The Relationship between Bacterial Sulfur Cycling and Ca/Mg Carbonate Precipitation—Old Tales and New Insights from Lagoa Vermelha and Brejo do Espinho, Brazil
Abstract
:1. Introduction
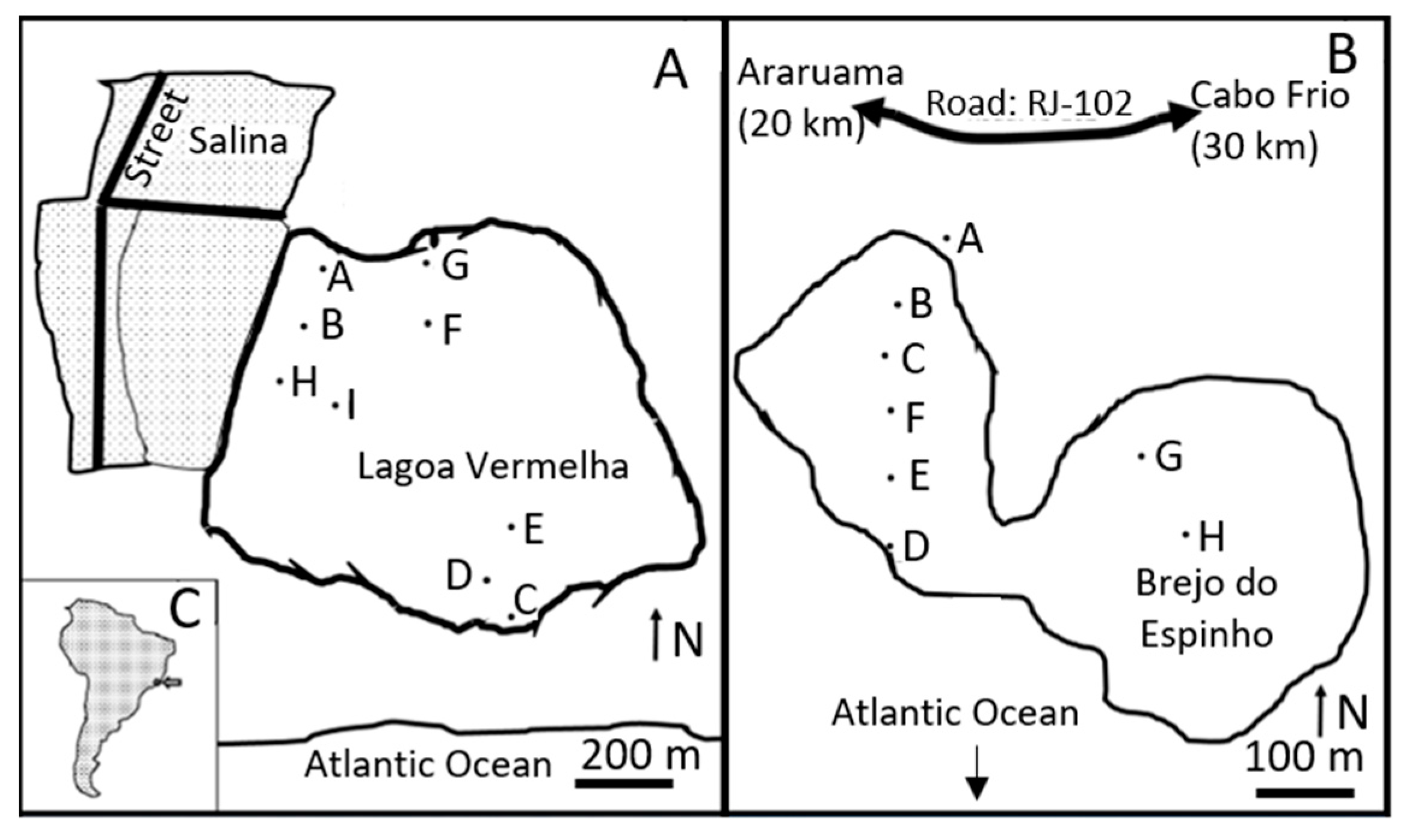
2. Study Area
3. Sampling and Methodology
3.1. Sampling Strategy and Fieldwork
3.2. Sample Preparation and Laboratory Analyses
3.3. Isotope Analysis
4. Results
4.1. Field Observations and Facies Description
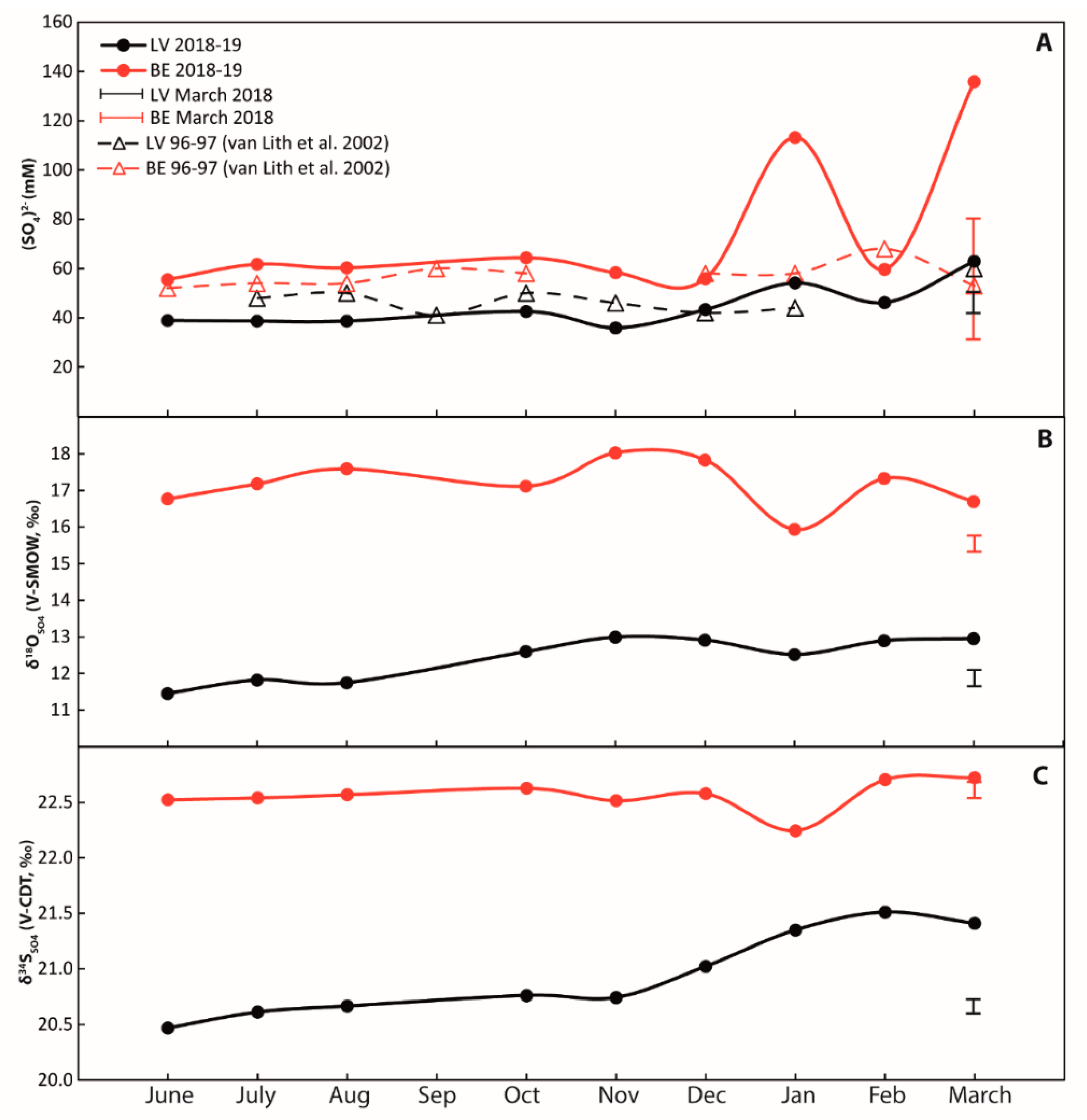
4.2. Geochemistry of Water Samples
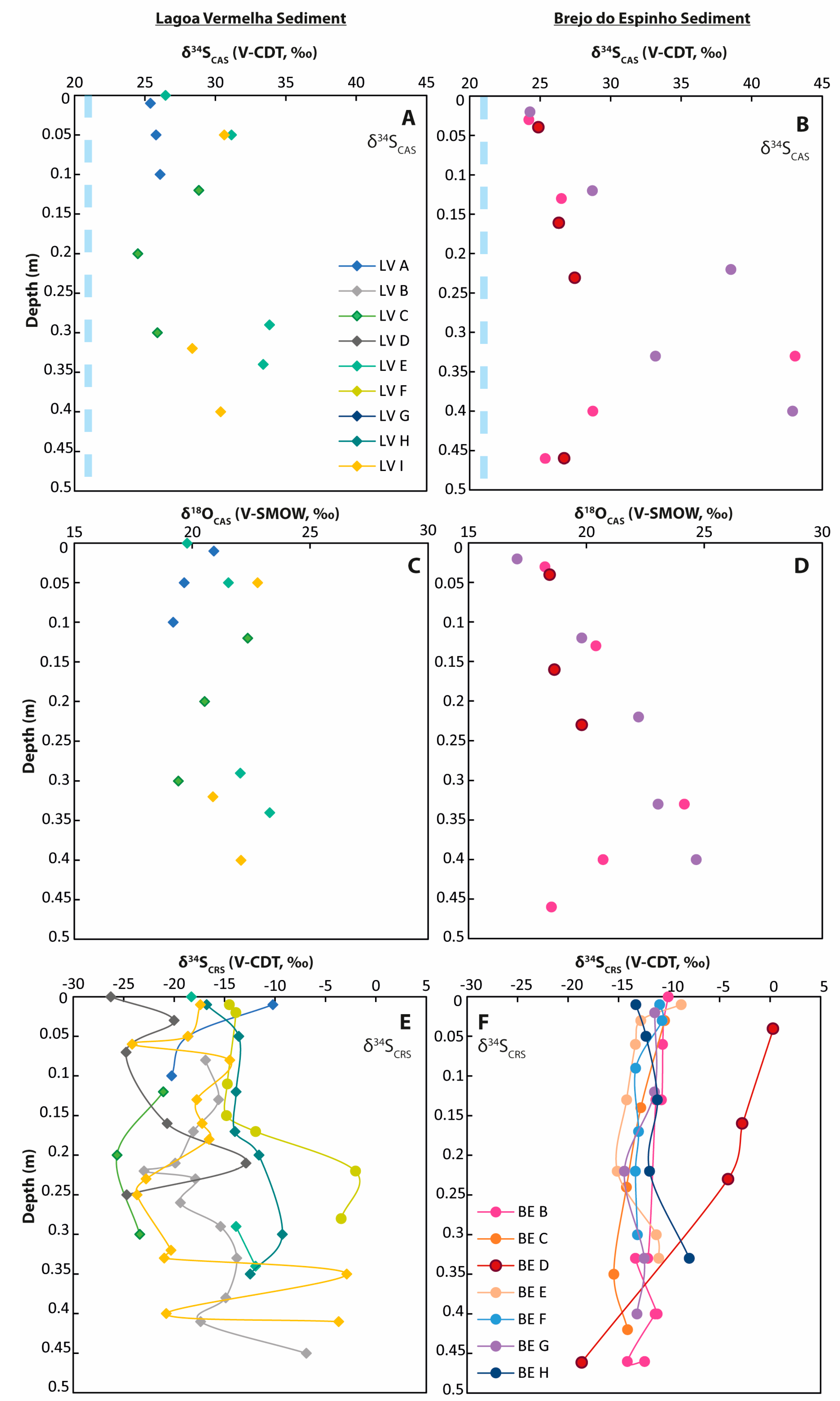
4.3. Geochemistry of Sediment Samples
5. Discussion
5.1. Sulfur Cycling in Surface Water
5.1.1. Sulfate Concentrations—Controlled by Evaporation and Rain Events
5.1.2. Sulfur and Oxygen Isotopes—Influenced by BSR, Sulfur Oxidation, and Evaporation
5.2. Pore-Water: Two Types of Sulfur Cycling
5.3. δ34S in Pyrite Revealing Differences in Sulfur Cycling
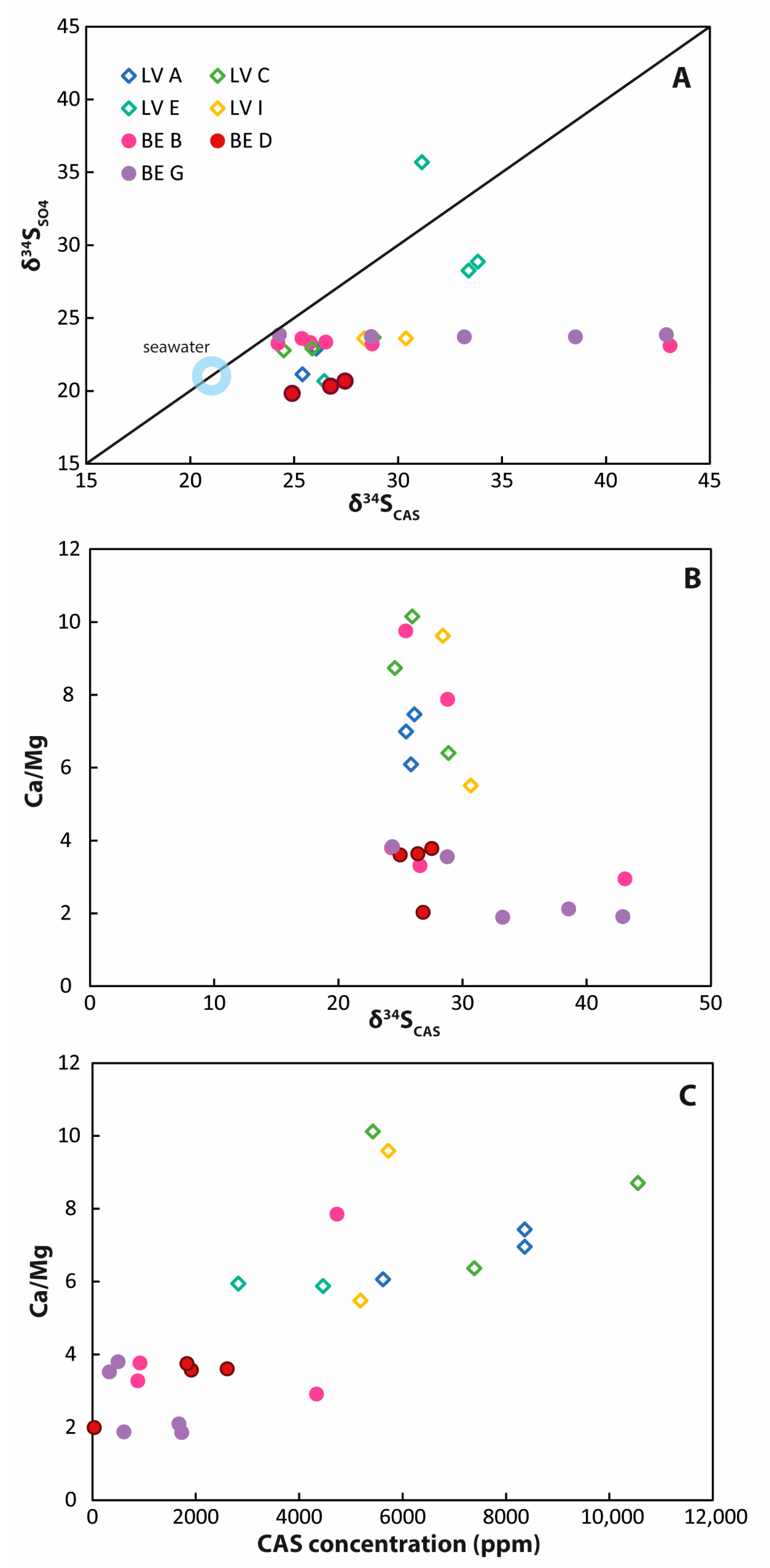
5.4. Carbonate-Associated Sulfate (CAS) Showing Different Formation Environments for Ca/Mg and Ca Carbonates
5.5. More Implications on Lagoonal Ca/Mg Carbonate Formation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Given, R.K.; Wilkinson, B.H. Dolomite abundance and stratigraphic age; constraints on rates and mechanisms of Phanerozoic dolostone formation. J. Sed. Res. 1987, 57, 1068–1078. [Google Scholar] [CrossRef]
- Warren, J. Dolomite: Occurrence, evolution and economically important associations. Earth-Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Tucker, M.E. Precambrian dolomites: Petrographic and isotopic evidence that they differ from Phanerozoic dolomites. Geology 1982, 10, 7–12. [Google Scholar] [CrossRef]
- Grotzinger, J.P. Facies and evolution of Precambrian carbonate depositional systems: Emergence of the modern platform archetype. In Controls on Carbonate Platforms and Basin Development; SEPM Special Publications: Tulsa, OK, USA, 1989; Volume 44, pp. 79–107. [Google Scholar]
- Chang, B.; Li, C.; Liu, D.; Foster, I.; Tripati, A.; Lloyd, M.K.; Maradiaga, I.; Luo, G.; An, Z.; She, Z.; et al. Massive formation of early diagenetic dolomite in the Ediacaran ocean: Constraints on the “dolomite problem”. Proc. Natl. Acad. Sci. USA 2020, 117, 14005–14014. [Google Scholar] [CrossRef]
- Schmoker, J.W.; Krystink, K.B.; Halley, R.B. Selected characteristics of limestone and dolomite reservoirs in the United States. Am. Assoc. Petrol. Geol. Bull. 1985, 69, 733–741. [Google Scholar]
- Lumsden, D.N. Secular variations in dolomite abundance in deep marine sediments. Geology 1985, 13, 766–769. [Google Scholar] [CrossRef]
