Excess 40Ar in Alkali Feldspar and 206,207Pb in Apatite Caused by Fluid-Induced Recrystallisation in a Semi-Closed Environment in Proterozoic (Meta)Granites of the Mt Isa Inlier, NE Australia
Abstract
:1. Introduction
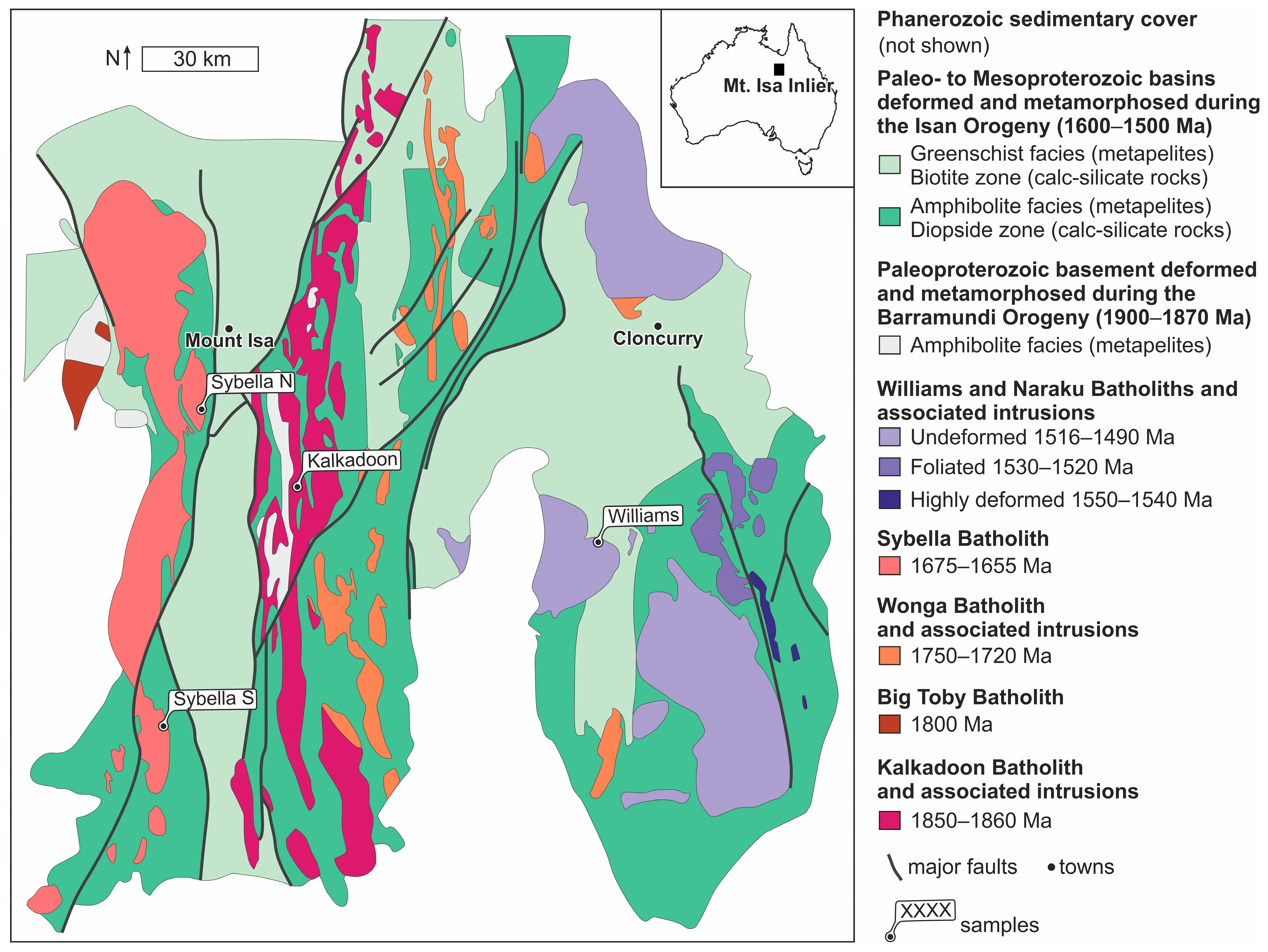
2. Sample Provenance
