Abstract
Mining is an important industry, accounting for 6.9% of global GDP. However, global development promotes accelerated demand, resulting in the accumulation of hazardous waste in land, sea, and air environments. It reached 7 billion tonnes of mine tailings generated yearly worldwide, and 19 billion solid tailings will be accumulated by 2025. Adding to this, the legacy of environmental damage from abandoned mines is worrying; there are around 10,000 abandoned mines in Canada, 50,000 in Australia, and 6000 in South Africa, as well as 9500 coal mines in China, reaching 15,000 by 2050. In this scenario, restoration techniques from mining tailings have become increasingly discussed among scholars due to their potential to offer benefits towards reducing tailing levels, thereby reducing environmental pressure for the correct management and adding value to previously discarded waste. This review paper explores the available literature on the main techniques of mining tailing recycling and reuse and discusses leading technologies, including the benefits and limitations, as well as emerging prospects. The findings of this review serve as a supporting reference for decision makers concerning the related sustainability issues associated with mining, mineral processing, and solid waste management.
1. Introduction
The products of mining activity are essential not only for the subsistence of modern society but also for its improvement. We can reflect on the impact of the absence of products in our daily lives, such as aircraft, ceramics, computers, building materials, medicines, agricultural products, asphalt, electronic products, metals, and paints [1,2]. Substantial mining activity is usually correlated with a region’s development, such that geologically privileged regions can count on a considerable part of their GDP from this activity [3]. For example, the European extractive industry includes more than 17,500 companies employing more than half a million people, and the development of the western United States was primarily due to the mining industry [4].
The activities involved in the intricate process of mining range from metal extraction (precious metals, ferrous alloys, and nonferrous materials), mineral beneficiation (gypsum, salt, kaolin, sulfur, and phosphate), fuels (hard coal, steam coal, petroleum, and coking coal), smelting, refining, and remediation [5,6,7]. The process of extraction produces significant amounts of wastes, typically consisting of (i) solid wastes in the form of waste rock, clouds of dust, sludges, and slags; (ii) liquid wastes in the form of wastewater and effluents; and (iii) gaseous emissions. Waste generated due to mining activity poses a serious issue due to the large amounts generated, and it is often associated with the risks posed by its storage and environmental management [8].
Global resources are finite, and greater extraction and use of virgin materials put significant pressure on the Earth’s resources, critically threatening future generational resource requirements [9]. Furthermore, population growth generates high consumption, putting pressures never seen before on natural resources. Consequently, the mining industry is generating vast quantities of tailings per year, representing one of the more prominent waste producers worldwide, reaching 7 billion tonnes per year [10]. Recent estimates point out that 19 billion solid tailings will be accumulated by 2025, and due to the structural complexity (chemical and physical), 20% cannot be recycled at all [11,12]. Among others, the consequences are apparent in the permanent impacts on soil exposure, vegetation, water sources (major impact), atmospheric pollution, and harming the lives of the population in its surroundings [1,13,14,15]. In this scenario, recycling mine tailings can help reduce the number of tailings for disposal. Circular economy, recyclability, recycling, and reuse have been identified as emerging solutions that can drive the multidimensional aspects of sustainability in the mining and metal extraction industries. As the residue is a heterogeneous, complex, and reactive mixture of minerals, each solution has its advantages and limitations of methods that are observed in waste feasibility studies.
In a feasibility study, waste characterization is initially done during mining, where the waste is stored above ground ore prior to treatment. At this phase, the concern is to determine if the residues will cause acid and metalliferous drainage (AMD), saline and sodic drainage, and leaching and mobilization of metals and toxic compounds. AMD is the formation and movement of highly acidic water rich in heavy metals and causes serious environmental problems around the world. It refers to effluents with low pH and high concentrations of hazardous and toxic elements that are generated when sulfide-rich wastes are exposed to the environment [16]. It is especially harmful when mining activity ceases, causing the water table to rise to normal levels, reacting with contaminated acid leachates that settle on rock walls when the AMD is present [17,18,19]. Australia, Canada, and China have 52,324; 10,129; and 5383 abandoned mines, respectively [20,21]. Neutralization, adsorption, ion exchange, membrane technology, biological mediation, and electrochemical remediation techniques have been used with relative success in tackling AMD [22,23,24,25,26]. As a disadvantage of these remediation techniques, they need to be applied for a long time due to the persistence of the reactivity of the elements that form AMD. As a result, prevention strategies have gained the attention of scholars due to their ability to limit the formation of AMD in the early stages [16].
Recently, as is hereafter reviewed, many researchers have proposed the recycling or reuse of mine wastes due to their potential to offer benefits over reducing tailing levels, thereby reducing environmental pressure for the correct management and adding value to previously discarded waste. Criticism occurs when some researchers argue that there are more opportunities to reduce the impact of tailing waste in the mining design phase than in the operational or post-processing phases, and the emphasis on post-processing strategies could undermine prevention opportunities [27]. Given the concerns listed, the aims of this review are to present recent technological advancements in recycling and reuse of mining tailings, to explore the environmental and economic implications of these strategies, and finally to discuss future perspectives for mine waste remediation technologies.
2. Methodology
A total of 90 published articles between 1990 and 2022 were analyzed. These papers were retrieved through scholarly databases, such as Scielo, Scopus, Google Scholar, Science Direct, ResearchGate, and Web of Science. The keywords used in the literature search included: “mining tailings,” “waste”, and “recycling techniques” with “metal recovery”, “construction materials”, “new applications”, “sustainable mining”, and “agricultural fertilizers”.
The articles were analyzed according to a series of characteristics. Initially, the types of tailing sources were identified, such as lead, zinc, copper from overload, rock waste, processing tailings, metallurgical slag, and water treatment residuals, among others. The disposal and treatment types of the mining waste were also noted. Next, the degree of the environmental impact of mining operations was noted: for example, the suppression or prevention of vegetation; the removal of large amounts of fertile soil; the contamination of water sources—waters in rivers or in reservoirs—by oil, grease, and heavy metals; the modification of the water-flow regimen; air pollution; and the risks arising from the accumulation of tailings in containment barriers. Finally, the recovery options observed in the articles were summarized, outlining the advantages and disadvantages of each application.
3. Discussion
3.1. Mining and Mineral Processing Wastes
Mining waste refers to all material that is extracted from the ground and processed to varying stages during the ore-processing and enrichment phases, having low or no economic value, as it is considered an unusable mineralized material and hence is stored or discarded rather than processed [28,29]. Usually, these products present themselves as fine suspended materials (1–600 μm), including dissolved metals and reagents, chemicals, and inorganic and organic additives, and are thus stored in the form of slurry in large man-made embankments, commonly referred to as tailings dams. Table 1 summarizes some types of mine waste, their classifications, and disposal options [30,31,32,33]. Table 2 shows the main applications of mine tailing identified in the articles.

Table 1.
Characterization of mining waste [30,31,32,33].

Table 2.
Tailings identified and their applications.
According to Table 1, mining waste can present itself as rock waste from the bedrock that has been mined and transported out of the pit. However, it does not have a metal concentration of economic interest. It is stored in a landfill site near mining production because it is not economically viable to transport to another site [34]. In the processing phase, an ore mill is located at the extraction site to produce the first marketable products (metallic concentrates, sorted ore, and ingots); the residues of this stage are called processing waste. In this phase, various types of waste, such as aqueous solutions, wastewater, and slurry composed of fine-grained particles mixed with additives as well as products of chemical reactions are produced, which need to be stored in ponds for dewatering. Acid mine drainage occurs when acid, sulfate, and metal wastewater (effluents with a low pH and high toxic element concentration) are released from the ponds into the environment. The mine may continue to generate AMD for decades even after it ceases its operation. It is a huge source of concern due to its high environmental impact [16,35]. Still, in the processing stage, roasting is applied in sulfides to extract metals and remove impurities from the ore. Therefore, toxic gases like SO2 come out; this is an example of mine waste in the form of atmospheric emissions [36]. Still, one of the residues from the burning of sulfides is classified as slag, and it usually accumulates together with ashes in the vicinity of the production center, rather than in tailings ponds.
Disposal refers to accumulating large amounts of waste in a concentrated area or filling spaces in inoperative mines. Tailings dams are the most common method of deposition of fine tailings from ore grinding. Here, the idea is to dispose of the waste in an optimized, accessible, and environmentally safe way to allow its reprocessing in the future with the advancement of new technologies. We will address some of them in this article. Underground backfilling is the most expensive method; it can only be used away from aquifers, and it is generally an option when geological stability and safety in operations are required. Submarine tailing disposal (STD) consists of the deposition of tailings in underwater marine bodies. Although there is a lot of criticism regarding the risks of the operation, and this has been increasing restrictions on the use of this solution over time [37,38], some authors emphasize the benefit because the underwater conditions favor the geochemical stabilization of sulfide mineral residues [39].
