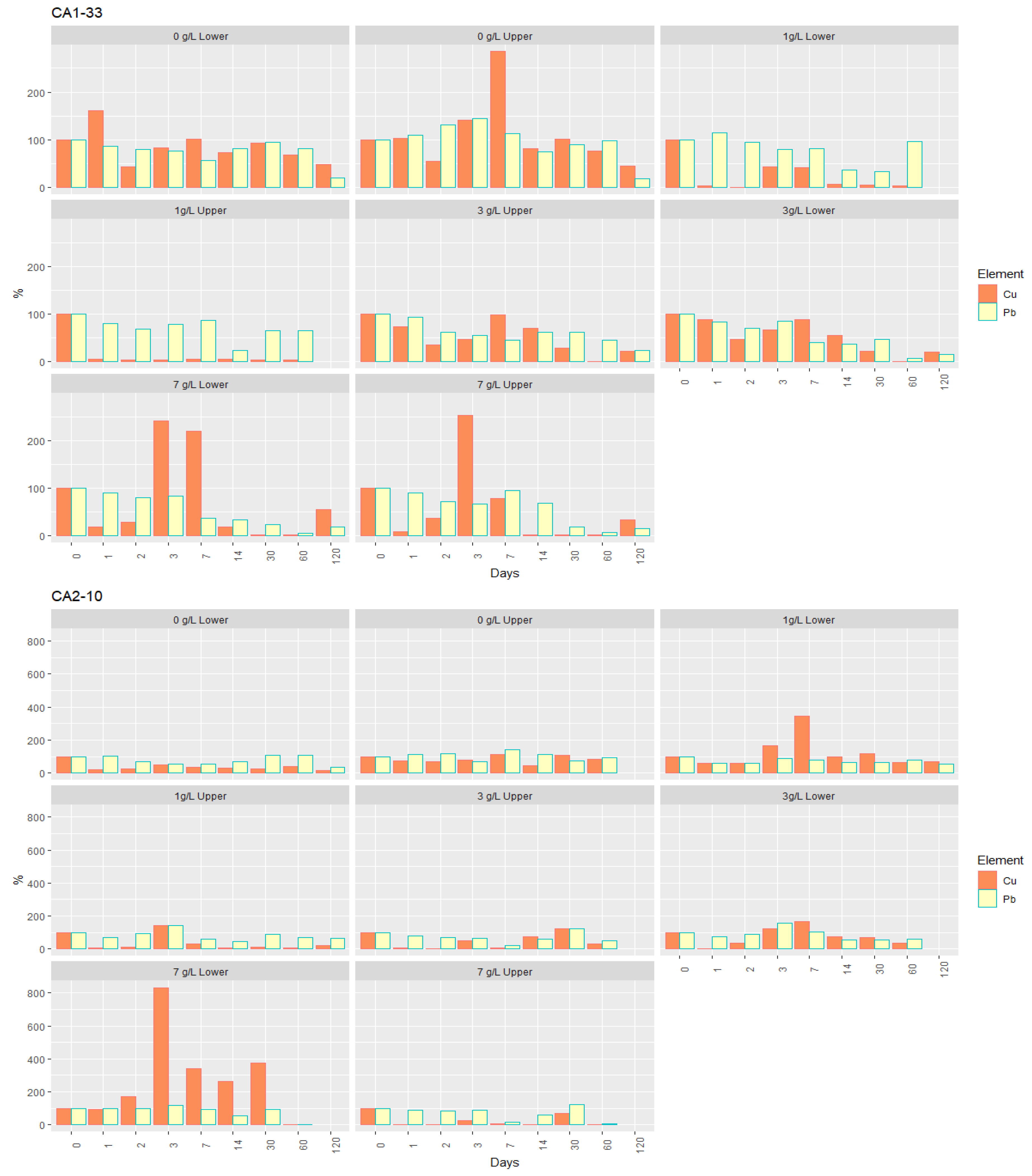
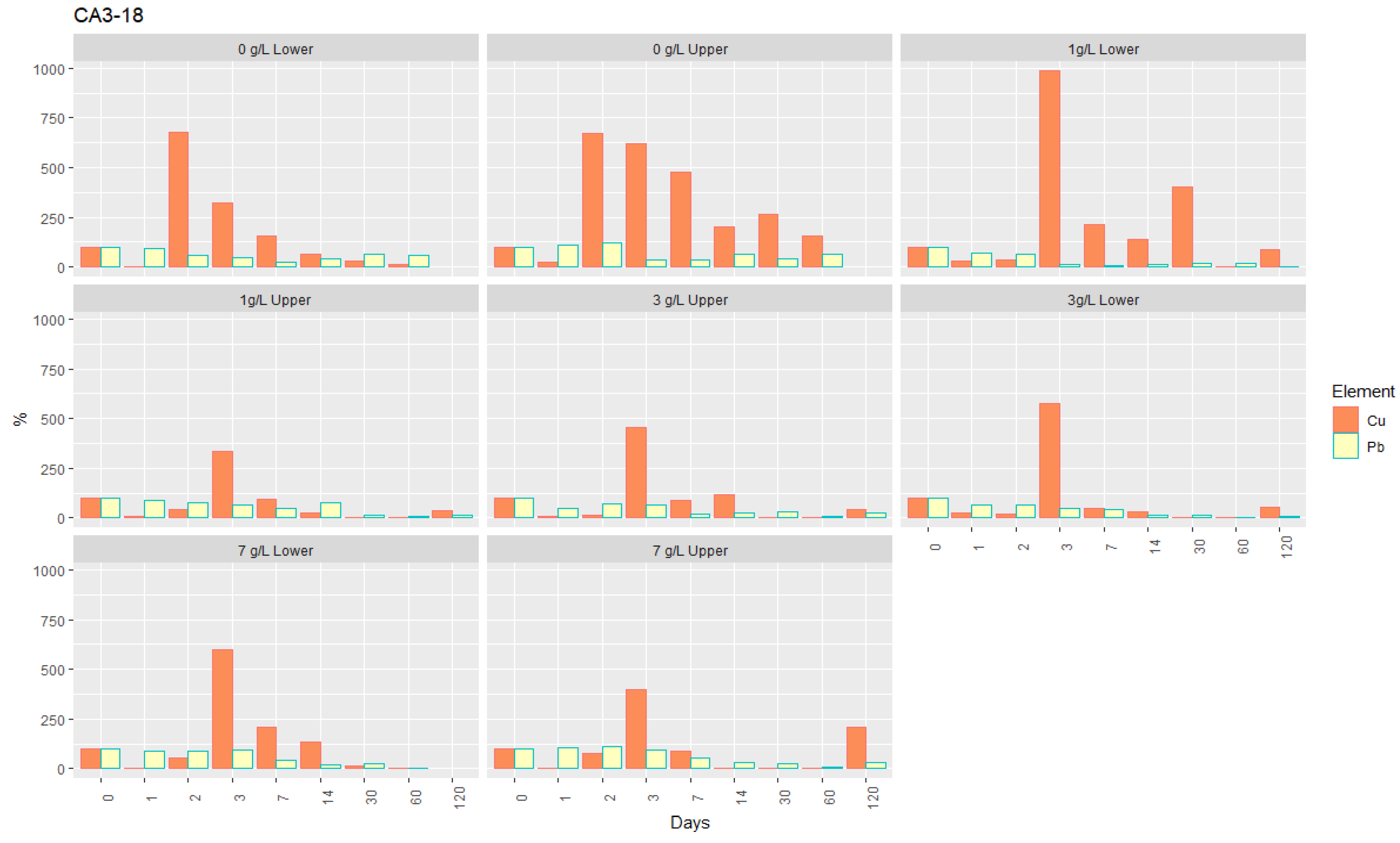
- For lead, the retention rate was directly proportional to the concentration of nZVI suspensions, and the suspensions with higher concentrations proved to be very efficient in its removal, in some samples, even after 120 days (Figure 7). This is the case of soil CA1-33, which, at the end of the test, showed a removal rate of 80–85%, corresponding to a concentration of 0.05–0.07 mg/L of Pb, albeit still higher than the regulated value of 0.01 mg/L [39]. Although with a maximum immobilization of 90% in the alluvium sample CA2-10, Pb decreased to about 0.08 mg/L. Only in sample CA3-18, values below the regulated value were reached, given the removal of almost 100% after 60 days, corresponding to a concentration of 0.001 mg/L of Pb. After this period, there was, however, a slight increase.