- Holland, H.D.; Zimmerman, H. The dolomite problem revisited. Int. Geol. Rev. 2000, 42, 37–41. [Google Scholar] [CrossRef]
- Gaines, A.M. Protodolomite redefined. J. Sediment. Petrol. 1977, 47, 543–546. [Google Scholar]
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review. Sedimentology 2015, 62, 1749–1769. [Google Scholar] [CrossRef]
- Petrash, D.A.; Bialik, O.M.; Bontognali, T.R.R.; Vasconcelos, C.; Roberts, J.A.; Mckenzie, J.A.; Konhauser, K.O. Microbially catalyzed dolomite formation: From near-surface to burial. Earth-Sci. Rev. 2017, 171, 558–582. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Mckenzie, J.A. Microbial mediation of modern dolomite precipitation and diagenesis under anoxic conditions (Lagoa Vermelha, Rio de Janeiro, Brazil). J. Sed. Res. 1997, 67, 378–390. [Google Scholar]
- Wright, D.T. The role of sulphate-reducing bacteria and cyanobacteria in dolomite formation in distal ephemeral lakes of the Coorong region, South Australia. Sediment. Geol. 1999, 126, 147–157. [Google Scholar] [CrossRef]
- Wright, D.T.; Wacey, D. Precipitation of dolomite using sulphate reducing bacteria from the Coorong Region, South Australia: Significance and implications. Sedimentology 2005, 52, 987–1008. [Google Scholar] [CrossRef]
- Warthmann, R.; van Lith, Y.; Vasconcelos, C.; McKenzie, J.A.; Karpoff, A.M. Bacterially induced dolomite precipitation in anoxic culture experiments. Geology 2000, 28, 1091–1094. [Google Scholar] [CrossRef]
- Moreira, N.F.; Walter, L.M.; Vasconcelos, C.; Mckenzie, J.A.; McCall, P.J. Role of sulfide oxidation in dolomitization: Sediment and pore-water geochemistry of a modern hypersaline lagoon system. Geology 2004, 32, 701–704. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Warthmann, R.; Mckenzie, J.A.; Visscher, P.T.; Bittermann, A.G.; van Lith, Y. Lithifying microbial mats in Lagoa Vermelha, Brazil: Modern Precambrian relics? Sed. Geol. 2006, 185, 175–183. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Vasconcelos, C.; Schmid, T.; Dittrich, M.; McKenzie, J.A. Aerobic microbial dolomite at the nanometer scale: Implications for the geologic record. Geology 2008, 36, 879–882. [Google Scholar] [CrossRef]
- Krause, S.; Liebetrau, V.; Gorb, S.; Sánchez-Román, M.; McKenzie, J.A.; Treude, T. Microbial nucleation of Ca/Mg dolomite in exopolymeric substances under anoxic modern seawater salinity: New insight into an old enigma. Geology 2012, 40, 587–590. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; McKenzie, J.A.; Warthmann, R.J.; Vasconcelos, C. Microbially influenced formation of Mg-calcite and Ca-dolomite in the presence of exopolymeric substances produced by sulfate-reducing bacteria. Terra Nova 2014, 26, 72–77. [Google Scholar] [CrossRef]