According to Table 2, construction materials are the main applications of mine waste. In this sector, the most significant research is related to additive incorporation in cement for concrete block manufacturing [33,40], followed by brick manufacturing [41,42]. Agricultural products are in second place. Tailings that are suitable for use in agriculture must possess more similar physicochemical, compositional, and morphological characteristics, primarily in being rich in silicates, calcium, iron, and aluminum, among other beneficial elements, to be desirable for soil remediation and remineralization purposes. Several agricultural applications were observed in the articles, such as improving soil structure and crop yield [43], reducing soil erosion [44], treating acidic or metal-rich soils [45,46], or increasing available S and P concentrations in the soil [47].
3.2. Recovery of Mine Wastes through Reuse and Recycling
Reusing mine waste means using the material in its entirety without processing it in a new application. Recycling, on the other hand, extracts new valuable components from the waste or uses the waste as an input to the manufacture of a valuable product or application through processing [48]
In the different mining phases (exploration, transport, processing, and beneficiation), measures are taken to manage the generated waste. Different parameters such, as geographic, geological, hydrogeological, and climatological disparities, are decisive for addressing the strategies. In the long term, the research and development (R&D) sectors of companies work to improve the efficiency of current exploration methods (drilling and extraction), while in the short term, planners and decision makers embrace management tools, as shown in Figure 1, aiming to add value to the production liability and reducing the risk of the operation.
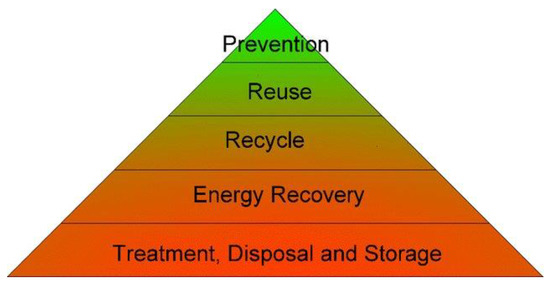
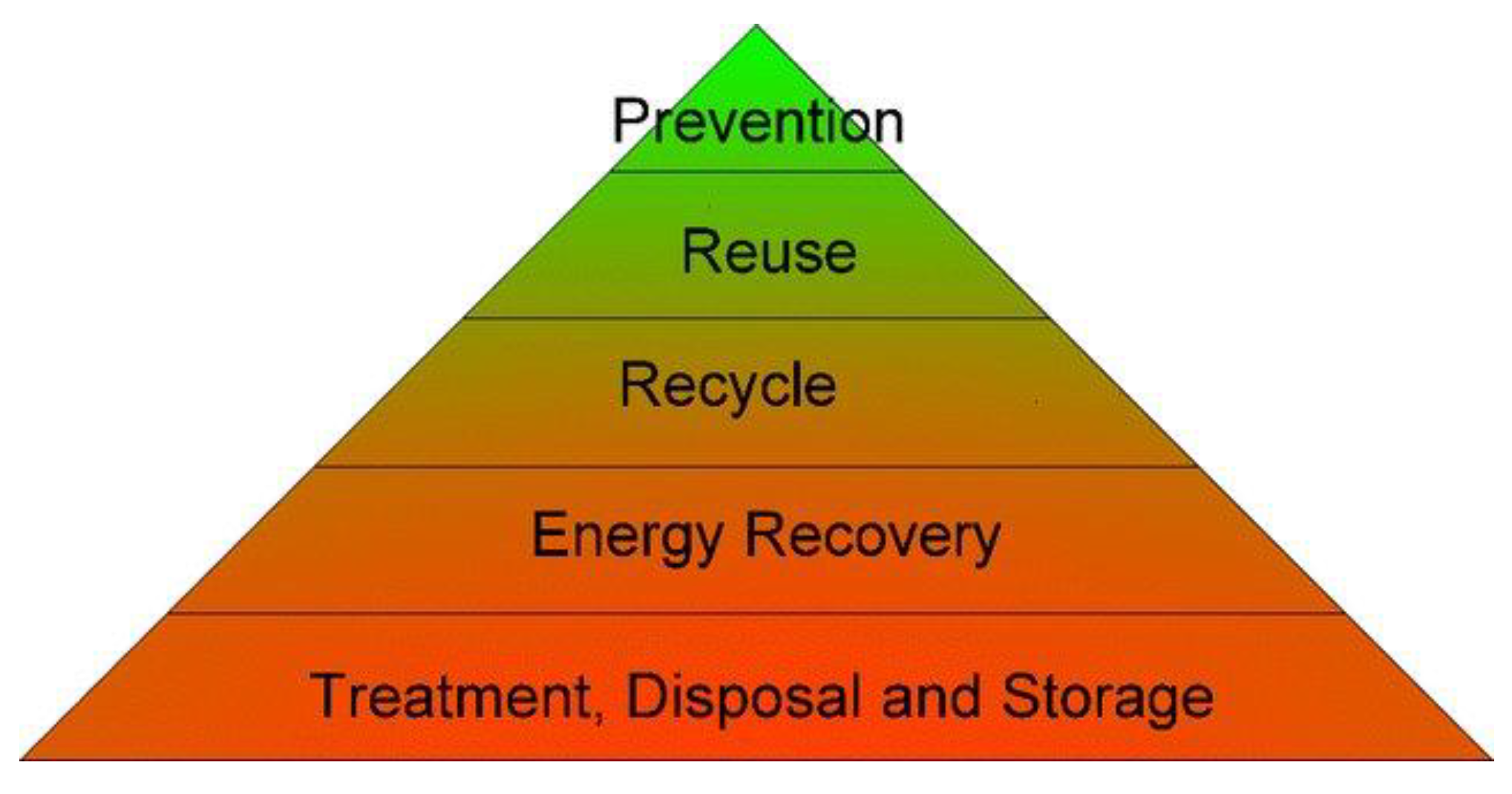
Figure 1.
Mine waste hierarchy, adapted from [49].
The triangle of Figure 1 represents a mine waste hierarchy serving as a guide for prioritizing waste management practices, representing the most favored at the top to the least favored at the bottom. As seen, the minimization of the creation of mine waste is the preferred option, whereas disposal and treatment are the least preferred option. Reuse and recycling are the top feasible options in waste management [48].
For it to be possible to reuse waste, there must be a guarantee of the quality of the material compared to the original condition. In this strategy, there is no biological, physical, or physical-chemical transformation. The advantages are the saving of natural resources and manufacturing of cheaper products.
Recycling, which aims to reintroduce a waste after undergoing transformations in its properties to a particular production chain and serve as raw material for the manufacture of other products, has the following advantages: generation of employment, encouragement of scientific development, and reduction of the need for extraction of minerals in mines, among others. However, the most common practice used in conventional mining is treatment, disposal, and storage, the least favored option.
Despite the consolidation of technologies for treatment, disposal, and storage, there is a growing evolution regarding the use of mining waste recycling, especially in developed countries [50,51,52], where an increase in the number of research activities in this area leads to the belief that structural modification of this pyramid is possible in the future [53,54].
Table 3 shows the main recycling and reuse processes of residues, outlining the advantages and drawbacks of each. These residues are by-products of mining or have an indirect relationship in sharing similar properties and/or composition to mining waste, thus facing similar technical and economic feasibility challenges and opportunities.

Table 3.
The relationship between types of mine waste and their main recovery techniques.
It is important to emphasize that even if a residue is not directly linked to mineral exploration, it entered the subsequent analysis of this section because its use in the composition of mineral residues in recycling processes was identified in several analyzed articles, signaling its importance: for example, metallurgical waste in [32,71,72] and steel slag in [73,74,75,76].
It is also worth noting that the choice of procedure depends on the physical-chemical characteristics/properties of the residue, in addition to the operational cost to recover these materials from the waste and their environmental impact. Some techniques are already well consolidated, while others are still under development, requiring further research for their use to be on a larger scale.
Next, we will detail the main types of waste and their respective methods of use, whether in recycling or reuse.
3.2.1. Metal Waste
In the past, the prices of non-ferrous metals were somewhat lower than they are today, and this is the main reason why the mining industry has left significant quantities of these in tailings dams around the world. The increased reprocessing of copper tailings is becoming an increasingly logical decision, with a sufficiently high content of valuable components so that tailings can be economically exploited, and new technologies for copper extraction and recycling are being developed [30]. It is also possible to recycle iron, copper, zinc, and gold mining waste to obtain bricks, tiles, and lightweight aggregates [12]. Regarding copper recovery, bioleaching has been widely used, where thermophilic bacteria are used to recover copper and other valuable metals [60,61].