- (3)
- Copper is the element with the most distinct behavior regarding its retention by nZVI (Figure 7): (1) all the tests showed effective removal; and (2) contrary to the other elements, there is no proportionality between the retention rate and the suspensions concentration. In all the samples and for all the nZVI concentrations, the retention rate was always high, and the temporal evolution and behavior of Cu were very similar. After 60 days of the injection and at the final stage of the test (120 days), Cu concentrations in the interstitial solution were always lower than the regulated value of 2.00 mg/L [53]. The retention rates were very similar in the soil CA1-33 (70–95%) and in the alluvium CA2-10 (80–90%), reaching 99% in CA3-18. The minimum concentrations attained after 60 days corresponded to 0.02 and 0.04 mg/L, 0.1 and 0.3 mg/L, and 0.001 mg/L, respectively. In the case of CA3-18, given the high efficiency of the Cu removal by applying nZVI, the final values were very similar for any concentration of the suspension injected.
- (4)
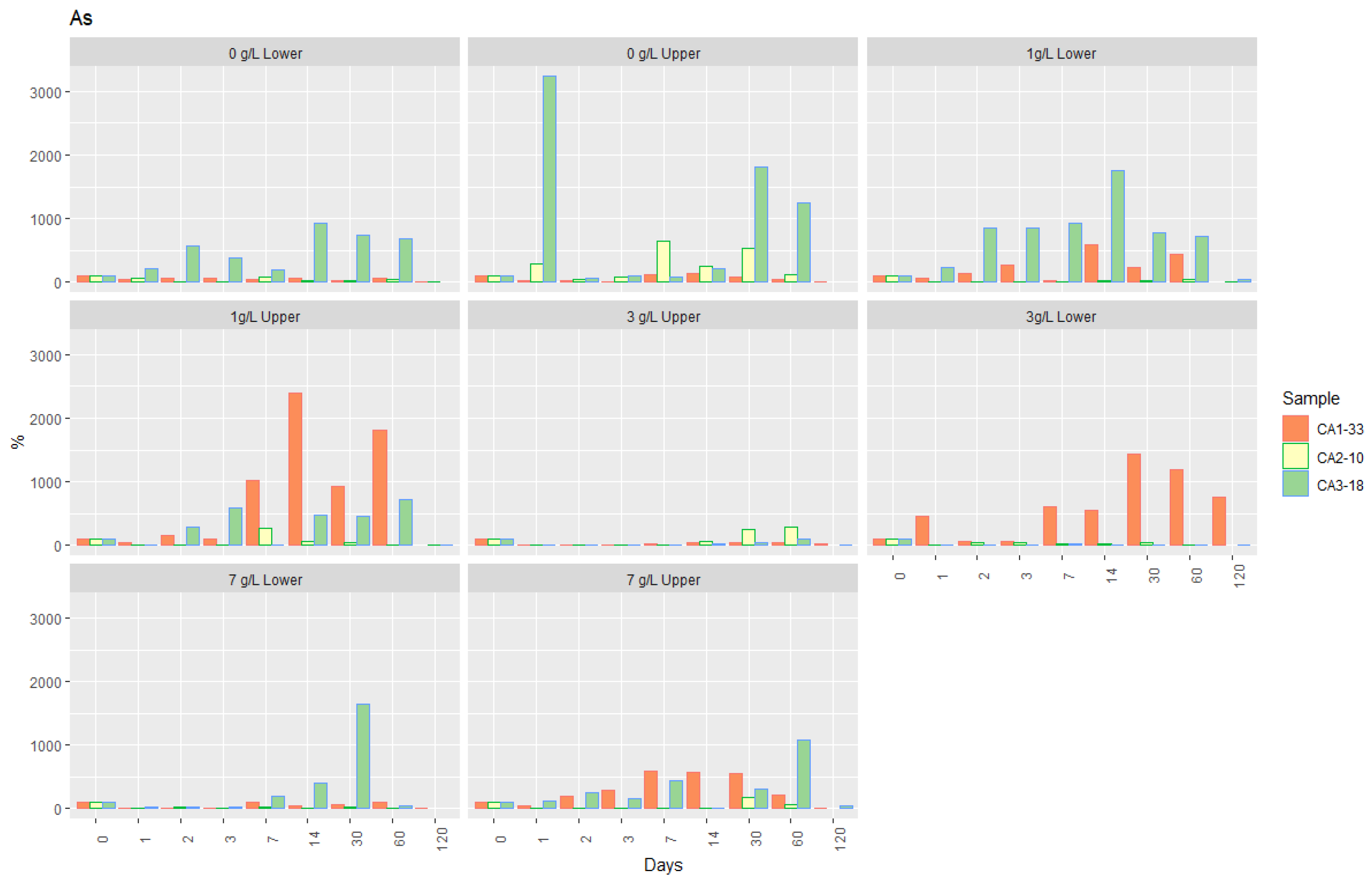
- Although with critical values in the sedimentary materials and soil arsenic contents in the aqueous phase were very low, some of them slightly higher than the limits proposed by Brazilian legislation for groundwater [53].