- Bahniuk, A.; Mckenzie, J.A.; Perri, E.; Bontognali, T.R.R.; Vögeli, N.; Rezende, C.E.; Rangel, T.P.; Vasconcelos, C. Characterization of environmental conditions during microbial Ca/Mg carbonate precipitation and early diagenetic dolomite crust formation: Brejo do Espinho, Rio de Janeiro, Brazil. Geol. Soc. London Spec. Publ. 2015, 418, 243–259. [Google Scholar] [CrossRef]
- Areias, C.; Barbosa, C.F.; Cruz, A.P.S.; McKenzie, J.A.; Ariztegui, D.; Eglinton, T.; Haghipour, N.; Vasconcelos, C.; Sánchez-Román, M. Organic matter diagenesis and precipitation of Ca/Mg carbonate and dolomite in modern hypersaline lagoons linked to climate changes. Geochim. Cosmochim. Acta 2022, 337, 14–32. [Google Scholar] [CrossRef]
- Han, Y.J.; Aizenberg, J. Effect of magnesium ions on oriented growth of calcite on carboxylic acid functionalized self-assembled monolayer. J. Am. Chem. Soc. 2003, 125, 4032–4033. [Google Scholar] [CrossRef] [PubMed]
- Arp, G.; Reimer, A.; Reitner, J. Microbialite formation of increased alkalinity, Satonda Crater Lake, Indonesia. J. Sediment. Res. 2003, 73, 105–127. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; DiMasi, E.; Han, Y.-J.; Aizenberg, J.; Kuzmenko, I. Orientation and mg incorporation of calcite grown on functionalized self-assembled monolayers: A synchrotron X-ray study. Cryst. Growth Des. 2005, 5, 2139–2145. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, H.; Konishi, H.; Kemp, J.M.; Roden, E.E.; Shen, Z. Dissolved sulfide-catalyzed precipitation of disordered dolomite: Implications for the formation mechanism of sedimentary dolomite. Geochim. Cosmochim. Acta 2012, 97, 148–165. [Google Scholar] [CrossRef]
- Kastner, M. Controls on dolomite formation. Nature 1984, 311, 410–411. [Google Scholar] [CrossRef]
- Burns, S.J.; Baker, P.A. A geochemical study of dolomites in the Monterey Formation, California. J. Sediment. Petrol. 1987, 57, 128–129. [Google Scholar]
- van Lith, Y.; Vasconcelos, C.; Warthmann, R.; Martins, J.C.F.; McKenzie, J.A. Bacterial sulfate reduction and salinity: Two controls on dolomite precipitation in Lagoa Vermelha and Brejo do Espinho (Brazil). Hydrobiologia 2002, 485, 35–49. [Google Scholar] [CrossRef]
- Barkan, Y.; Paris, G.; Webb, S.M.; Adkins, J.F.; Halevy, I. Sulfur isotope fractionation between aqueous and carbonate-associated sulfate in abiotic calcite and aragonite. Geochim. Cosmochim. Acta 2020, 280, 317–339. [Google Scholar] [CrossRef] [Green Version]