Furthermore, copper waste has been used with relative success replacing granite used in road and highway pavement concretes and brick production [32,33,77,78]. In these studies, the suitability of copper ore tailings as an additive mixture in concrete preparation was tested by replacing common Portland cement at different grades. Up to 20% is saved when we use copper ore tailings instead of Portland cement. The copper tailings produced in this way have good strength and durability characteristics. Regarding the anticorrosive characteristics and cost reduction, the use as an additive product, rather than as raw material, was considered a good option [79]. Copper slag is also being used in the manufacture of roof tiles, mine filling materials, and granular materials [80].
In most cases, iron ore tailings have fine granulometry, high silica content, iron oxides, alumina, and other smaller minerals [81]. This composition facilitates its use in the construction industry [82]. However, this waste generates water and soil pollution in the form of dust, leaching water runoff from mining waste, and infiltration of iron-contaminated water, which consequently affects living things [83]. When using iron ore tailings as mortar, up to 85% of tailings can be applied with good results, with the option of manufacturing different types of products, such as paving blocks and masonry blocks [84]. Clay and shale are essential in producing bricks and must be subjected to a high firing temperature [85]. Extracting materials consumes a lot of energy, negatively affecting the environment and releasing a worrying amount of residue and greenhouse gases [41,86]. Thus, it is interesting to defend the development of ecologically correct materials and construction processes, where iron tailings represent an option of raw material to produce bricks.
Similar to copper waste, iron ore residues can be mixed in concrete, floors, and ceramic tiles with relative mechanical, physical, and chemical performance [87,88,89]. The compressive strength of concrete from iron ore tailings (Figure 2) showed an improvement of 11.56% compared to concrete with conventional aggregates, showing that it is possible to obtain quality materials from mineral tailings in relation to certain mechanics properties [87]. However, some characteristics disqualify processing residues as aggregates, such as metal composition, variability, particle size, leaching of trace metals, and adjacent chemical reactions that can generate unwanted acids [56]. Because the iron content in this type of material is very low, reprocessing can become complicated due to the huge volume of waste generated to extract an economically viable amount of iron ore. This excessive volume of waste will require good storage management.

Figure 2.
(a) Fine and (b) coarse iron ore tailings, and (c) tailings aggregate concrete. Source: [87]. CC-BY.
Sulfides from mining oxidize more easily in tailings facilities, exposing tailings to air and water. This disturbing phenomenon in the mining industry is known as acid mine drainage (AMD). A solution to reduce the formation of acid mine drainage is the use/recycling of mine waste into building materials and geopolymers [90]. In addition, there are other industrial materials with recovery potential from tailings: for example, sulfur, sulfuric acid, metallic pigments, sulfates, calcium carbonate and magnesium hydroxides, agricultural materials (e.g., fertilizers), and adsorbents [91].
3.2.2. Gypsum Waste
Gypsum is by far one of the most widely produced materials in the world. The total amount of unwanted by-product, the phosphogypsum (PG) solid waste, produced up to 2006 is estimated at 6 billion tonnes, of which 2.2 billion tonnes (37%) were produced in the United States [63].
Due to the high content of calcium, phosphorus, and sulfur, PG has been successfully used as a soil amendment, in addition to having a fertilizer value due to the ammonium sulfate content [11]. Recent studies [92] have used mixtures of cement—OPC (Ordinary Portland Cement) and phosphogypsum—in the stabilization of soils with high water content and low strength, known as degraded soils. The authors characterized different types of sedimentary soils, with different plasticity indexes, where the soil sludge was mechanically mixed with cement and phosphogypsum powder, obtaining a homogeneous paste. The specimens obtained from the above procedure were subjected to different tests, such as unconfined compressive strength (UCT), pH measurement, SEM, and XRD. Significant improvements were obtained in terms of mechanical strength, density improvement, and obtaining ettringite (AFt) as the main cementitious product of the pozzolanic reaction between cement, phosphogypsum, and clay minerals.
It is economically attractive to develop studies to seek the use of PG in construction materials, roads [93], agriculture [63], re-obtaining mineral resources that were previously underexploited, or environmental applications. However, it is important to know that the percentage of use is still low (15%), and the remainder, in most cases, is accumulated in abandoned storage areas [63,94].
3.2.3. Metallurgical Waste
Metallurgical powders are generated from materials added in foundry furnaces. They are heterogeneous mixtures and oxides with a relative degree of complexity [56]. Generally, the two main options for recovering valuable metals from ferrous powders are pyrometallurgical and hydrometallurgical processes. The principal gain of pyrometallurgical processes is the ability to process in a viable way metallurgical dust containing high amounts of Zn [56,65].
However, they are special processes that require high temperatures, efficient dust filtration systems, and volatilized return steps for the additional recovery of metals present in the flue gases [56]. There are advantages related to hydrometallurgical processes, which places them highly rated compared to other technologies. Flexibility, economy, and low emission of toxic gases, dust, and noise are some of the advantages. As a disadvantage, we can mention the high-water consumption, in addition to making it impossible to use it in products with a high added value, such as glass and ceramic materials, among others. Due to the considerable concentration of Pb, Cr, Zn, and Cd, these residues are classified as hazardous. Thus, its disposal is controlled through pre-treatment or stabilization [56,66,95,96,97]. Although these powders are economically valuable, recycling can be done directly, but the process is generally limited by the accumulation of harmful metals and other materials.
3.2.4. Steel Slag
The energy consumed in high-temperature metal processing is distributed between metal, slag, off-gas, and natural losses to the atmosphere. The thermal energy of the slag accounts for about 10 and 90% of the output energy, depending on the slag-to-metal ratio and the discharge temperature. Ferrous slags account for over 90% of the output thermal energy. The available energy associated with slag alone constitutes 50% of that energy. Thus, the investigation to recover part of this energy is recurrent, as well as the development of more energy-efficient processes based on the physical and chemical aspects of the slag. These methodologies include dry granulation processes, air jet granulation, impact granulation of solid slag, and centrifugal granulation [69,70]. Liquid slag is also studied as it has a good property of sensitive heat recovery through chemical methods [70].
Solidified metallurgical slag contains significant amounts of metal-based contaminants, which can harm the environment. For example, the presence of dissolved toxic metal species, such as chromium in stainless steel, steel, and ferrochromium alloy slag, can cause serious environmental problems [56,98,99].
The recovery of slag from metallurgical processes is recurrent due to the number of consolidated techniques. The application of slag as building materials [32] and in the manufacture of ceramics [100,101] is often identified. However, the recycling and reuse of the slag are strongly hampered by the presence of dissolved toxic metallic elements [31].
Radiation monitoring of waste from the blast furnace is necessary, and there is already a study that proves it established safe values for building materials, which indicates that the cement compounds studied do not have a significant effect on increasing the exposure risk of the population [102]. Metallurgical slag also has great potential as a raw material in building new engineering materials: for example, glass ceramics, porous ceramic materials, ceramic bricks, functional zeolites for wastewater treatment, and refractory materials.
Blast furnace slag is used in the composition of cement, adding special properties such as increased mechanical strength, morphology, and resistance to abrasion [103,104,105]. Converter slag (obtained by the pig iron industry) is not used as much for recycling due to a high free lime content, but there is potential if the free lime is stabilized by carbonation [106,107].