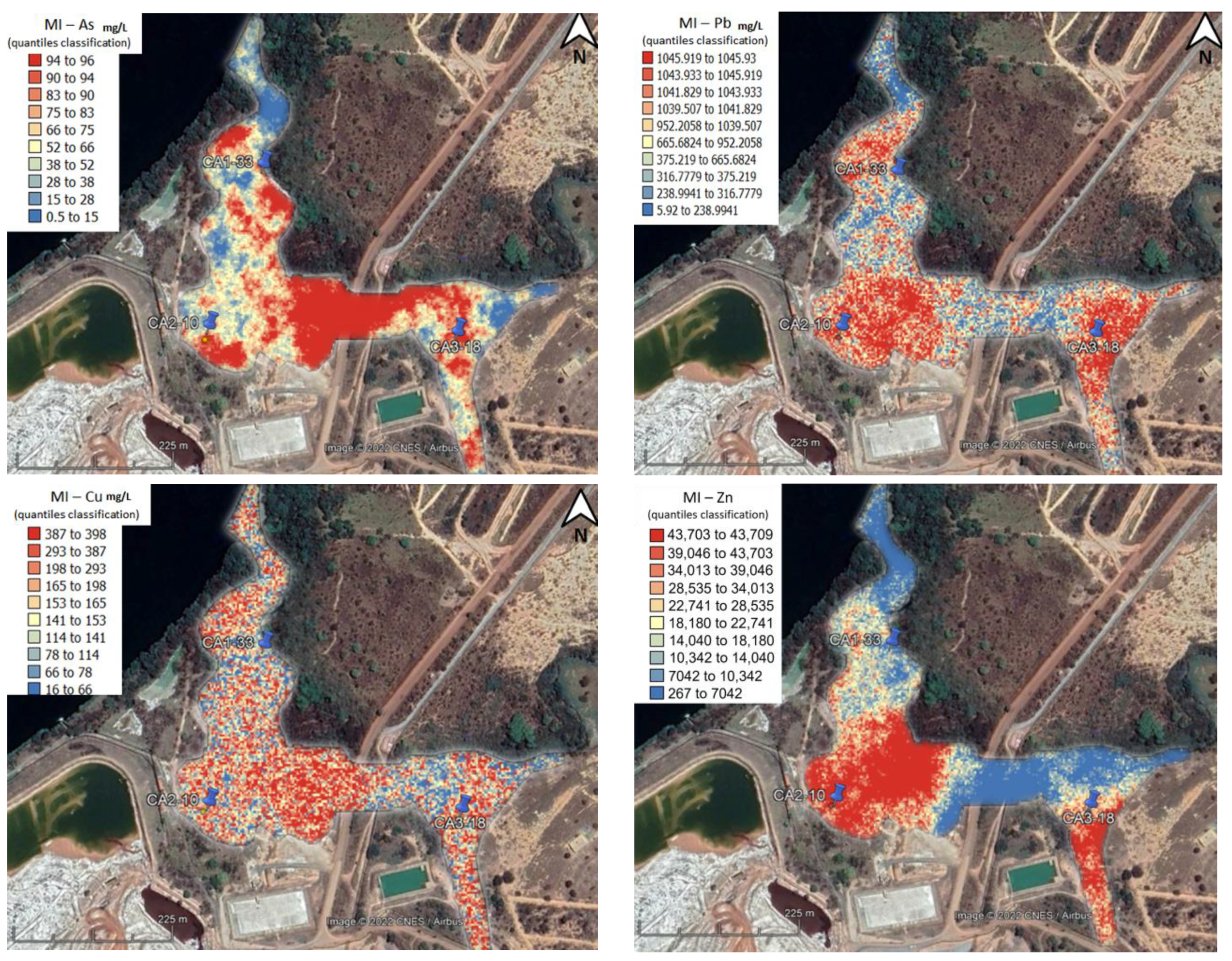
4.3. Test Data Evaluation—Risk Spatial Projection
5. Discussion
5.1. Effect of a Laboratory-Scale Injection of nZVI Solutions into Heavily Metal-Contaminated Materials from a Tropical Climate
- (1)
- In this experiment a drought situation was simulated, since the system was closed, and the extracted water was not replaced. The interstitial water became more concentrated over time and the material was always moist; however, there was no simulation of water loss by infiltration and water replacement by the rain effect. The most extreme situation of a closed system was recreated, and thus, the concentrations of metals in soluble phase reached the highest values. If there is a marked decrease of the metal concentrations over time after nanoparticles application, this decrease should have greater expression at a real scale. Since not all the conditions of a real situation could be reproduced, the achieved data cannot be considered as absolute.
- (2)
- The extractions of the interstitial water were always conducted in the same points of the sedimentary columns, in an upper and lower position; thus, the values obtained for the different metals do not reflect the whole column and they have to be considered as point sample values; this consideration results from the fact that aggregation and nanoparticles nuclei was verified in several areas of the columns and the diffusion of the particles through the soil and alluvial sediments was not uniform, due, mainly, to their low permeability. Models of aggregation of small particles have been published in many works and in most of them it is mentioned that a surface charge established on the surface of particles causes repulsive electrostatic forces between them. However, the iron particles corrode in the water, and this process can produce changes on the surface charge and on the aggregation rate [62]. Because particles are made from iron, they also have magnetic properties, which significantly affects the aggregation rate [28,63,64]. As a result of aggregate formation, the specific surface area will also decrease, resulting in a reactivity decrease [65].
- (3)
- These two factors led to the development of distinct chemical environments in the same sample, which certainly conditioned different retention reactions and rates of the various metals. Thus, the irregularities verified for pH, redox, and element concentrations in the upper and lower layers of the columns corroborate the occurrence of different removal and solubilization reactions with distinct behaviors and intensities throughout the sample. The poor diffusion of the suspension may have been due, apart from the low permeability of the material, to the injection speed, which was possibly too high. However, since the injection was made in an upward direction, a lower speed could cause sedimentation of the particles in the peristaltic pump tubes.
- (4)
- The data that will best reflect the real situation of metals immobilization correspond to the analysis of the composite porewater (Table 4) and levels of the target metals analyzed in digested samples, resulting from the homogenization of all the material in each column. However, these could only be carried out before the injection (t = 0) and in the final phase of the test (t = 120), when the soil and alluvium were removed from the columns after 4 months of the nanoparticles’ injection.
5.2. Factors Affecting the Variation of Physic-Chemical Parameters over the Batch Column Experiment
- (1)
- Chemical composition characterized by high levels of iron oxides (Fe2O3: 16–52%);
- (2)
- (3)
- Excess of metals in solution in a confined environment, where no infiltration, diffusion, and leaching of soluble ions could occur, and where permanent water saturation might decrease oxidation conditions at an early stage. After 3 days, the ZVI might have reacted directly with the metallic elements in solution in cationic form, leading to H+ release, with subsequent pH decrease and dissolution of some iron oxides, with high contents in these materials. This may also justify the increase of oxidation conditions, as can be demonstrated by Equation (2):
- (4)
- In the presence of some metals, such as Pb, with high contents in these sedimentary materials, the reduction-oxidation reaction with the nZVI particles can lead to the formation of H+, which may lower the pH (Equation (3)):
5.3. Removal Mechanisms of Contaminants by nZVI
- Metals that have an E0 that is more negative than, or similar to, that of Fe0 (e.g., Cd and Zn) are removed from solution by adsorption onto the iron oxide (hydroxide) layer surrounding the zero-valence iron (Fe0) core. Upon binding to the FeOOH layer, these metals bond through electrostatic interactions without undergoing changes in their valence state. To a lesser extent, complexation at the surface of the nanoparticles and co-precipitation may also occur.