3. Methods
4. Results
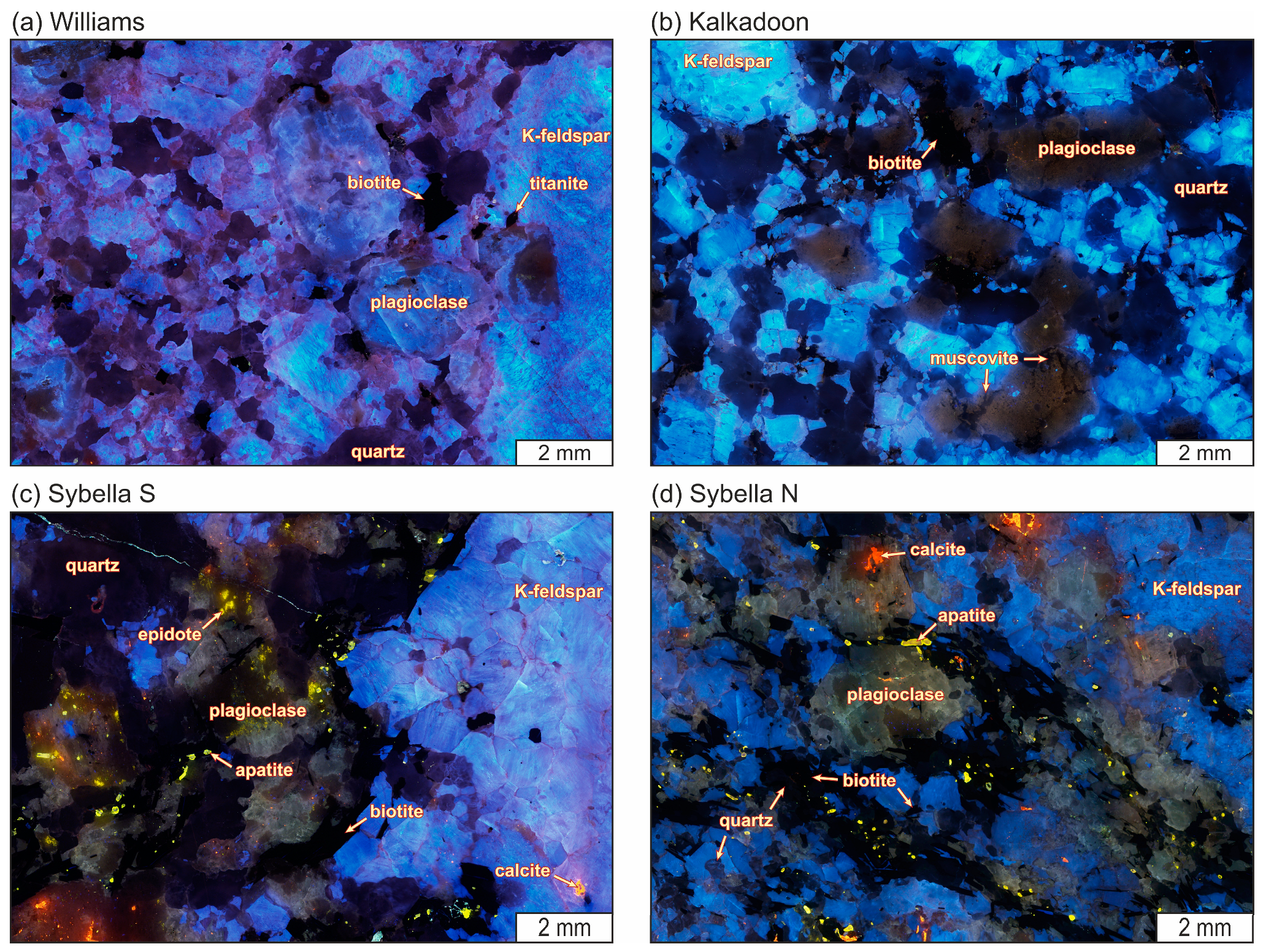
4.1. Rock and Mineral Textures
4.1.1. Hand Specimens