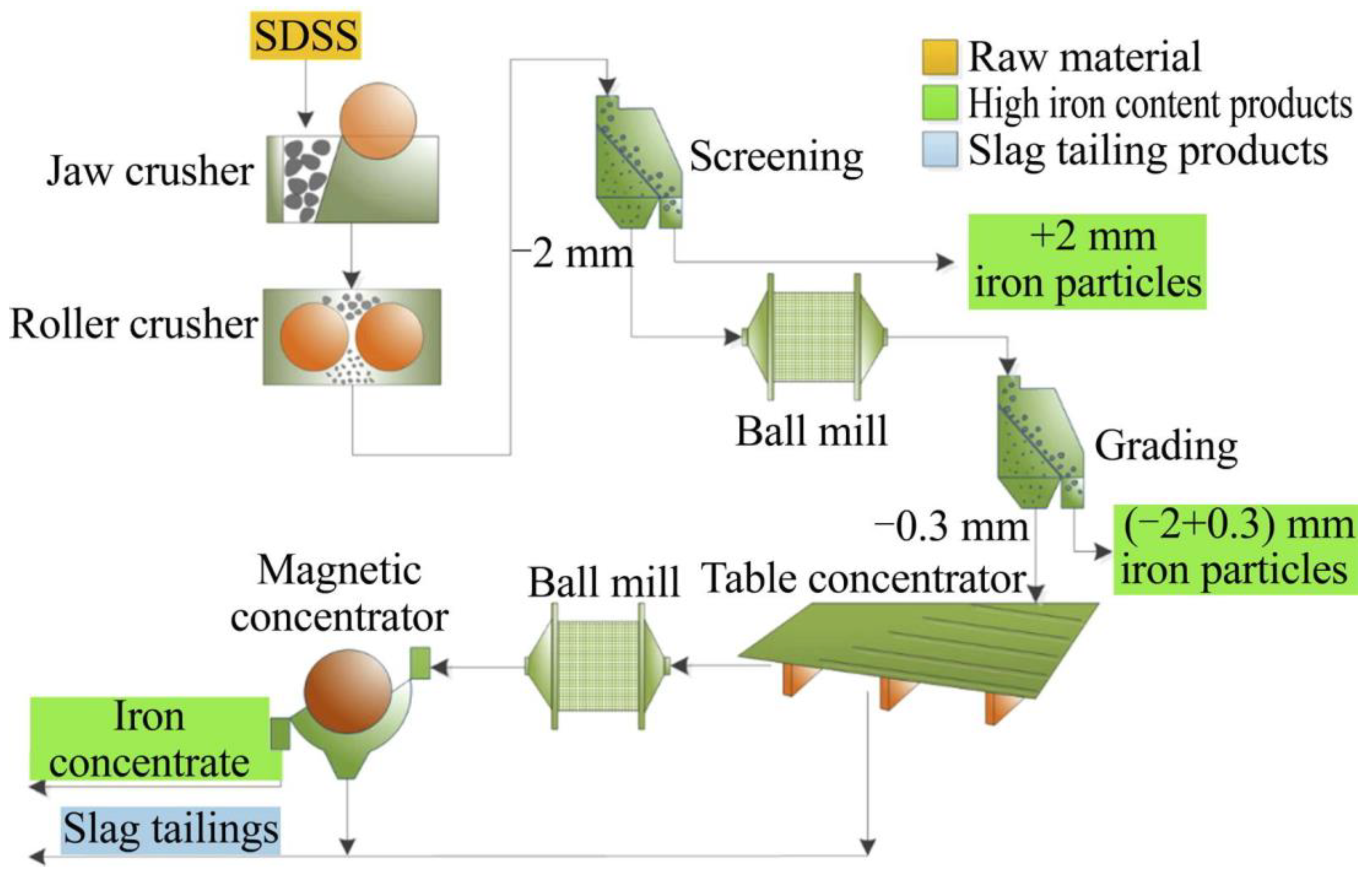
The slag from an electric arc furnace (EAF) and slag from desulfurization and slag skimming (SDSS) generated by steel mills have been used as a fine aggregate or concrete filling material in construction industries [12,108], but beneficiation treatment can be a requirement either due to unsuitable properties or to improve properties, safety, and market value, as exemplified in Figure 3. Excellent results were observed for slag rejects obtaining good homogeneity, good mechanical properties, and the possibility of up to 40% cement reduction in the application in concretes [109].
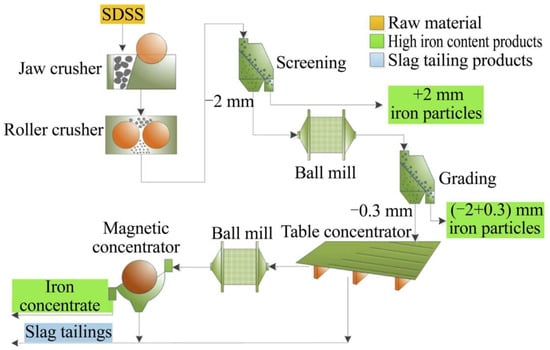
Figure 3.
Flowsheet of SSDS beneficiation experiment. Source: [108]. CC-BY-NC-ND 4.0.
In the mining process itself, there is the formation of slag, a waste material produced in ore extraction. This mining waste can be used as construction material (road construction), both in infrastructure and in the reclamation of land damaged by mining [110,111]. However, the use of slag from metallurgical processes is more common for the recovery/recycling of tailings from mining, so steel slag is among the more commonly used waste types [32,75]. For example, there is a study that was carried out recycling gold mining waste by slag atomization, being a process without harmful environmental impacts. However, a thorough evaluation of the process is necessary to obtain various types of materials, such as asphalt concrete, among others [74].
3.3. Future Perspectives of Mine Tailing Remediation Techniques
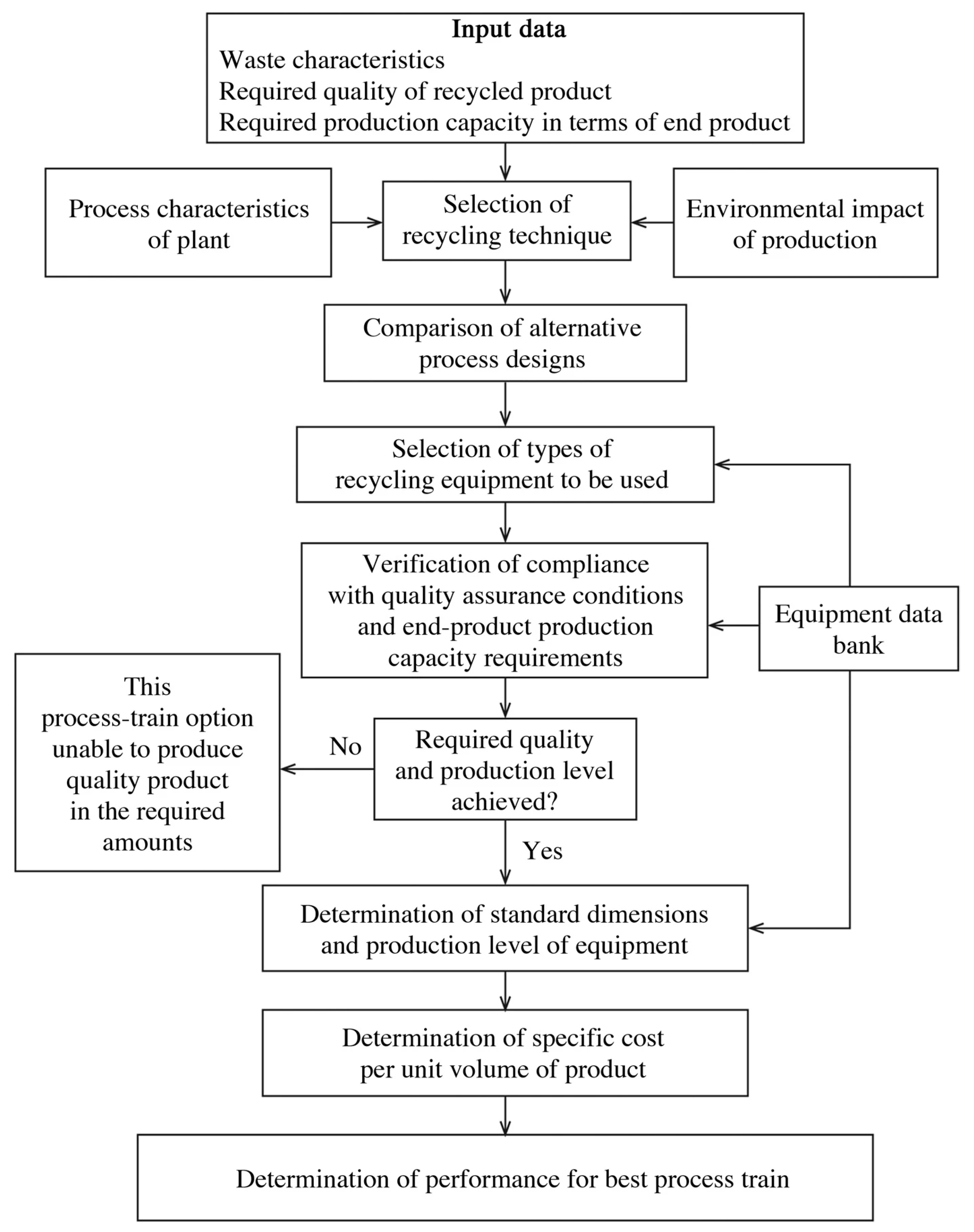
Mining waste generation and disposal must always be reviewed and updated periodically. Although several methods can be suggested for recycling mine waste, the key point is the presence of up-to-date feasibility and technical, operational, economic, and environmental studies in line with local legislation. The waste recycling technique used depends on the production capacity required, the type and volume of final product generated, and the public health and environmental regulations applicable to the production process [112,113]. Figure 4 shows a decision-making organogram for aiding in the design of upgrading processes for mine wastes [113].