- Kampschulte, A.; Strauss, H. The sulfur isotopic evolution of Phanerozoic seawater based on the analysis of structurally substituted sulfate in carbonates. Chem. Geol. 2004, 204, 255–286. [Google Scholar] [CrossRef]
- Present, T.M.; Adkins, J.F.; Fischer, W.W. Variability in sulfur isotope records of Phanerozoic seawater sulfate. Geophys. Res. Let. 2020, 47, e2020GL088766. [Google Scholar] [CrossRef]
- Present, T.M.; Gutierrez, M.; Paris, G.; Kerans, C.; Grotzinger, J.P.; Adkins, J.F. Diagenetic controls on the isotopic composition of carbonate-associated sulphate in the Permian Capitan Reef Complex, West Texas. Sedimentology 2019, 66, 2605–2626. [Google Scholar] [CrossRef]
- Barbiére, E.B. Condições climáticas dominantes na porção oriental da lagoa de Araruama (RJ) e suas implicações na diversidade do teor de salinidade. Cad. Ciências Terra 1985, 59, 3–35. [Google Scholar]
- Höhn, A.; Tobschall, H.J.; Maddock, J.E.L. Biogeochemistry of a hypersaline lagoon east of Rio de Janeiro, Brazil. Sci. Total Environ. 1986, 58, 175–185. [Google Scholar] [CrossRef]
- Moreira, I.; Patchineelam, R.; Luca Rebello, A. Preliminary investigations on the occurrence of diagenetic dolomite in surface sediments of Lagoa Vermelha, Brazil. GeoJournal 1987, 14, 357–360. [Google Scholar] [CrossRef]
- Castro, J.W.A.; Suguio, K.; Seoane, J.; Cunha, A.M.; Dias, F.F. Sea-level fluctuations and coastal evolution in the state of Rio de Janeiro, southeastern Brazil. An. Acad. Bras. Ciências 2014, 86, 671–683. [Google Scholar] [CrossRef]
- Seeberg-Elverfeldt, J.; Schlüter, M.; Feseker, T.; Kölling, M. Rhizon sampling of pore-waters near the sediment-water interface of aquatic systems. Limnol. Oceanogr. Methods 2005, 3, 361–371. [Google Scholar] [CrossRef]
- Canfield, D.E.; Raiswell, R.; Westrich, J.T.; Reaves, C.M.; Berner, R.A. The use of chromium reduction in the analysis of reduced inorganic sulfur in sediments and shales. Chem. Geol. 1986, 54, 149–155. [Google Scholar] [CrossRef]
- Rees, C.E.; Jenkins, W.J.; Monster, J. The sulphur isotopic composition of ocean water sulphate. Geochim. Cosmochim. Acta 1978, 42, 377–381. [Google Scholar] [CrossRef]
- Böttcher, M.E.; Brumsack, H.J.; Dürselen, C.D. The isotopic composition of modern seawater sulfate: I. Coastal waters with special regard to the North Sea. J. Mar. Syst. 2007, 67, 73–82. [Google Scholar] [CrossRef]
- Wortmann, U.G.; Chernyavsky, B.; Bernasconi, S.M.; Brunner, B.; Böttcher, M.E.; Swart, P.K. Oxygen isotope biogeochemistry of pore-water sulfate in the deep biosphere: Dominance of isotope exchange reactions with ambient water during microbial sulfate reduction (ODP Site 1130). Geochim. Cosmochim. Acta 2007, 71, 4221–4232. [Google Scholar] [CrossRef]