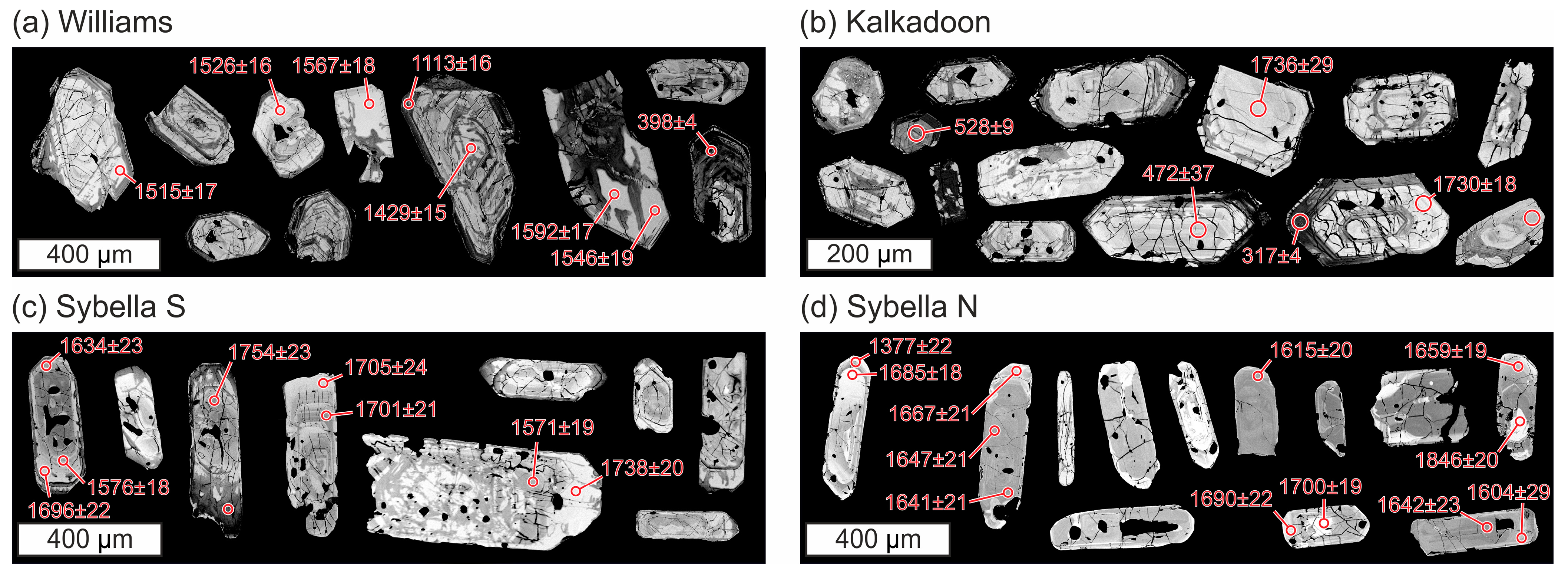
4.1.2. Zircon
4.1.3. Other U-Th-Bearing Silicates
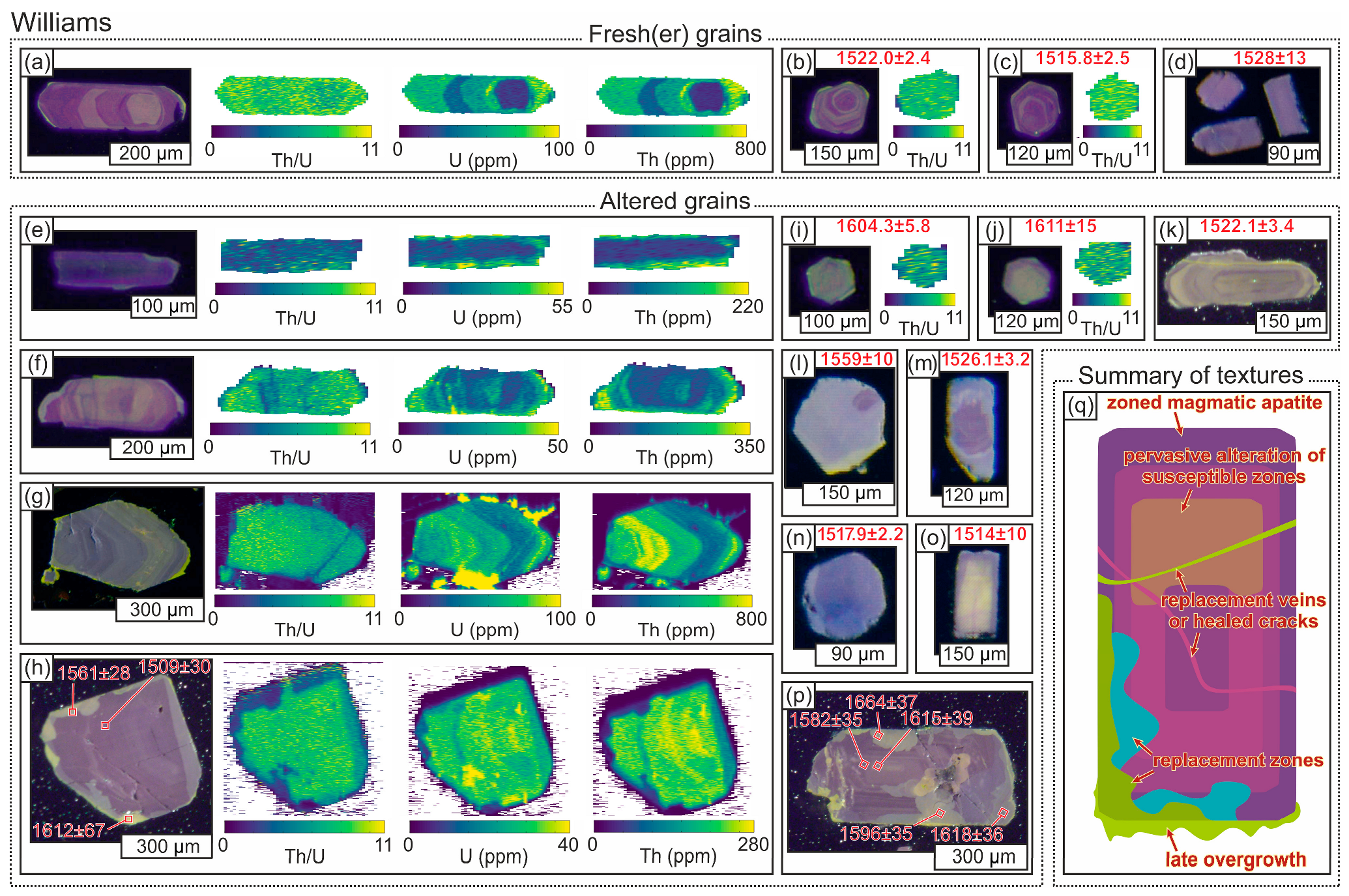
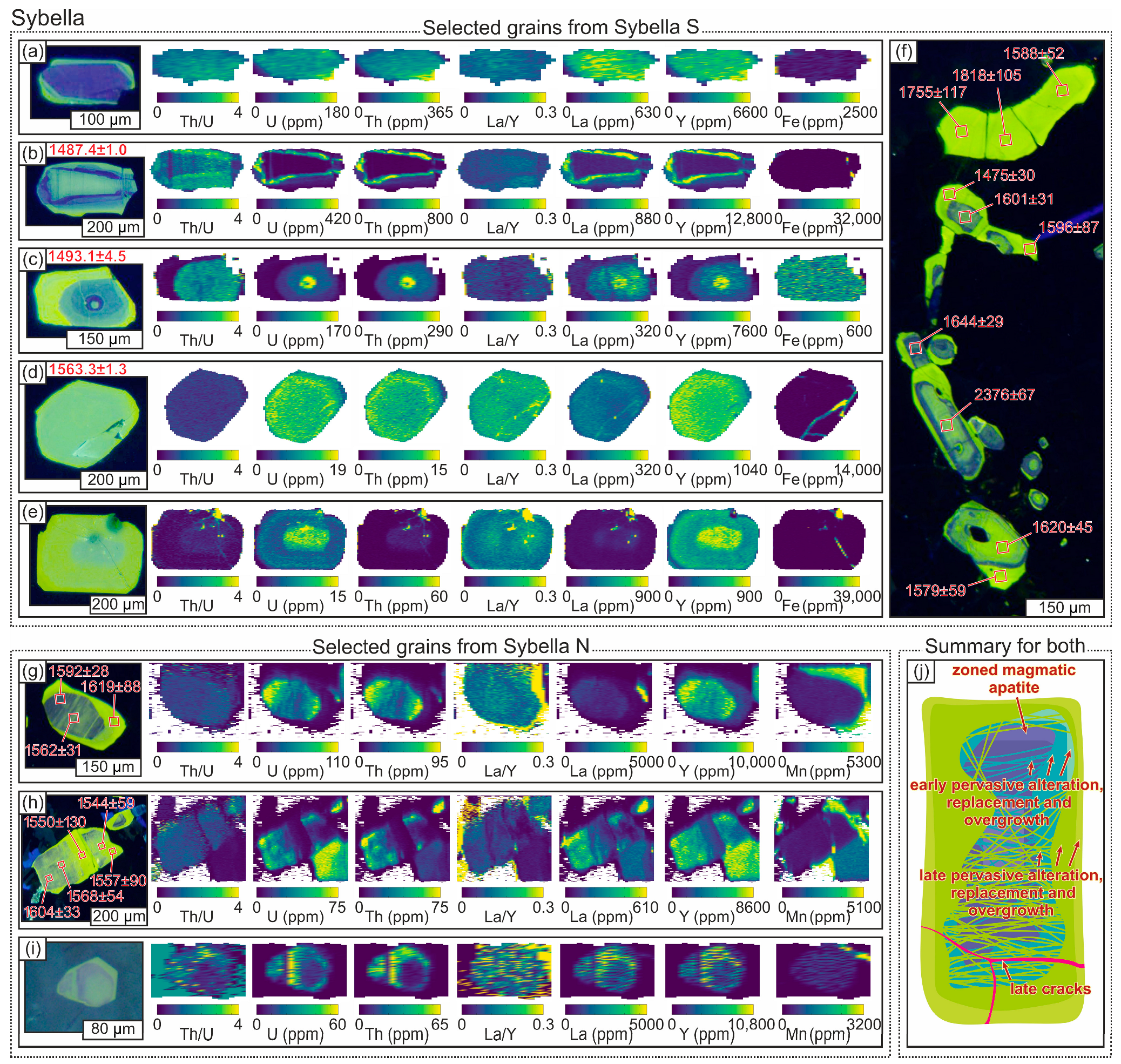