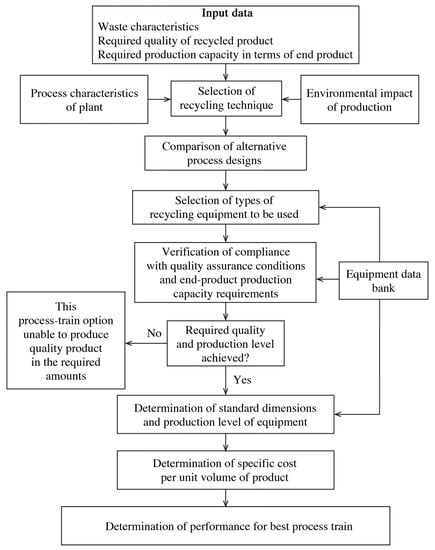
Figure 4.
Flow chart for mineral waste recycling process-train design algorithm. Source: [113]. Re-used with permission from Springer Nature (5342261298332).
Geopolymerization is a promising technology that is currently being studied to convert tailings or dumps into new raw materials. It consists of mixtures of a solution of sodium silicate and sodium hydroxide to which water is added. Iron ore tailings are blended in geopolymers as fine aggregates, fillers, and precursors, forming new materials that can be used as construction materials, such as roads and highways. This procedure can be used to recover several types of tailings, mainly copper (binder materials, bricks, and road-construction materials), iron (binder materials, bricks, and backfills for building foundations), and phosphate (binder materials), among other applications [114].
A practical example of the above is the study carried out by Figueiredo et al. [115], where a commercial metakaolin (MK)-based geopolymeric cement was prepared as precursor material, and sodium silicate, Na2SiO3, (SS), and sodium hydroxide, NaOH, (SH), as activators. Three different iron ore tailings (IOT) obtained from flotation processes of three mines located in the province of Quadrilátero Ferrífero, in the state of Minas Gerais, Brazil, were added to the above to use the mine tailings as filler. Subsequently, the chemical composition of the MK and IOT was evaluated by X-ray fluorescence analysis (XRF) with a Philips spectrometer. The incorporation of tailings into the geopolymer cements promoted an increase in compressive strength, which is promising for the development of better mine tailings management practices for alternative applications, but the authors state that further research is required to better understand the interactions between geopolymer matrices and backfills from mine tailings.
Another remarkably recent technology is the one that uses microorganisms, but it is still in the laboratory study phase. In addition, the lack of greater investment affects the development of this study, also being a chronic problem in a broader way. Although the economic return is satisfactory, there is a need for an initial investment that often limits advances in this area. To achieve a long-term aesthetic solution, the use of live plants or microorganisms/biomass, which can be implemented in situ for remediation of tailings and mill tailings, is proving to be a promising strategy. In this recent phenomenon, phytostabilization has emerged as an alternative recovery technique for the stabilization of environmental toxins using green plants, which is proving to be economical and self-sustaining and aims to rehabilitate the entire terrestrial as well as aquatic ecosystem [14,116].
It is remarkable that a greater effort is still needed to increase the recycling of solid waste. Mine tailings technologies can be improved, where there are many discrepancies in recycling rates and application of waste reduction technologies between a few developed countries (US, Japan, Western Europe, China) and most countries [117,118]. It is necessary to involve industrial waste in use because of its potentially valuable consumer properties to develop and implement low-waste technologies in cooperation with scientific organizations [119].
4. Conclusions
In recent years, several adequate resource management approaches have been used for recycling mine waste, most notably the recovery of valuable minerals and metals, production of cost-effective building materials, and preparation of soil modifiers and agricultural fertilizers [120,121,122].
A greater concern of the countries for the better use of this waste to provide profitability to the use of these components in various areas of application is notable. From the analyzed works, studies were observed in relation to the use of waste in almost 40 countries, mainly highlighting China, India, the United States, Spain, Japan, Australia, the United Kingdom, Russia, Canada, and South Africa. Many of these countries produce large amounts of solid waste, but in parallel, they are evolving the issue of waste recycling, with the aim of reducing environmental impacts.
Despite the high demand from the construction industry (1.5 billion tonnes), the use of tailings as construction material does not reach 1% in volume [118]. This situation occurs due to factors such as the value of building materials being relatively low compared to other products with higher added value and transportation costs [118,123].
In this review, the successful or potentially implementable approaches covered predominantly included those where the material properties resulted in ecologically friendly and low-cost resource recovery, compared to traditional materials. To this end, it has been clear from the literature that the recycling or recovery of tailings from mines has been applied as construction materials, despite low profitability, and agricultural applications, but this is limited to tailings having non-hazardous compositions and being in reasonably close locations to their end use.
To tackle these wastes, engineered technologies to reprocess them are needed, and the most innovative examples of these from the literature have been bioleaching, flotation, and magnetic separation. Even then, limitations arise when it comes to the complexity of the recovery process, or of the mineral composition itself, and the still generation of residues (sludges and wastewaters) with burdensome contaminants (metals, ligands, surfactants, acids, microbes, etc.).
There is a need for more research on the recycling and recovery of tailings from mines, with little R&D being observed in different chemical compositions and the geotechnical characterization of tailings. Tailing recycling is thus still incipient in most countries in terms of volume, and local regulatory pressures have been the main drivers for action in wealthier nations and those with strong environmental advocation groups.
Despite a relatively vast amount of literature on the research subject of mine tailing reuse and resource recovery, the subject also remains quite broadly tackled. This leaves several research gaps in need of more attention, mainly those concerning technological transfer from academia to practice and improved efficiency in the recovery and use of valuable compounds from tailings, including chromium, cobalt, manganese, nickel, bauxite, aluminum, zinc, silver, feldspar, and bentonite, among others.
The reuse of mining waste involves integrated and properly controlled management, which does not always lead to the desired results. To improve the current situation, the integration of different methodologies and available technologies will be necessary, and inspiration could be found in the principles of process intensification [124], which, despite their origins in the field of chemical engineering, have the potential to bring disruptive solutions to the fields of mineral and solid waste processing.