- Metals with an E0 much more positive than Fe0 (e.g., As and Cu) are preferentially removed by precipitation and layer-mediated reduction (reductive precipitation) on the surface of nZVI [45].
- Metals with E0 slightly more positive than Fe0 (e.g., Pb) can be removed by both adsorption and partial chemical reduction.
- Reduction—As, Cu, and Pb.
- Adsorption—As, Pb, Cd, and Zn.
- Oxidation/reoxidation—As and Pb.
- Co-precipitation—As.
- Precipitation—Cu, Pb, Cd, and Zn.
- Immobilization of zinc and cadmium
- (1)
- Extremely high contents, much higher than the toxicity limits.
- (2)
- The high levels of these two elements in soluble forms may influence the retention capacity of nZVI, through competition between the two cations for chemo-adsorption sites in the (oxy)iron hydroxide layer formed on the nanoparticles surface. It has been observed by several authors [17] that, in the presence of these two cations and in view of the competitiveness between both, there is a more efficient removal and selectivity of nZVI particles for Zn2+ than for Cd2+.
- (3)
- Low pH values that decreased after the first day of the test and remained low until about 60 days after injection. Low pH accelerates the corrosion and the dissolution of the oxide layer of the nZVI, increasing the reaction rates due to greater availability of electrons from the Fe0 core [56]. Therefore, the general decrease of pH after 2 days of injection may have contributed to intense reactions that may have rapidly immobilized metallic cations by adsorption onto the iron oxide (hydroxide) layer surrounding the zero-valence iron (Fe0) core. However, the impact of pH on metal removal by nZVI depends on the oxidation state of the metal and the removal mechanism [56]. For Zn and Cd, besides the very high values in solution, both cations are easily mobilized at pH < 5.5 [70]. Thus, although a very significant immobilization of most metals was observed, the concentrations of Zn and Cd, with retention rates ranging from 80–100% for Zn and 50–80% for Cd, were not able to reach the legislated values. The high reaction rates due to the low pH values are likely to have been one of the factors responsible for the decreased reactivity of nZVI after 2 months.
- (4)
- Presence of high levels of sulphates, which is also an inhibiting factor for Zn and Cd retention, since it is a competitive anion for receiving electrons transferred by the nZVI [56]. Sulphates, in contact with nZVI, may be reduced to sulphides, which precipitate with metal cations on the surface of nanoparticles, reducing their adsorption capacity.
- Immobilization of Lead
- Immobilization of Copper
- Immobilization of Arsenic
5.4. Aging Time of nZVI
5.5. Reduction of the Risk Level of Sediments and Soils after nZVI Injection
6. Conclusions
- This was a laboratory batch study where it was not possible to simulate the drainage, diffusion, and precipitation conditions that naturally occur. This corresponded to a very complex and closed system, tested under extreme conditions, simulating a prolonged period of drought, in which there was no replacement of water during the entire test; thus, the interstitial water of the materials became progressively more concentrated.
- This experiment reflected the real situation of the surrounding area of a metallurgical plant, where most of the soils, alluvium, and river sediments showed very high concentrations of heavy metals and sulphates of anthropic origin, including high levels of lithogenic iron and manganese. The contaminant metals include elements with different standard redox potentials (E0) relative to that of Fe0, which led to multiple retention mechanisms, namely, adsorption, desorption, reduction, oxidation, complexation, and co-precipitation. This high diversity under soluble phase decreased the reactivity of the nZVI particles, probably by competition among the various cations for the exchange sites or, in the case of anionic complexes such as sulphates, by reduction and transformation into sulphides that may be precipitated together with metallic cations on the surface of the nanoparticles.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fonseca, R.; Pinho, C.; Albuquerque, T.; Araújo, J. Environmental Factors and Metal Mobilisation in Alluvial Sediments—Minas Gerais, Brazil. Geosci. J. 2021, 11, 110. [Google Scholar] [CrossRef]
- Votorantim Metais. Zoneamento da Distribuição da Contaminação de Sedimentos do Leito Submerso do Rio São Francisco; Golder Associates Brasil Consultoria e Projetos LTDA, Belo Horizonte, Brazil, RT-079-515-6012-0015-00-J; Final Report to Votorantim Metais Zinco S/A: Três Marias, Brazil, 2007. [Google Scholar]
- Votorantim Metais. Available online: http://www.vmetais.com.br/pt-BR/Paginas/default.aspx (accessed on 10 December 2013).
- Abreu, C.B.; Martins, A.H. Recuperação de sulfato de cálcio a partir do resíduo gerado no processamento de zinco primário. Estud. Technol. 2009, 5, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.F. Perfil do Minério de Zinco. Ministério de Minas e Geologia, 2010. (Technical Report N°25). Available online: www.mme.gov.br/sgm/galerias/arquivos/plano_duo_decenal/a_mineracao_brasileira/P16_RT25_Perfil_do_Minério_de_Zinco.pdf (accessed on 3 September 2014).
- Oliveira, M.A.; Horn, A.H. Comparação da Concentração de Metais Pesados nas Águas do rio São Francisco em Três Marias, desde 1991 até hoje, relacionando a atuação da CMM-Três Marias. Geonomos 2006, 14, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, R.; Araújo, A.; Martins, L.; Dias, N.; Gomes, C.; Carneiro, J.; Cavacundo, O.; Borges, J.; Caldeira, B.; Costa, I.; et al. Relatório Final do Projeto de Consultoria “Estratégias de Remediação dos Córregos Consciência e Barreiro Grande– 2ª fase; University of Évora, Évora, Portugal; Empresa Votorantim Metais, Inc.: Três Marias, Brazil, 2015; p. 587. [Google Scholar]
- Fonseca, R.; Araújo, A.; Martins, L.; Dias, N.; Pinho, C.; Matos, J. Environmental impact assessment of a metal alloy production plant on the water quality of a tributary of the largest Brazilian river (São Francisco River). In Proceedings of the From DGT Research to Environmental Assessment, San Sebastian, Spain, 28 September–1 October 2015. [Google Scholar]
- Ribeiro da Costa, I.; Fonseca, R.; Pinho, C.; Araújo, A.; Martins, L.C.; Dias, N.; Janeiro, A.I.; Freitas, G. Contaminated soils and sediments associated with Zn ore metallurgy near the São Francisco River, Minas Gerais (Brazil). Environ. Earth Sci. 2018, 77, 202. [Google Scholar] [CrossRef]
- Pinho, C.; Fonseca, R.; Carneiro, J.; Araújo, A. Assessment of the Environmental Risk of a Floodplain Contaminated by Metals Based on Different Indices and Environmental Classification Factors, Minas Gerais, Brazil. In Mine Water Solutions; Pope, J., Wolkersdorfer, C., Sartz, L., Weber, A., Wolkersdorfer, K., Eds.; IMWA: Lakewood, CO, USA, 2020; pp. 63–69. [Google Scholar]
- Pinho, C.; Fonseca, R.; Carneiro, J.; Araújo, A. Evaluation of the environmental risks of contaminated materials: An advice on the most appropriate environmental remediation techniques. Geosci. J. 2021, 11, 164. [Google Scholar] [CrossRef]
- Fonseca, R.; Palma, C. Problemas ambientais relacionados com a dragagem de sedimentos poluídos. In Proceedings of the 5ª Jornadas de Engenharia Hidrográfica, Instituto Hidrográfico, Lisboa, Portugal, 19–21 June 2018. [Google Scholar]