- Froelich, P.N.; Klinkhammer, G.P.; Luedtke Bender, M.; Luedtke, N.A.; Heath, G.R.; Cullen, D.; Dauphin, P.; Hammond, D.; Hartman, B.; Maynard, V. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: Suboxic diagenesis. Geochim. Cosmochim. Acta 1979, 43, 1075–1090. [Google Scholar] [CrossRef]
- Berner, R.A. A rate model for organic matter decomposition during bacterial sulfate reduction in marine sediments. Colloq. Int. CNRS 1980, 293, 35–44. [Google Scholar]
- Lloyd, R.M. Oxygen isotope behavior in the Sulfate-Water System. J. Geophys. Res. 1968, 73, 6099–6110. [Google Scholar] [CrossRef]
- Fritz, P.; Basharmal, G.M.; Drimmie, R.J.; Ibsen, J.; Qureshi, R.M. Oxygen isotope exchange between sulphate and water during bacterial reduction of sulphate. Chem. Geol. Isot. Geosci. 1989, 79, 99–105. [Google Scholar] [CrossRef]
- Antler, G.; Turchyn, A.V.; Rennie, V.; Herut, B.; Sivan, O. Coupled sulfur and oxygen isotope insight into bacterial sulfate reduction in the natural environment. Geochim. Cosmochim. Acta 2013, 118, 98–117. [Google Scholar] [CrossRef] [Green Version]
- Antler, G.; Turchyn, A.V.; Ono, S.; Sivan, O.; Bosak, T. Combined 34S, 33S and 18O isotope fractionations record different intracellular steps of microbial sulfate reduction. Geochim. Cosmochim. Acta 2017, 203, 364–380. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Findlay AJl Pellerin, A. The biogeochemical sulfur cycle of marine sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, B.B.; Revsbech, N.P.; Cohen, Y. Photosynthesis and structure of benthic microbial mats: Microelectrode and SEM studies of four cyanobacterial communities. Limnol. Oceanogr. 1983, 28, 1075–1093. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Jorgensen, B.B.; Blackburn, T.H.; Cohen, Y. Microelectrode studies of the photosynthesis and O2, H2S, and pH profiles of a microbial mat 1. Limnol. Oceanogr. 1983, 28, 1062–1074. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, B.B.; Gallardo, V.A. Thioploca spp.: Filamentous sulfur bacteria with nitrate vacuoles. FEMS Microbiol. Ecol. 1999, 28, 301–313. [Google Scholar] [CrossRef]
- Jørgensen, B.B. Sulfur biogeochemical cycle of marine sediments. Geochem. Perspect. 2021, 10, 145–146. [Google Scholar] [CrossRef]
- Brunner, B.; Bernasconi, S.M.; Kleikemper, J.; Schroth, M.H. A model for oxygen and sulfur isotope fractionation in sulfate during bacterial sulfate reduction processes. Geochim. Cosmochim. Acta 2005, 69, 4773–4785. [Google Scholar] [CrossRef]
- Farquhar, J.; Canfield, D.E.; Masterson, A.; Bao, H.; Johnston, D. Sulfur and oxygen isotope study of sulfate reduction in experiments with natural populations from Faellestrand, Denmark. Geochim. Cosmochim. Acta 2008, 72, 2805–2821. [Google Scholar] [CrossRef]