Author Contributions
Conceptualization, F.S.M.A. and R.M.S.; methodology, F.S.M.A. and I.T.-L.; investigation, F.S.M.A., I.T.-L., and E.B.N.; writing—original draft preparation, F.S.M.A. and I.T.-L.; writing—review and editing, E.B.N. and R.M.S.; visualization, I.T.-L.; supervision, R.M.S.; project administration, R.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
ITL was sponsored by the Mitacs Globalink program and “Convocatoria Jóvenes Investigadores e Innovadores en el marco de la Reactivación Económica 2021–Mecanismo 1, Minciencias–Ministerio de Ciencia, Tecnología e Innovación”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank Hugo Fantucci for providing advice and language revisions on the first version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cadeias, C.; Ávila, P.; Coelho, P.; Teixeira, J.P. Mining Activities: Health Impacts. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J.O., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 788–802. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Osanloo, M.; Azimi, Y. Evaluation of positive and negative impacts of mining on sustainable development by a semi-quantitative method. J. Clean. Prod. 2022, 366, 132955. [Google Scholar] [CrossRef]
- Shavina, E.; Prokofev, V. Implementation of environmental principles of sustainable development in the mining region. E3S Web Conf. 2020, 174, 1–6. [Google Scholar] [CrossRef]
- Garbarino, E.; Orveillon, G.; Saveyn, H.G. Management of waste from extractive industries: The new European reference document on the Best Available Techniques. Resour. Policy 2020, 69, 101782. [Google Scholar] [CrossRef]
- Agboola, O.; Babatunde, D.E.; Fay+omi, O.S.I.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Yahaya, A.; Mamudu, O.A. A review on the impact of mining operation: Monitoring, assessment and management. Results Eng. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Vitti, C.; Arnold, B.J. The Reprocessing and Revalorization of Critical Minerals in Mine Tailings. Mining, Met. Explor. 2022, 39, 1–6. [Google Scholar] [CrossRef]
- Agboola, A.A. Farming Systems in NIGERIA. Agronomy in Nigeria; Wiley and Sons: Hoboken, NJ, USA; University of Ibadan: Ibadan, Nigeria, 2000; pp. 25–34. [Google Scholar]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Luthra, S.; Mangla, S.K.; Sarkis, J.; Tseng, M.-L. Resources melioration and the circular economy: Sustainability potentials for mineral, mining and extraction sector in emerging economies. Resour. Policy 2022, 77, 1–4. [Google Scholar] [CrossRef]
- Marín, O.A.; Kraslawski, A.; Cisternas, L.A. Estimating processing cost for the recovery of valuable elements from mine tailings using dimensional analysis. Miner. Eng. 2022, 184, 107629. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Tanaka, M.; Shekdar, A.V. Global trends in waste generation. In Recycling, Waste Treatment and Clean Technology; Gaballah, I., Mishar, B., Solozabal, R., Tanaka, M., Eds.; TMS Mineral, Metals and Materials Publishers: Madrid, Spain, 2004; pp. 1541–1552. [Google Scholar]
- Pappu, A.; Saxena, M.; Asolekar, S.R. Solid wastes generation in India and their recycling potential in building materials. Build. Environ. 2007, 42, 2311–2320. [Google Scholar] [CrossRef]
- Galán, J.E. The benefits are at the tail: Uncovering the impact of macroprudential policy on growth-at-risk. J. Financ. Stab. 2020, 100831. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.; Xu, H.; Cui, X.; Li, J.; Chen, J.; Zheng, B. Characterizations of heavy metal contamination, microbial com-497 munity, and resistance genes in a tailing of the largest copper mine in China. Environ. Pollut. 2021, 280, 116947. [Google Scholar] [CrossRef] [PubMed]
- Jawadand, S.; Randive, K. A Sustainable Approach to Transforming Mining Waste into Value-Added Products. In Innovations in Sustainable Mining; Springer: Cham, Switzerland, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Shengo, L.M. Review of Practices in the Managements of Mineral Wastes: The Case of Waste Rocks and Mine Tailings. Wat. Air Soil Poll. 2021, 232, 273. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Mal, U.; Adhikari, K. Groundwater quality and hydrological stress induced by Lower Gondwana open cast coal mine. J. Earth Syst. Sci. 2021, 130, 32. [Google Scholar] [CrossRef]
- Tatsuhara, T.; Arima, T.; Igarashi, T.; Tabelin, C.B. Combined neutralization–adsorption system for the disposal of hydrothermally altered excavated rock producing acidic leachate with hazardous elements. Eng. Geol. 2012, 139–140, 76–84. [Google Scholar] [CrossRef]
- Lottermoser, B.G.; Ashley, P.M. Mobility and retention of trace elements in hardpan-cemented cassiterite tailings, north Queensland, Australia. Environ. Earth Sci. 2006, 50, 835–846. [Google Scholar] [CrossRef]
- Mackasey, W.O. Abandoned Mines in Canada; Unpublished Report prepared for Mining Watch Canada; WOM Geological Associates, Inc.: Sudbury, OM, Canada, 2000. [Google Scholar]
- Chartrand, M.M.G. Electrochemical remediation of acid mine drainage. J. Appl. Electrochem. 2003, 33, 259–264. [Google Scholar] [CrossRef]
- Wu, P.; Tang, C.; Liu, C.; Zhu, L.; Pei, T.; Feng, L. Geochemical distribution and removal of As, Fe, Mn and Al in a surface water system affected by acid mine drainage at a coalfield in Southwestern China. Environ. Earth Sci. 2008, 57, 1457–1467. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Zagury, G.J.; Kulnieks, V.I.; Neculita, C.M. Characterization and reactivity assessment of organic substrates for sul-phate-reducing bacteria in acid mine drainage treatment. Chemosphere 2006, 64, 944–954. [Google Scholar] [CrossRef]
- Zhong, C.M.; Xu, Z.L.; Fang, X.H.; Cheng, L. Treatment of acid mine drainage (AMD) by ultra-low pressure reverse osmosis and nanofiltration. Environ. Eng. Sci. 2007, 24, 1297–1306. [Google Scholar] [CrossRef]
- McLellan, B.; Corder, G.; Giurco, D.; Green, S. Incorporating sustainable development in the design of mineral processing operations—Review and analysis of current approaches. J. Clean. Prod. 2009, 17, 1414–1425. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2010; 400p. [Google Scholar]
- BRGM. Management of Mining, Quarrying and Ore-Processing Waste in the European Union; 7 Figs., 17 Tables, 7 annexes, 1 CD-ROM (Collected data); BRGM: Orlèans, France, 2001; 79p. [Google Scholar]
- Hann, D. Copper tailings reprocessing. RMZ Mater. Geoenviron. 2021, 67, 1–10. [Google Scholar] [CrossRef]
- Matinde, E.; Simate, G.; Ndlovu, S. Mining and metallurgical wastes: A review of recycling and re-use practices. J. S. Afr. Inst. Min. Met. 2018, 118, 825–844. [Google Scholar] [CrossRef]
- Oluwasola, E.A.; Hainin, M.R.; Aziz, M.M.A. Evaluation of asphalt mixtures incorporating electric arc furnace steel slag and copper mine tailings for road construction. Transp. Geotech. 2015, 2, 47–55. [Google Scholar] [CrossRef]
- Çelik, Ö.; Elbeyli, I.Y.; Piskin, S. Utilization of gold tailings as an additive in Portland cement. Waste Manag. Res. J. Sustain. Circ. Econ. 2006, 24, 215–224. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.; Alfredsson, M.; Edwards, S.; Macklin, M.; Hudson-Edwards, K. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Shamsuddin, M. Physical Chemistry of Metallurgical Processes, 1st ed.; The Minerals, Metals & Materials Society; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Submarine Tailings Disposal Toolkit. Available online: http://www.miningwatch.ca/files/01.STDtoolkit.intr_.pdf (accessed on 12 June 2014).
- MiningWatch Canada Webpage. Troubled Waters: How Mine Waste Dumping is Poisoning Our Oceans, Rivers, and Lakes. Available online: http://www.miningwatch.ca/news/troubled-watershow-mine-waste-dumping-poisoning-our-oceans-rivers-and-lakes (accessed on 12 June 2014).
- Dold, B. Submarine Tailings Disposal (STD)—A Review. Minerals 2014, 4, 642–666. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Zhang, L. Production of bricks from waste materials—A review. Constr. Build. Mater. 2013, 47, 643–655. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Production of eco-friendly bricks from copper mine tailings through geopolymerization. Constr. Build. Mater. 2012, 29, 323–331. [Google Scholar] [CrossRef]
- Tang, Z.; Lei, T.; Yu, J.; Shainberg, I.; Mamedov, A.I.; Ben-Hur, M.; Levy, G.J.; Mamedov, A.I. Runoff and Interrill Erosion in Sodic Soils Treated with Dry PAM and Phosphogypsum. Soil Sci. Soc. Am. J. 2006, 70, 679–690. [Google Scholar] [CrossRef]