- Chen, S.Y.; Chen, W.H.; Shih, C.J. Heavy metal removal from wastewater using zero-valent iron nanoparticles. Water Sci. Technol. 2008, 58, 1947–1954. [Google Scholar] [CrossRef]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Review. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Houben, D.; Pircar, J.; Sonnet, P. Heavy metal immobilization by cost-effective amendments in a contaminated soil: Effects on metal leaching and phytoavailability. J. Geochem. Explor. 2012, 123, 87–94. [Google Scholar] [CrossRef]
- Houben, D.; Sonnet, P. Metal immobilization and nitrate reduction in a contaminated soil amended with zero-valent iron (Fe0). Ecotoxicol. Environ. Saf. 2020, 201, 110868. [Google Scholar] [CrossRef]
- Soto-Hidalgo, K.T.; Cabrera, C.R. Nanoscale Zero Valent Iron for Environmental Cadmium Metal Treatment. In Green Chemistry; Saleh, H.E.M., Koller, M., Eds.; IntechOpen: London, UK, 2018; pp. 135–146. [Google Scholar]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Zeng, G.; Wan, J.; Cheng, M.; Zhang, C.; Hu, C.; Li, J. Performance and toxicity assessment of nanoscale zero valent iron particles in the remediation of contaminated soil: A review. Chemosphere 2018, 210, 1145–1156. [Google Scholar] [CrossRef]
- Kumpiene, J.; Antelo, J.; Brännvall, E.; Carabante, I.; Ek, K.; Komárek, M.; Söderberg, C.; Wårell, L. In situ chemical stabilization of trace element-contaminated soil—Field demonstrations and barriers to transition from laboratory to the field—A review. Appl. Geochem. 2019, 100, 335–351. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Elliott, D.W. Applications of iron nanoparticles for groundwater remediation. Remediat. J. 2006, 16, 7–21. [Google Scholar] [CrossRef]
- Yan, W.L.; Herzing, A.A.; Kiely, C.J.; Zhang, W.-X. Nanoscale zero-valent iron (nZVI): Aspects of the core-shell structure and reactions with inorganic species in water. J. Contam. Hydrol. 2010, 118, 96–104. [Google Scholar] [CrossRef]
- Mueller, N.C.; Braun, J.; Bruns, J.; Černík, M.; Rissing, P.; Rickerby, D.; Nowack, B. Application of nanoscale zero valent iron (nZVI) for groundwater remediation in Europe. Environ. Sci. Pollut. Res. 2012, 19, 550–558. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-P.; Li, X.; Cao, J.; Zhang, W.-X.; Wang, H.P. Characterization of zero-valent iron nanoparticles. Adv. Colloid Interface Sci. 2006, 120, 47–56. [Google Scholar] [CrossRef]
- Li, X.-Q.; Elliott, D.W.; Zhang, W.-X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Kebria, D.Y.; Darvishi, G.; Kootenaei, F.G. The Use of Nano Zero Valent Iron in Remediation of Contaminated Soil and Groundwater. Int. J. Environ. Sci. 2013, 1, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Gholami, P.; Khataee, A.; Vahid, B. Integration of Polydopamine and Fe3O4 Nanoparticles with Graphene Oxide to Fabricate an Efficient Recoverable Catalyst for the Degradation of Sulfadiazine. Ind. Eng. Chem. Res. 2020, 59, 183–193. [Google Scholar] [CrossRef]
- Rosická, D.; Šembera, J. Assessment of Influence of Magnetic Forces on Aggregation of Zero-valent Iron Nanoparticles. Nanoscale Res. Lett. 2011, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, J.R. The Soil and Groundwater Remediation with Zero Valent Iron Nanoparticles. Procedia Eng. 2016, 143, 1268–1275. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Fan, M.; Brown, R.C.; VAN Leeuwen, J.H.; Wang, J.; Wang, W.; Song, Y.; Zhang, P. Synthesis, Properties, and Environmental Applications of Nanoscale Iron-Based Materials: A Review. Crit. Rev. Environ. Sci. Technol. 2006, 36, 405–431. [Google Scholar] [CrossRef]
- Noubactep, C. The Suitability of Metallic Iron for Environmental Remediation. Environ. Prog. Sustain. Energy 2010, 29, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Cissoko, N.; Zhang, Z.; Zhang, J.H.; Xu, X.H. Removal of Cr(VI) from simulative contaminated groundwater by iron metal. Process Saf. Environ. 2009, 87, 395–400. [Google Scholar] [CrossRef]
- Liu, W.; Tian, S.; Zhao, X. Application of Stabilized Nanoparticles for In Situ Remediation of Metal-Contaminated Soil and Groundwater: A Critical Review. Curr. Pollut. Rep. 2015, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Fua, F.; Dionysioub, D.; Liuc, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 194–205. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Liu, W.; Zhang, W.-X. Zero-valent iron nanoparticles (nZVI) for the treatment of smelting wastewater: A pilot-scale demonstration. Chem. Eng. J. 2014, 254, 115–123. [Google Scholar] [CrossRef]
- Ling, L.; Huang, X.; Li, M.; Zhang, W.-X. Mapping the Reactions in a Single Zero-Valent Iron Nanoparticle. Environ. Sci. Technol. 2017, 51, 14293–14300. [Google Scholar] [CrossRef]
- Bartzas, C.; Komnitsas, K.; Paspaliaris, I. Laboratory evaluation of Fe0 barriers to treat acidic leachates. Miner. Eng. 2006, 19, 505–514. [Google Scholar] [CrossRef]
- Gil-Díaz, M.M.; Pérez-Sanz, A.; Vicente, M.Á.; Lobo, M.C. Immobilisation of Pb and Zn in soils using stabilised zero-valent iron nanoparticles: Effects on soil properties. Clean Soil Air Water 2014, 42, 1776–1784. [Google Scholar] [CrossRef]
- Huang, D.; Hu, Z.; Peng, Z.; Zeng, G.; Chen, G.; Zhang, C.; Cheng, M.; Wan, J.; Wang, X.; Qin, X. Cadmium immobilization in river sediment using stabilized nanoscale zero-valent iron with enhanced transport by polysaccharide coating. J. Environ. Manag. 2018, 210, 191–200. [Google Scholar] [CrossRef]
- Lahr, J.; Kooistra, L. Environmental risk mapping of pollutants: State of the art and communication aspects. Sci. Total Environ. 2010, 408, 3899–3907. [Google Scholar] [CrossRef]
- Moen, J.E.T.; Ale, B.J.M. Risk maps and communication. J. Hazard. Mater. 1998, 61, 271–278. [Google Scholar] [CrossRef]
- Zambon, I.; Colantoni, A.; Carlucci, M.; Morrow, N.; Sateriano, A.; Salvati, L. Land quality, sustainable development and environmental degradation in agricultural districts: A computational approach based on entropy indexes. EIA Rev. 2017, 64, 37–46. [Google Scholar] [CrossRef]
- Journel, A.G.; Huijbregts, C.J. Mining Geostatistics; Academic Press: San Diego, CA, USA, 1978. [Google Scholar]
- Almeida, F.F.M. O Cráton do São Francisco. Ver. Bras. Geoc. 1977, 7, 349–364. [Google Scholar]
- Trindade, W.M. Concentração e Distribuição de Metais Pesados em Sedimentos do rio São Francisco entre Três Marias e Pirapora/MG: Factores Naturais e Antrópicos. Master’s Thesis, Federal University of Minas Gerais, Belo Horizonte, Brasil, 2010. [Google Scholar]
- Batista, A.A.M.; Ribeiro, M.Q.C.; Macedo, A.T.M.; Tonidandel, D. Parecer Técnico GEDIN N° 00107/2008: Barragem Murici; Technical Report; FEAM: Belo Horizonte, Brazil, 2008. [Google Scholar]
- Signorelli, N.; Tuller, M.P.; Silva, P.C.; Justo, L.J. Geological Map; Scale 1:250 000, sheet SE.23-Y-B-Três Marias; CPRM—Geological Souvey of Brazil, Ministery of Mines and Energy: Belo Horizonte, Brazil, 2003. [Google Scholar]
- Chiavegatto, J.R.S. Análise Estratigráfica das Seqüências Tempestíticas da Formação Três Marias (Proterozóico Superior), na Porção Meridional da Bacia do São Francisco. Master’s Thesis, School of Mines, Federal Univeresity of Ouro Preto, Ouro Preto, Brazil, 1992. [Google Scholar]
- Conflitos Ambientais Minas Gerais. Luta Contra a Poluição Provocada por Barragens de Rejeitos Operadas pela Votorantim Metais. Available online: http://conflitosambientaismg.lcc.ufmg.br/conflito/?id=194 (accessed on 10 December 2013).