4.1.4. Apatite
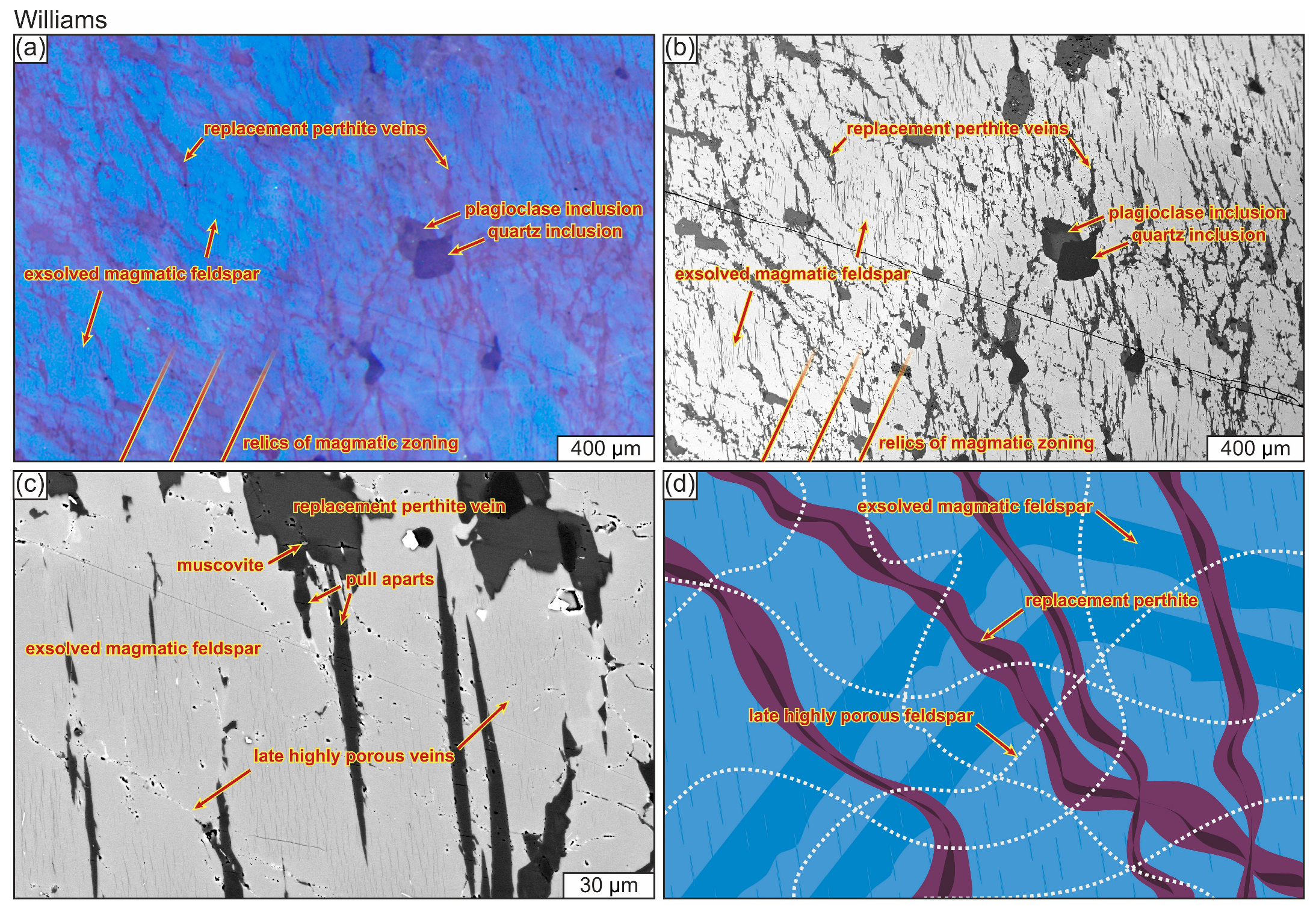
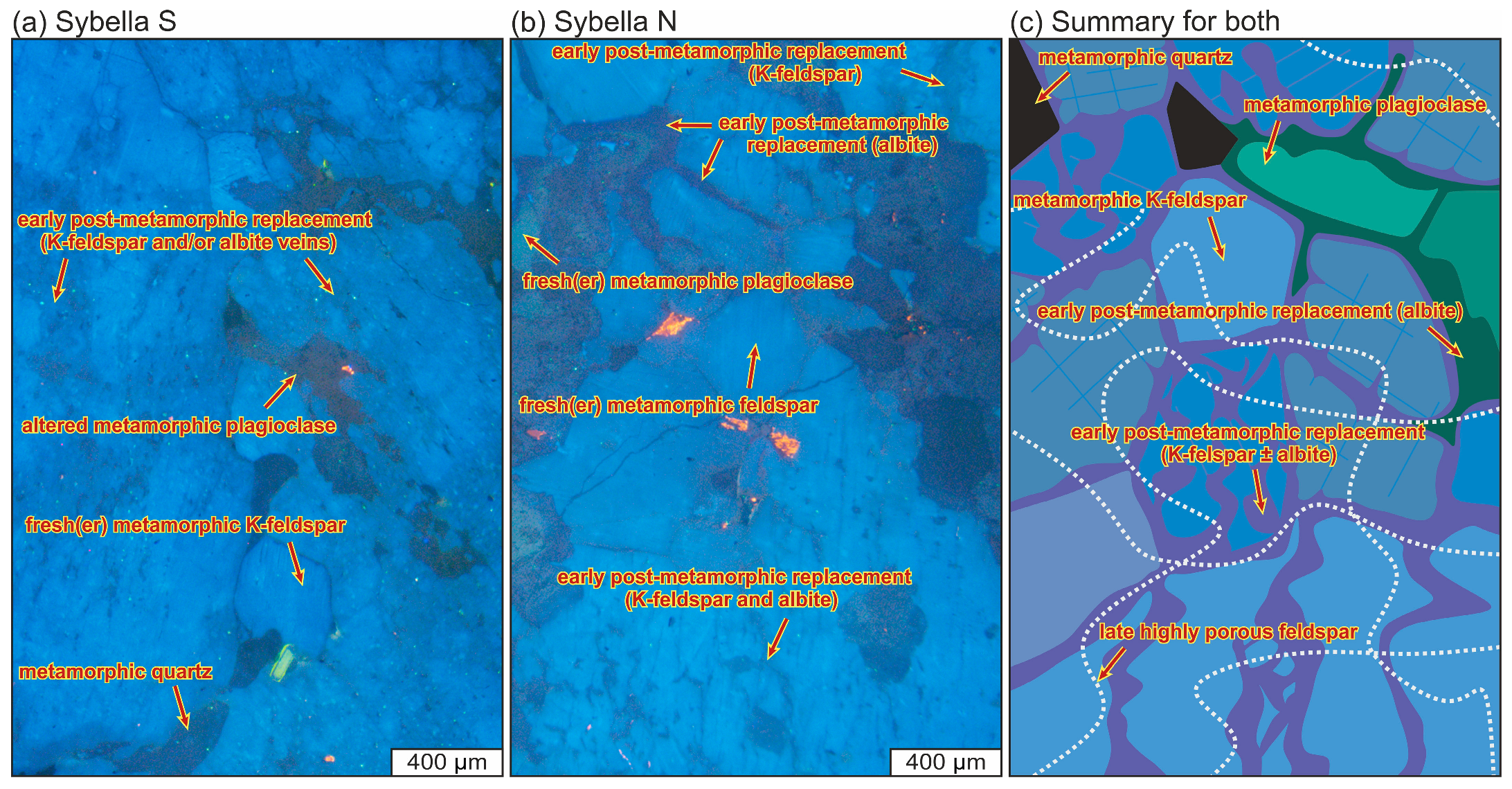
4.1.5. Alkali Feldspar