- Turchyn, A.V.; Brüchert, V.; Lyons, T.W.; Engel, G.S.; Balci, N.; Schrag, D.P.; Brunner, B. Kinetic oxygen isotope effects during dissimilatory sulfate reduction: A combined theoretical and experimental approach. Geochim. Cosmochim. Acta 2010, 74, 2011–2024. [Google Scholar] [CrossRef]
- Böttcher, M.E.; Thamdrup, B.O.; Vennemann, T.W. Oxygen and sulfur isotope fractionation during anaerobic bacterial disproportionation of elemental sulfur. Geochim. Cosmochim. Acta 2001, 65, 1601–1609. [Google Scholar] [CrossRef]
- Dole, M.; Lane, G.A.; Rudd, D.P.; Zaukelies, D.A. Isotopic composition of atmospheric oxygen and nitrogen. Geochim. Cosmochim. Acta 1954, 6, 65–78. [Google Scholar] [CrossRef]
- Kroopnick, P.; Craig, H. Atmospheric oxygen: Isotopic composition and solubility fractionation. Science 1972, 175, 54–55. [Google Scholar] [CrossRef]
- Mizutani, Y.; Rafter, T.A. Isotopic behaviour of sulphate oxygen in the bacterial reduction of sulphate. Geochem. J. 1973, 6, 183–191. [Google Scholar] [CrossRef]
- Böttcher, M.E.; Smock, A.M.; Cypionka, H. Sulfur isotope fractionation during experimental precipitation of iron (II) and manganese (II) sulfide at room temperature. Chem. Geol. 1998, 146, 127–134. [Google Scholar] [CrossRef]
- Bertran, E.; Waldeck, A.; Wing, B.A.; Halevy, I.; Leavitt, W.D.; Bradley, A.S.; Johnston, D.T. Oxygen isotope effects during microbial sulfate reduction: Applications to sediment cell abundances. ISME J. 2020, 14, 1508–1519. [Google Scholar] [CrossRef]
- Wood, W.W.; Sanford, W.E.; Habshi, A.R.S.A. Source of solutes to the coastal sabkha of Abu Dhabi. Geol. Soc. Am. Bull. 2002, 114, 259–268. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A.; Warthmann, R.; Bernasconi, S.M. Calibration of the d18O paleothermometer for dolomite precipitated in microbial cultures and natural environments. Geology 2005, 33, 317–320. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; Vasconcelos, C.; Warthmann, R.J.; Bernasconi, S.M.; Dupraz, C.; Strohmenger, C.J.; Mckenzie, J.A. Dolomite formation within microbial mats in the coastal sabkha of Abu Dhabi (United Arab Emirates). Sedimentology 2010, 57, 824–844. [Google Scholar] [CrossRef]
- Harrison, A.G.; Thode, H.G. Mechanism of the bacterial reduction of sulphate from isotope fractionation studies. Trans. Faraday Soc. 1958, 54, 84–92. [Google Scholar] [CrossRef]
- Betts, R.H.; Voss, R.H. The kinetics of oxygen exchange between the sulfite ion and water. Canadian J. Chem. 1970, 48, 2035–2041. [Google Scholar] [CrossRef] [Green Version]