- Li, Y.; Cai, Y. Mobility of toxic metals in sediments: Assessing methods and controlling factors. J. Environ. Sci. 2015, 31, 203–205. [Google Scholar] [CrossRef]
- Matheron, G. The Theory of Regionalized Variables and Its Applications; Les Cahiers du Centre de Morphologie Mathématique; Ecole des Mines de Paris: Paris, France, 1971; 211p. [Google Scholar]
- U.S. EPA. Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges and Soils. 2007. Available online: www.epa.gov/osw/hazard/testmethods/sw846/pdfs/3051a.pdf (accessed on 15 November 2013).
- COPAM. Normative Resolution, COPAM n° 166, 29 June 2011; State Environmental Policy Council: Belo Horizonte, Brazil, 2011; 5p. [Google Scholar]
- CONAMA. Resoluções do CONAMA: Resoluções Vigentes Publicadas Entre Setembro de 1984 e Janeiro de 2012; Ministério do Meio Ambiente: Brasília, Brazil, 2012; 1125p. [Google Scholar]
- Elliott, D.W.; Zhang, W.-X. Field assessment of nanoscale bimetallic particles for groundwater treatment. Environ. Sci. Technol. 2001, 35, 4922–4926. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S.; Crane, R. Nanoscale Metallic Iron for Environmental Remediation: Prospects and Limitations. Wat. Air Soil Poll. 2012, 223, 1363–1382. [Google Scholar] [CrossRef] [Green Version]
- Grieger, K.D.; Fjordbøge, A.; Hartmann, N.B.; Eriksson, E.; Bjerg, P.L.; Baun, A. Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: Risk mitigation or trade-off? J. Contam. Hydrol. 2010, 118, 165–183. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, W.X. Sequestration of metal cations with zerovalent iron nanoparticles a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J. Phys. Chem. C 2007, 111, 6939–6946. [Google Scholar] [CrossRef]
- Liu, A.; Liu, J.; Han, J.; Zhang, W. Evolution of nanoscale zero-valent iron (nZVI) in water: Microscopic and spectroscopic evidence on the formation of nano- and micro-structured iron oxides. J. Hazard. Mater. 2017, 322, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reardon, E.J.; Fagan, R.; Vogan, J.L.; Przepiora, A. Anaerobic corrosion reaction kinetics of nanosized iron. Environ. Sci. Technol. 2008, 42, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef]
- Cundya, A.B.; Hopkinsona, L.; Whitby, R.L.D. Use of iron-based technologies in contaminated land and groundwater remediation: A review. Sci. Total Environ. 2008, 400, 42–51. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Tratnyek, P.G.; Sarathy, V.; Baer, D.R.; Amonette, J.E.; Pecher, K.; Wang, C.; Linehan, J.C.; Matson, D.W.; Penn, R.L.; et al. Characterization and properties of metallic iron nanoparticles: Spectroscopy, electrochemistry, and kinetics. Environ. Sci. Technol. 2005, 39, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; Collins, R.N.; Waite, T.D.; Hanna, K. Advances in Surface Passivation of Nanoscale Zerovalent Iron: A Critical Review. Environ. Sci. Technol. 2018, 52, 12010–12025. [Google Scholar] [CrossRef]
- Song, H.; Carraway, E.R. Reduction of chlorinated ethanes by nanosized zerovalent iron: Kinetics, pathways, and effects of reaction conditions. Environ. Sci. Technol. 2005, 39, 6237–6245. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef]
- Speight, J.G. Hydrolysis. In Reaction Mechanisms in Environmental Engineering Analysis and Prediction; Butterworth Heinemann: Oxford, UK, 2018; pp. 203–229. [Google Scholar]
- Siegel, F. Environmental Geochemistry of Potentially Toxic Metals; Springer: Berlin/Heidelberg, Germany, 2002; 218p. [Google Scholar]
- Rangsivek, R.; Jekel, M.R. Removal of dissolved metals by zero-valent iron (ZVI): Kinetics, equilibria, processes and implications for stormwater runoff treatment. Water Res. 2005, 39, 4153–4163. [Google Scholar] [CrossRef] [PubMed]
- Hoerle, S.; Mazaudier, F.; Dillmann, P.; Santarini, G. Advances in understanding atmospheric corrosion of iron. II: Mechanistic modelling of wet–dry cycles. Corros. Sci. 2004, 46, 1431–1465. [Google Scholar] [CrossRef]
- Sarathy, V.; Tratnyek, P.G.; Nurmi, J.T.; Baer, D.R.; Amonette, J.E.; Chun, C.L.; Penn, R.L.; Reardon, E.J. Aging of iron nanoparticles in aqueous solution: Effects on structure and reactivity. J. Phys. Chem. C 2008, 112, 2286–2293. [Google Scholar] [CrossRef]