- Zhang, X.C.; Miller, W.P.; Nearing, M.A.; Norton, L.D. Effects of surface treatment on surface sealing, runoff, and interrill erosion. Trans. ASAE 1998, 41, 989–994. [Google Scholar] [CrossRef]
- Takahashi, T.; Ikeda, Y.; Nakamura, H.; Nanzyo, M. Efficiency of gypsum application to acid Andosols estimated using aluminum release rates and plant root growth. Soil Sci. Plant Nutr. 2006, 52, 584–592. [Google Scholar] [CrossRef]
- Rodríguez-Jordá, M.; Garrido, F.; García-González, M. Potential use of gypsum and lime rich industrial by-products for induced reduction of Pb, Zn and Ni leachability in an acid soil. J. Hazard Mater. 2010, 175, 762–769. [Google Scholar] [CrossRef]
- Delgado, A.; Madrid, A.; Kassem, S.; Andreu, L.; del Campillo, M.C. Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant Soil 2002, 245, 277–286. [Google Scholar] [CrossRef]
- Gorakhki, M.H.; Bareither, C.A. Sustainable Reuse of Mine Tailings and Waste Rock as Water-Balance Covers. Minerals 2017, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Lottermoser, B. Recycling, Reuse and Rehabilitation of Mine Wastes. Elements 2011, 7, 405–410. [Google Scholar] [CrossRef]
- Owen, J.; Kemp, D.; Lèbre, É.; Svobodova, K.; Murillo, G.P. Catastrophic tailings dam failures and disaster risk disclosure. Int. J. Disaster Risk Reduct. 2019, 42, 101361. [Google Scholar] [CrossRef]
- Kumar, U.; Singh, D.N. E-waste management through regulations. Int. J. Eng. Invent. 2013, 3, 6–14. [Google Scholar] [CrossRef]
- Azcue, J.M. Environmental Impacts of Mining Activities: Emphasis on Mitigation and Remedial Measures; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Cobîrzan, N.; Muntean, R.; Thalmaier, G.; Felseghi, R.-A. Recycling of Mining Waste in the Production of Masonry Units. Materials 2022, 15, 594. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Sánchez, J.A.; García-Gómez, J.J.; Velasco-Muñoz, J.F.; Carretero-Gómez, A. Mining Waste and Its Sustainable Management: Advances in Worldwide Research. Minerals 2018, 8, 284. [Google Scholar] [CrossRef]
- Wang, H.-G.; Liu, W.; Jia, N.; Zhang, M.; Guo, M. Facile synthesis of metal-doped Ni-Zn ferrite from treated Zn-containing electric arc furnace dust. Ceram. Int. 2017, 43, 1980–1987. [Google Scholar] [CrossRef]
- Ndlovu, S.; Simate, G.S.; Matinde, E. Waste Production and Utilization in the Metal Extraction Industry, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Mackay, I.; Mendez, E.; Molina, I.; Videla, A.R.; Cilliers, J.J.; Brito-Parada, P.R. Dynamic froth stability of copper flotation tailings. Miner. Eng. 2018, 124, 103–107. [Google Scholar] [CrossRef]
- Kalisz, S.; Kibort, K.; Mioduska, J.; Lieder, M.; Małachowska, A. Waste management in the mining industry of metals ores, coal, oil and natural gas—A review. J. Environ. Manag. 2021, 304, 114239. [Google Scholar] [CrossRef]
- Rao, G.V.; Markandeya, R.; Kumar, R. Modeling and Optimisation of Multigravity Separator for Recovery of Iron Values from Sub Grade Iron Ore Using Three Level Three Factor Box Behnken Design. Int. J. Miner. Process. Extr. Met. 2017, 2, 46. [Google Scholar] [CrossRef]
- Duarte, J.C.; Estrada, P.; Beaumont, H.; Sitima, M.; Pereira, P. Biotreatment of tailings for metal recovery. Mine Water Environ. 1990, 9, 193–206. [Google Scholar] [CrossRef]
- Stankovic, S.; Moric, I.; Pavic, A.; Vojnovic, S.; Vasiljevic, B.; Cvetkovic, V. Bioleaching of copper from old flotation tailings samples (Copper Mine Bor, Serbia). J. Serbian Chem. Soc. 2015, 80, 391–405. [Google Scholar] [CrossRef]
- Pulungan, L.; Pramusanto, P.; Hermana, F.A. The research of gold processing from tailings of iron sand processing from South Kalimantan by using amalgamation methods in West Java. J. Phys. Conf. Ser. 2019, 1375, 12047. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Macías, F.; Pérez-López, R.; Basallote, M.D.; Millán-Becerro, R. Valorization of wastes from the fertilizer industry: Current status and future trends. J. Clean. Prod. 2018, 174, 678–690. [Google Scholar] [CrossRef]
- Garg, M.; Singh, M.; Kumar, R. Some aspects of the durability of a phosphogypsum-lime-fly ash binder. Constr. Build. Mater. 1996, 10, 273–279. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Yan, J.; Li, Z.; Hwang, J.-Y.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Clean. Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- de Buzin, P.J.W.K.; Heck, N.C.; Vilela, A.C.F. EAF dust: An overview on the influences of physical, chemical and mineral features in its recycling and waste incorporation routes. J. Mater. Res. Technol. 2017, 6, 194–202. [Google Scholar] [CrossRef]
- Rodríguez, O.; Alguacil, F.J.; Baquero, E.E.; García-Díaz, I.; Fernández, P.; Sotillo, B.; López, F.A. Recovery of niobium and tantalum by solvent extraction from Sn–Ta–Nb mining tailings. RSC Adv. 2020, 10, 21406–21412. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.; Rojo, A.; Ottosen, L. Electrodialytic Remediation of Copper Mine Tailings. Procedia Eng. 2012, 44, 2053–2055. [Google Scholar] [CrossRef]
- Bisio, G. Energy recovery from molten slag and exploitation of the recovered energy. Energy 1997, 22, 501–509. [Google Scholar] [CrossRef]
- Barati, M.; Esfahani, S.; Utigard, T. Energy recovery from high temperature slags. Energy 2011, 36, 5440–5449. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Q.; Wang, Z. Comprehensive Analysis of the Coal Particle in Molten Blast Furnace Slag To Recover Waste Heat. Energy Fuels 2017, 31, 8813–8819. [Google Scholar] [CrossRef]
- Šajn, R.; Ristović, I.; Čeplak, B. Mining and Metallurgical Waste as Potential Secondary Sources of Metals—A Case Study for the West Balkan Region. Minerals 2022, 12, 547. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Vaughan, J.; Southam, G.; van der Ent, A.; Nkrumah, P.N.; Ma, X.; Parbhakar-Fox, A. Review on metal extraction technologies suitable for critical metal recovery from mining and processing wastes. Miner. Eng. 2022, 182, 107537. [Google Scholar] [CrossRef]
- Kim, S.-K.; Yang, D.-H.; Rao, S.; Nam, C.-W.; Rhee, K.-I.; Sohn, J.-S. A new approach to the recycling of gold mine tailings using red mud and waste limestone as melting fluxes. Geosystem Eng. 2012, 15, 44–49. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Yliniemi, J.; Ismailov, A.; Levänen, E.; Tanskanen, P.; Kinnunen, P.; Röning, J.; Illikainen, M. Recycling lithium mine tailings in the production of low temperature (700–900 °C) ceramics: Effect of ladle slag and sodium compounds on the processing and final properties. Constr. Build. Mater. 2019, 221, 332–344. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kim, Y.J.; Choi, O.; Lee, K.M.; Lachemi, M. Utilization of tailings from tungsten mine waste as a substitution material for cement. Constr. Build. Mater. 2009, 23, 2481–2486. [Google Scholar] [CrossRef]
- Qiu, G.; Luo, Z.; Shi, Z.; Ni, M. Utilization of coal gangue and copper tailings as clay for cement clinker calcinations. J. Wuhan Univ. Technol. Sci. Ed. 2011, 26, 1205–1210. [Google Scholar] [CrossRef]
- Prahallada, M.C.; Shanthappa, B.C. Use of copper ore tailings—As an excellent pozzolana in the preparation of concrete. Int. J. Adv. Eng. 2014, 3, 1–10. [Google Scholar]
- Onuaguluchi, O.; Eren, Ö. Reusing copper tailings in concrete: Corrosion performance and socioeconomic implications for the Lefke-Xeros area of Cyprus. J. Clean. Prod. 2016, 112, 420–429. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R. Premchand Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- da Silva, F.; Araújo, F.; Teixeira, M.; Gomes, R.; von Krüger, F. Study of the recovery and recycling of tailings from the concentration of iron ore for the production of ceramic. Ceram. Int. 2014, 40, 16085–16089. [Google Scholar] [CrossRef]
- Shewalul, Y.W. Experimental study of the effect of waste steel scrap as reinforcing material on the mechanical properties of concrete. Case Stud. Constr. Mater. 2021, 14, e00490. [Google Scholar] [CrossRef]
- Rouaiguia, I.; Bounouala, M.; Abdelmalek, C.; Idres, A.; Benselhoub, A. Optical sorting technology for waste management from the Boukhadra iron ore mine (NE Algeria). REM—Int. Eng. J. 2022, 75, 55–65. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Mineração (IBRAM). Gestão e Manejo de Rejeitos da Mineração, 1st ed.; Instituto Brasileiro de Mineração, IBRAM: Brasilia, Brazil, 2016; p. 128. [Google Scholar]
- Mueller, H.; Maithy, S.; Prajapati, S.; Bhatta, A.D.; Shrestha, B.L. Greenbrick Making Manual; Hillside Press: Kathmandu, Nepal, 2008; Available online: http://www.ecobrick.in/resource_data/KBAS100046.pdf. (accessed on 1 November 2021).