- Horner, D.A.; Connick, R.E. Kinetics of oxygen exchange between the two isomers of bisulfite ion, disulfite ion (S2O52−), and water as studied by oxygen-17 nuclear magnetic resonance spectroscopy. Inorg. Chem. 2003, 42, 1884–1894. [Google Scholar] [CrossRef]
- Kohl, I.E.; Asatryan, R.; Bao, H. No oxygen isotope exchange between water and APS–sulfate at surface temperature: Evidence from quantum chemical modeling and triple-oxygen isotope experiments. Geochim. Cosmochim. Acta 2012, 95, 106–118. [Google Scholar] [CrossRef]
- Brunner, B.; Bernasconi, S.M. A revised isotope fractionation model for dissimilatory sulfate reduction in sulfate reducing bacteria. Geochim. Cosmochim. Acta 2005, 69, 4759–4771. [Google Scholar] [CrossRef]
- Habicht, K.S.; Canfield, D.E. Sulfur isotope fractionation during bacterial sulfate reduction in organic-rich sediments. Geochim. Cosmochim. Acta 1997, 61, 5351–5361. [Google Scholar] [CrossRef]
- van Lith, Y.; Warthmann, R.; Vasconcelos, C.; Mckenzie, J.A. Sulfate-reducing bacteria induce low-temperature Ca-dolomite and high Mg-calcite formation. Geobiology 2003, 1, 71–79. [Google Scholar] [CrossRef]
- Nordeng, S.H.; Sibley, D.F. Dolomite stoichiometry and Ostwald’s step rule. Geochim. Cosmochim. Acta 1994, 58, 191–196. [Google Scholar] [CrossRef]
- Aloisi, G.; Gloter, A.; Kruger, M.; Wallmann, K.; Guyot, F.; Zuddas, P. Nucleation of calcium carbonate on bacterial nanoglobules. Geology 2006, 34, 1017–1020. [Google Scholar] [CrossRef]
- Algeo, T.J.; Luo, G.M.; Song, H.Y.; Lyons, T.W.; Canfield, D.E. Reconstruction of secular variation in seawater sulfate concentrations. Biogeosciences 2015, 12, 2131–2151. [Google Scholar] [CrossRef] [Green Version]
- Habicht, K.S.; Canfield, D.E. Isotope fractionation by sulfate-reducing natural populations and the isotopic composition of sulfide in marine sediments. Geology 2001, 29, 555–558. [Google Scholar] [CrossRef]
- Drake, H.; Tullborg, E.L.; Whitehouse, M.; Sandberg, B.; Blomfeldt, T.; Åström, M.E. Extreme fractionation and micro-scale variation of sulphur isotopes during bacterial sulphate reduction in deep groundwater systems. Geochim. Cosmochim. Acta 2015, 161, 1–18. [Google Scholar] [CrossRef]
- Baldermann, A.; Deditius, A.P.; Dietzel, M.; Fichtner, V.; Fischer, C.; Hippler, D.; Leis, A.; Baldermann, C.; Mavromatis, V.; Stickler, C.P.; et al. The role of bacterial sulfate reduction during dolomite precipitation: Implications from Upper Jurassic platform carbonates. Chem. Geol. 2015, 412, 1–14. [Google Scholar] [CrossRef]