- Liu, Y.; Majetich, S.A.; Tilton, R.D.; Sholl, D.S.; Lowry, G.V. TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environ. Sci. Technol. 2005, 39, 1338–1345. [Google Scholar] [CrossRef]
- Liu, Y.; Lowry, G.V. Effect of particle age (Fe0 content) and solution pH on NZVI reactivity: H2 evolution and TCE dechlorination. Environ. Sci. Technol. 2006, 40, 6085–6090. [Google Scholar] [CrossRef]
- Phenrat, T.; Long, T.C.; Lowry, G.V.; Veronesi, B. Partial Oxidation (“Aging”) and Surface Modification Decrease the Toxicity of Nanosized Zerovalent Iron. Environ. Sci. Technol. 2009, 43, 195–200. [Google Scholar] [CrossRef]
- Parida, K.M.; Das, N.N. Reductive dissolution of hematite in hydrochloric acid medium by some inorganic and organic reductants: A comparative study. Indian J. Eng. Mater. Sci. 1996, 3, 243–247. [Google Scholar]



| Chemical Composition of Fe0 Nanoparticles | Fe (Core), FeO (Capsule) |
|---|---|
| Mass percentage of the solution | 20% |
| Fe0 mass in solid fraction | 80% |
| Other substances of the solid fraction | Fe3O4, FeO, C |
| Other substances in the liquid fraction | Organic Stabilizer |
| Particle shape | Spherical |
| Fe0 particle size | D50 nm < 50 |
| Specific surface | >25 m2/g |
| Color | Black |
| Solution density | 1210 kg/m3 |
| Fe0 Density | 7870 kg/m3 |
| Fe3O4 Density | 5700 kg/m3 |
| Sediments and Alluvium (CONAMA, 2012) | As (mg kg−1) | Cd (mg kg−1) | Cr (mg kg−1) | Cu (mg kg−1) | Ni (mg kg−1) | Pb (mg kg−1) | Zn (mg kg−1) |
|---|---|---|---|---|---|---|---|
| Normal | <5.9 | <0.6 | <33.0 | <17.0 | <14.0 | <8.4 | <58.0 |
| Intermediate | 33.0–37.3 | 17.0–35.7 | 14.0–18.0 | 8.4–35.0 | 58.0–123.0 | ||
| Caution | 5.9–17.0 | 0.6–3.5 | 37.3–90.0 | 35.7–197.0 | 18.0–35.9 | 35.0–91.3 | 123.0–315.0 |
| Critical | >17.0 | >3.5 | >90.0 | >197.0 | >35.9 | >91.3 | >315.0 |
| ZVI | Layers | pH | Eh (mV) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t0 | t24H | t48H | t72H | t7d | t14d | t30d | t60d | t120d | t0 | t24H | t48H | t72H | t7d | t14d | t30d | t60d | t120d | ||
| CA1-33 | |||||||||||||||||||
| 1 g/L | Upper | 4.44 | 6.61 | 6.90 | 6.53 | 2.70 | 6.22 | 3.20 | 3.91 | 5.87 | 554.0 | 476.6 | 276.2 | 287.9 | 464.4 | 253.3 | 384.3 | 685.4 | 305.4 |
| Lower | 4.07 | 6.76 | 6.32 | 6.26 | 6.09 | 5.62 | 4.76 | 3.27 | 5.65 | 431.2 | 424.9 | 272.8 | 466.0 | 296.4 | 233.8 | 375.4 | 639.7 | 321.6 | |
| 3 g/L | Upper | 3.50 | 6.88 | 7.10 | 6.72 | 4.11 | 3.00 | 3.33 | 3.23 | 4.77 | 417.4 | 169.4 | 187.8 | 184.6 | 346.3 | 444.7 | 398.7 | 449.1 | 264.8 |
| Lower | 6.52 | 6.97 | 7.04 | 6.57 | 3.64 | 3.37 | 3.22 | 6.38 | 6.39 | 354.4 | 163.2 | 193.3 | 160.5 | 383.5 | 411.0 | 412.3 | 131.1 | 161.6 | |
| 7 g/L | Upper | 5.79 | 6.89 | 6.79 | 2.54 | 2.56 | 3.06 | 5.79 | 6.34 | 6.78 | 235.4 | 141.4 | 160.9 | 486.6 | 458.8 | 422.6 | 223.6 | 124.4 | 317.7 |
| Lower | 6.71 | 6.17 | 7.05 | 2.13 | 2.07 | 3.25 | 4.43 | 6.42 | 6.60 | 233.9 | 102.5 | 160.0 | 506.0 | 444.6 | 462.8 | 288.4 | 121.1 | 305.2 | |
| 0 g/L | Upper | 6.70 | 6.48 | 2.94 | 3.05 | 2.50 | 3.41 | 2.85 | 3.61 | 4.61 | 245.7 | 204.8 | 277.7 | 496.7 | 683.2 | 485.5 | 567.8 | 157.6 | 301.5 |
| Lower | 6.65 | 6.73 | 3.01 | 4.72 | 5.96 | 4.18 | 3.51 | 3.19 | 4.56 | 231.5 | 207.8 | 290.5 | 449.4 | 610.6 | 477.0 | 539.4 | 166.3 | 301.5 | |
| CA2-10 | |||||||||||||||||||
| 1 g/L | Upper | 5.21 | 5.84 | 5.07 | 2.76 | 3.91 | 3.00 | 2.95 | 3.35 | 3.58 | 284.7 | 99.7 | 206.7 | 382.9 | 322.3 | 369.2 | 402.2 | 359.3 | 189.0 |
| Lower | 5.16 | 5.64 | 4.61 | 2.60 | 3.90 | 3.25 | 3.99 | 3.44 | 3.71 | 310.6 | 201.9 | 216.6 | 410.8 | 357.9 | 409.8 | 418.7 | 450.8 | 234.8 | |
| 3 g/L | Upper | 5.51 | 6.28 | 6.13 | 2.57 | 4.19 | 4.08 | 3.26 | 4.04 | 4.87 | 245.8 | 51.3 | 129.7 | 384.6 | 290.4 | 407.4 | 430.4 | 152.3 | 275.4 |
| Lower | 5.23 | 5.93 | 4.91 | 2.45 | 1.84 | 3.98 | 3.42 | 2.95 | 4.07 | 234.8 | 76.4 | 173.8 | 390.1 | 439.7 | 436.0 | 458.2 | 286.0 | 380.2 | |
| 7 g/L | Upper | 5.94 | 6.10 | 6.23 | 2.63 | 3.08 | 3.02 | 2.98 | 3.21 | 4.76 | 261.6 | 129.3 | 133.7 | 395.3 | 314.2 | 383.4 | 471.7 | 281.5 | 397.3 |
| Lower | 5.91 | 5.79 | 6.23 | 2.25 | 2.53 | 3.77 | 3.25 | 4.38 | 5.10 | 229.7 | 147.0 | 167.2 | 457.4 | 399.1 | 417.4 | 489.2 | 128.1 | 321.5 | |
| 0 g/L | Upper | 2.57 | 2.93 | 2.72 | 2.77 | 2.72 | 3.20 | 3.05 | 3.63 | 3.54 | 226.2 | 551.8 | 545.3 | 643.1 | 627.0 | 593.9 | 181.6 | 569.1 | 320.7 |