- Bennet, J.M.; Sudhakar, M.; Natarajan, C. Development of coal ash—GGBS based geopolymer bricks. EJOSAT 2013, 2, 133–139. [Google Scholar]
- Kuranchie, F.A.; Shukla, S.; Habibi, D.; Mohyeddin, A. Utilisation of iron ore tailings as aggregates in concrete. Cogent Eng. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Ugama, T.I.; Ejeh, S.P.; Amartey, D.Y. Effect of iron ore tailing on the properties of concrete. Civ. Environ. Res. 2014, 6, 7–13. [Google Scholar]
- Utilization of iron ore tailings as replacement to fine aggregates in cement concrete pavements. Int. J. Res. Eng. Technol. 2014, 3, 369–376. [CrossRef]
- Niu, H.; Helser, J.; Corfe, I.J.; Kuva, J.; Butcher, A.R.; Cappuyns, V.; Kinnunen, P.; Illikainen, M. Incorporation of bioleached sulfidic mine tailings in one-part alkali-activated blast furnace slag mortar. Constr. Build. Mater. 2022, 333, 127195. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Bian, X.; Zeng, L.; Ji, F.; Xie, M.; Hong, Z. Plasticity role in strength behavior of cement-phosphogypsum stabilized soils. J. Rock Mech. Geotech. Eng. 2022. [Google Scholar] [CrossRef]
- Kovler, K. Radiological constraints of using building materials and industrial by-products in construction. Constr. Build. Mater. 2009, 23, 246–253. [Google Scholar] [CrossRef]
- Vásconez-Maza, M.D.; Bueso, M.C.; Mulas, J.; Faz, Á.; Martínez-Segura, M.A. Characterising an abandoned phosphogypsum deposit by combining radiological, geophysical, geochemical, and statistical techniques. CATENA 2022, 216, 106401. [Google Scholar] [CrossRef]
- Nyirenda, R. The processing of steelmaking flue-dust: A review. Miner. Eng. 1991, 4, 1003–1025. [Google Scholar] [CrossRef]
- Beukes, J.P.; van Zyl, P.G.; RAS, M. Treatment of Cr (VI)-containing wastes in the South African ferrochrome industry—A review of currently applied methods. J. S. Afr. Inst. Min. Metall. 2012, 112, 347–352. [Google Scholar]
- Saxena, S.; Jotshi, C. Management and combustion of hazardous wastes. Prog. Energy Combust. Sci. 1996, 22, 401–425. [Google Scholar] [CrossRef]
- Reuter, M.; Xiao, Y.; Boin, U. Recycling and environmental issues of metallurgical slags and salt fluxes. In VII International Conference on Molten Slags, Fluxes and Salts; Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2004; Volume 2004, pp. 349–356. [Google Scholar]
- Durinck, D.; Engström, F.; Arnout, S.; Heulens, J.; Jones, P.T.; Björkman, B.; Blanpain, B.; Wollants, P. Hot stage processing of metallurgical slags. Resour. Conserv. Recycl. 2008, 52, 1121–1131. [Google Scholar] [CrossRef]
- Quijorna, N.; Miguel, G.S.; Andrés, A. Incorporation of Waelz Slag into Commercial Ceramic Bricks: A Practical Example of Industrial Ecology. Ind. Eng. Chem. Res. 2011, 50, 5806–5814. [Google Scholar] [CrossRef]
- Karayannis, V.; Ntampegliotis, K.; Lamprakopoulos, S.; Papapolymerou, G.; Spiliotis, X. Novel sintered ceramic materials in-693 corporated with EAF carbon steel slag. Mater. Res. Express. 2017, 4, 015505. [Google Scholar] [CrossRef]
- Estokova, A.; Singovszka, E. Assessment of risk from irradiation originating from mortars with mineral waste addition. Indoor Built Environ. 2021, 31, 219–229. [Google Scholar] [CrossRef]
- Dubey, S.S.A.; Kushwah, S.S. Utilisation of Iron and Steel Slag in Building Construction. Proceedings of the International Conference on Sustainable Materials and Structures for Civil Infrastructures (SMSCI2019). AIP Conf. Proc. 2019, 2158, 20032. [Google Scholar]
- Rosales, J.; Agrela, F.; Entrenas, J.A.; Cabrera, M. Potential of Stainless-Steel Slag Waste in Manufacturing Self-Compacting Concrete. Materials 2020, 13, 2049. [Google Scholar] [CrossRef]
- Fahad, M.B.; Abdulkarem, A.M.; Hamed, T.H. A review on wastes as sustainable construction materials. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 12014. [Google Scholar] [CrossRef]
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef]
- Bodor, M.; Santos, R.M.; Cristea, G.; Salman, M.; Cizer, Ö.; Iacobescu, R.I.; Chiang, Y.W.; Van Balen, K.; Vlad, M.; Van Gerven, T. Laboratory investigation of carbonated BOF slag used as partial replacement of natural aggregate in cement mortars. Cem. Concr. Compos. 2016, 65, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Gao, P.; Han, Y. Resource utilization of slag from desulphurization and slag skimming: A comprehensive recycling process of all components. Int. J. Min. Sci. Technol. 2022, 32, 585–593. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Z.; Liu, X.; Sheng, M.; Li, H.; Xu, X.; Ai, L.; Yan, Q.; Yang, Y. Preparation of composite micro-slag based on the application of tailings slag in cement and concrete. Constr. Build. Mater. 2022, 322, 126515. [Google Scholar] [CrossRef]
- Bian, Z.; Miao, X.; Lei, S.; Chen, S.-E.; Wang, W.; Struthers, S. The Challenges of Reusing Mining and Mineral-Processing Wastes. Science 2012, 337, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.; Gilmour, S.; Parbhakar-Fox, A.; Olin, P. Geometallurgical Characterization of Non-Ferrous Historical Slag in Western Tasmania: Identifying Reprocessing Options. Minerals 2019, 9, 415. [Google Scholar] [CrossRef]
- Kouziyev, D.; Krivenko, A.; Chezganova, D.; Valeriy, B. Sensing of Dynamic Loads in the Open-Cast Mine Combine. E3S Web Conf. 2019, 105, 3014. [Google Scholar] [CrossRef]
- Bardovsky, A.D.; Gorbatyuk, S.M.; Albul, S.V.; Gorbatyuk, N.V. Optimization of Process Flow Diagrams for Processing of Mineral Wastes. Metallurgist 2021, 65, 465–472. [Google Scholar] [CrossRef]
- Qaidi, S.; Tayeh, B.A.; Zeyad, A.M.; de Azevedo, A.R.; Ahmed, H.U.; Emad, W. Recycling of mine tailings for the geopolymers production: A systematic review. Case Stud. Constr. Mater. 2022, 16, e00933. [Google Scholar] [CrossRef]
- Figueiredo, R.A.; Silveira, A.B.; Melo, E.L.; Costa, G.Q.; Brandão, P.R.; Aguilar, M.T.; Henriques, A.B.; Mazzinghy, D.B. Mechanical and chemical analysis of one-part geopolymers synthesised with iron ore tailings from Brazil. J. Mater. Res. Technol. 2021, 14, 2650–2657. [Google Scholar] [CrossRef]
- Sheoran, V.; Choudhary, R. Phytostabilization of mine tailings. In Phytorestoration of Abandoned Mining and Oil Drilling Sites., 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 307–324. [Google Scholar]
- Shettima, A.U.; Hussin, M.W.; Ahmad, Y.; Mirza, J. Evaluation of iron ore tailings as replacement for fine aggregate in concrete. Constr. Build. Mater. 2016, 120, 72–79. [Google Scholar] [CrossRef]
- Samir, M.; Alama, F.; Buysse, P.; Van Nylen, T.; Ostanin, O. Disposal of Mining Waste: Classification and International Recycling Experience. E3S Web Conf. 2018, 41, 2012. [Google Scholar] [CrossRef]
- Tsukerman, V.A.; Ivanov, S.V. Use of Mineral Waste of Industrial Enterprises in the Arctic Zone of the Russian Federation. IOP Conf. Series: Earth Environ. Sci. 2022, 988, 32001. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Bai, J.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings: Part 1. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. J. Hazard Mater. 2010, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, L.; Wu, A.; Kabwe, E.; Chen, X.; Yan, R. Copper recycle from sulfide tailings using combined leaching of ammonia solution and alkaline bacteria. J. Clean. Prod. 2018, 189, 746–753. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Wu, K. Evaluation of the possibility of copper recovery from tailings by flotation through bench-scale, commissioning, and industrial tests. J. Clean. Prod. 2017, 171, 1039–1048. [Google Scholar] [CrossRef]
- Tyulenev, M.; Lesin, Y.; Litvin, O.; Maliukhina, E.; Abay, A. Increasing the Reliability of the Work of Artificial Filtering Arrays for the Purification of Quarry Waste Water. E3S Web Conf. 2017, 21, 2019. [Google Scholar] [CrossRef]
- Rivas, D.F.; Boffito, D.C.; Faria-Albanese, J.; Glassey, J.; Afraz, N.; Akse, H.; Boodhoo, K.; Bos, R.; Cantin, J.; Chiang, Y.W.; et al. Process intensification education contributes to sustainable development goals. Part 1. Educ. Chem. Eng. 2020, 32, 1–14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).