- Fichtner, V.; Strauss, H.; Immenhauser, A.; Buhl, D.; Neuser, R.D.; Niedermayr, A. Diagenesis of carbonate associated sulfate. Chem. Geol. 2017, 463, 61–75. [Google Scholar] [CrossRef]
- Arvidson, R.S.; MacKenzie, F.T. The dolomite problem: Control of precipitation kinetics by temperature and saturation state. Am. J. Sci. 1999, 299, 257–288. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.A.; Kenward, P.A.; Fowle, D.A.; Goldstein, R.H.; González, L.A.; Moore, D.S. Surface chemistry allows for abiotic precipitation of dolomite at low temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 14540–14545. [Google Scholar] [CrossRef]
- Soetaert, K.; Hofmann, A.F.; Middelburg, J.J.; Meysman, F.J.R.; Greenwood, J. The effect of biogeochemical processes on pH (reprinted from marine chemistry, Vol. 105, pp. 30–51, 2007). Mar. Chem. 2007, 106, 380–401. [Google Scholar] [CrossRef]
- Ku, T.C.W.; Walter, L.M.; Coleman, M.L.; Blake, R.E.; Martini, A.M. Coupling between sulfur recycling and syndepositional carbonate dissolution: Evidence from oxygen and sulfur isotope composition of pore-water sulfate, South Florida Platform, USA. Geochim. Cosmochim. Acta 1999, 63, 2529–2546. [Google Scholar] [CrossRef]
- Fike, D.A.; Grotzinger, J.P.; Pratt, L.M.; Summons, R.E. Oxidation of the Ediacaran ocean. Nature 2006, 444, 744–747. [Google Scholar] [CrossRef]
- Gellatly, A.M.; Lyons, T.W. Trace sulfate in mid-Proterozoic carbonates and the sulfur isotope record of biospheric evolution. Geochim. Cosmochim. Acta 2005, 69, 3813–3829. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fichtner, V.; Schurr, S.L.; Strauss, H.; Vasconcelos, C.; Goetschl, K.E.; Areias, C.; Barbosa, C.F.; Immenhauser, A. The Relationship between Bacterial Sulfur Cycling and Ca/Mg Carbonate Precipitation—Old Tales and New Insights from Lagoa Vermelha and Brejo do Espinho, Brazil. Geosciences 2023, 13, 229. https://doi.org/10.3390/geosciences13080229
Fichtner V, Schurr SL, Strauss H, Vasconcelos C, Goetschl KE, Areias C, Barbosa CF, Immenhauser A. The Relationship between Bacterial Sulfur Cycling and Ca/Mg Carbonate Precipitation—Old Tales and New Insights from Lagoa Vermelha and Brejo do Espinho, Brazil. Geosciences. 2023; 13(8):229. https://doi.org/10.3390/geosciences13080229
Chicago/Turabian StyleFichtner, Vanessa, Simon L. Schurr, Harald Strauss, Crisógono Vasconcelos, Katja E. Goetschl, Camila Areias, Catia F. Barbosa, and Adrian Immenhauser. 2023. "The Relationship between Bacterial Sulfur Cycling and Ca/Mg Carbonate Precipitation—Old Tales and New Insights from Lagoa Vermelha and Brejo do Espinho, Brazil" Geosciences 13, no. 8: 229. https://doi.org/10.3390/geosciences13080229