| Lower | 2.57 | 3.64 | 4.78 | 3.45 | 3.06 | 3.07 | 3.70 | 3.60 | 3.26 | 331.0 | 486.5 | 503.4 | 579.6 | 584.1 | 524.0 | 202.0 | 574.1 | 336.5 | |
| CA3-18 | |||||||||||||||||||
| 1 g/L | Upper | 4.31 | 6.56 | 6.78 | 2.55 | 4.54 | 2.74 | 3.73 | 6.29 | 5.21 | 360.4 | 170.7 | 206.0 | 423.7 | 296.3 | 392.9 | 418.9 | 306.0 | 388.8 |
| Lower | 5.79 | 6.73 | 6.87 | 2.87 | 5.51 | 3.19 | 3.08 | 3.61 | 6.65 | 338.8 | 179.4 | 213.2 | 475.2 | 281.6 | 478.5 | 488.9 | 442.4 | 374.1 | |
| 3 g/L | Upper | 5.81 | 6.65 | 6.91 | 2.19 | 4.33 | 4.30 | 2.93 | 6.38 | 5.92 | 348.3 | 133.2 | 159.1 | 486.9 | 387.3 | 336.0 | 417.3 | 121.0 | 305.3 |
| Lower | 6.71 | 6.86 | 7.07 | 2.40 | 3.37 | 3.74 | 3.71 | 6.55 | 6.69 | 331.3 | 138.1 | 159.5 | 489.6 | 455.0 | 419.6 | 400.8 | 125.9 | 322.8 | |
| 7 g/L | Upper | 5.27 | 6.65 | 6.97 | 2.24 | 2.47 | 3.08 | 3.79 | 5.19 | 6.90 | 272.6 | 63.9 | 146.7 | 453.8 | 445.1 | 380.9 | 395.8 | 130.5 | 271.4 |
| Lower | 6.04 | 6.79 | 6.95 | 2.28 | 2.68 | 3.52 | 3.13 | 6.37 | 6.98 | 255.4 | 95.1 | 148.6 | 456.7 | 458.9 | 429.7 | 502.2 | 127.4 | 262.3 | |
| 0 g/L | Upper | 4.65 | 6.69 | 5.22 | 2.36 | 3.75 | 3.48 | 3.41 | 3.90 | 3.87 | 343.0 | 212.6 | 258.3 | 527.0 | 537.8 | 521.7 | 528.4 | 574.9 | 543.7 |
| Lower | 6.43 | 6.70 | 2.32 | 2.69 | 4.19 | 3.15 | 3.31 | 3.77 | 3.52 | 298.0 | 196.3 | 448.6 | 521.6 | 459.9 | 540.6 | 531.0 | 579.6 | 568.2 | |
| ZVI | As (mg/L) | As (%) | Cd (mg/L) | Cd (%) | Cu (mg/L) | Cu (%) | Pb (mg/L) | Pb (%) | Zn (mg/L) | Zn (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t = 0 | t = 120 | t = 120 | t = 0 | t = 120 | t = 120 | t = 0 | t = 120 | t = 120 | t = 0 | t = 120 | t = 120 | t = 0 | t = 120 | t = 120 | |
| CA1-33 | |||||||||||||||
| 1 g/L | 0.005 | 0.001 | 20 | 0.137 | 0.081 | 59 | 0.375 | 0.044 | 12 | 0.271 | 0.111 | 41 | 29.300 | 31.957 | 109 |
| 3 g/L | 0.055 | 0.001 | 2 | 0.110 | 0.041 | 38 | 0.102 | 0.028 | 27 | 0.304 | 0.031 | 10 | 24.001 | 8.852 | 37 |
| 7 g/L | 0.023 | 0.001 | 4 | 0.093 | 0.041 | 44 | 0.061 | 0.023 | 38 | 0.290 | 0.020 | 7 | 27.596 | 9.205 | 33 |
| 0 g/L | 0.061 | 0.001 | 2 | 0.115 | 0.040 | 35 | 0.064 | 0.019 | 29 | 0.235 | 0.041 | 18 | 22.023 | 17.001 | 77 |
| CA2-10 | |||||||||||||||
| 1 g/L | 0.057 | 0.003 | 5 | 4.568 | 0.813 | 18 | 0.420 | 0.123 | 29 | 1.388 | 0.750 | 54 | 950.101 | 309.307 | 33 |
| 3 g/L | 0.057 | 0.001 | 2 | 3.622 | 2.699 | 75 | 0.373 | 0.474 | 127 | 1.908 | 1.267 | 66 | 774.770 | 610.141 | 79 |
| 7 g/L | 0.062 | 0.001 | 2 | 3.808 | 2.134 | 56 | 0.185 | 0.190 | 103 | 1.125 | 0.648 | 58 | 829.836 | 546.192 | 66 |
| 0 g/L | 0.052 | 0.001 | 2 | 1.748 | 1.359 | 78 | 0.500 | 0.214 | 43 | 2.033 | 0.892 | 44 | 489.003 | 444.873 | 91 |
| CA3-18 | |||||||||||||||
| 1 g/L | 0.004 | 0.001 | 23 | 2.340 | 1.382 | 59 | 0.059 | 0.068 | 116 | 0.709 | 0.060 | 8 | 49.718 | 35.870 | 72 |
| 3 g/L | 0.093 | 0.001 | 1 | 2.315 | 1.184 | 51 | 0.081 | 0.050 | 62 | 0.549 | 0.036 | 7 | 47.229 | 24.658 | 52 |
| 7 g/L | 0.003 | 0.001 | 40 | 2.560 | 1.868 | 73 | 0.061 | 0.142 | 233 | 0.414 | 0.094 | 23 | 38.016 | 48.646 | 128 |
| 0 g/L | 0.003 | 0.001 | 33 | 2.724 | 1.438 | 53 | 0.077 | 0.098 | 127 | 0.484 | 0.055 | 11 | 46.985 | 39.998 | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, R.; Araújo, J.; Pinho, C.; Albuquerque, T. Evaluating the Effectiveness of Nanotechnology in Environmental Remediation of a Highly Metal-Contaminated Area—Minas Gerais, Brazil. Geosciences 2022, 12, 287. https://doi.org/10.3390/geosciences12080287
Fonseca R, Araújo J, Pinho C, Albuquerque T. Evaluating the Effectiveness of Nanotechnology in Environmental Remediation of a Highly Metal-Contaminated Area—Minas Gerais, Brazil. Geosciences. 2022; 12(8):287. https://doi.org/10.3390/geosciences12080287
Chicago/Turabian StyleFonseca, Rita, Joana Araújo, Catarina Pinho, and Teresa Albuquerque. 2022. "Evaluating the Effectiveness of Nanotechnology in Environmental Remediation of a Highly Metal-Contaminated Area—Minas Gerais, Brazil" Geosciences 12, no. 8: 287. https://doi.org/10.3390/geosciences12080287
APA StyleFonseca, R., Araújo, J., Pinho, C., & Albuquerque, T. (2022). Evaluating the Effectiveness of Nanotechnology in Environmental Remediation of a Highly Metal-Contaminated Area—Minas Gerais, Brazil. Geosciences, 12(8), 287. https://doi.org/10.3390/geosciences12080287