4.1.6. Fluorite
4.1.7. Relationships Between Alteration Textures in Different Minerals
4.2. Isotopic Data
4.2.1. Zircon
4.2.2. Pb Isotopes in Alkali Feldspar
4.2.3. Apatite
4.2.4. Fluorite
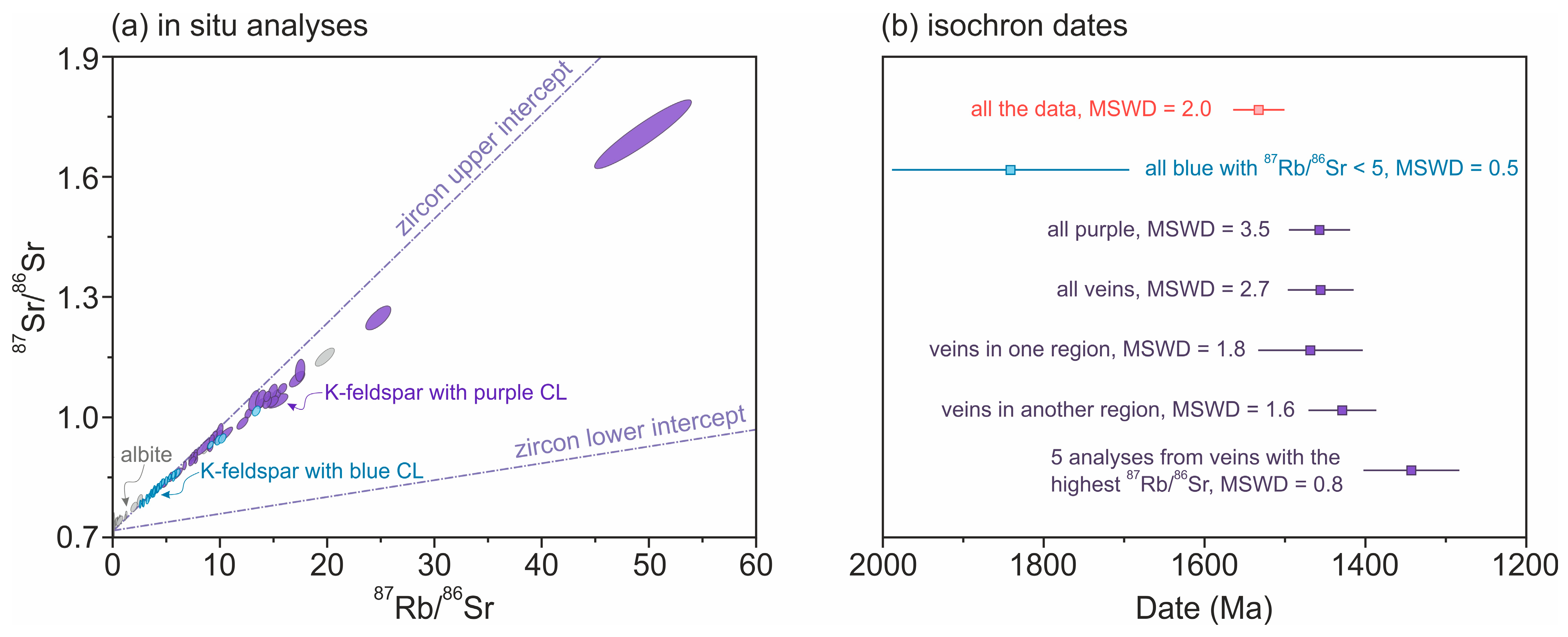
4.2.5. Rb-Sr Results for Alkali Feldspar
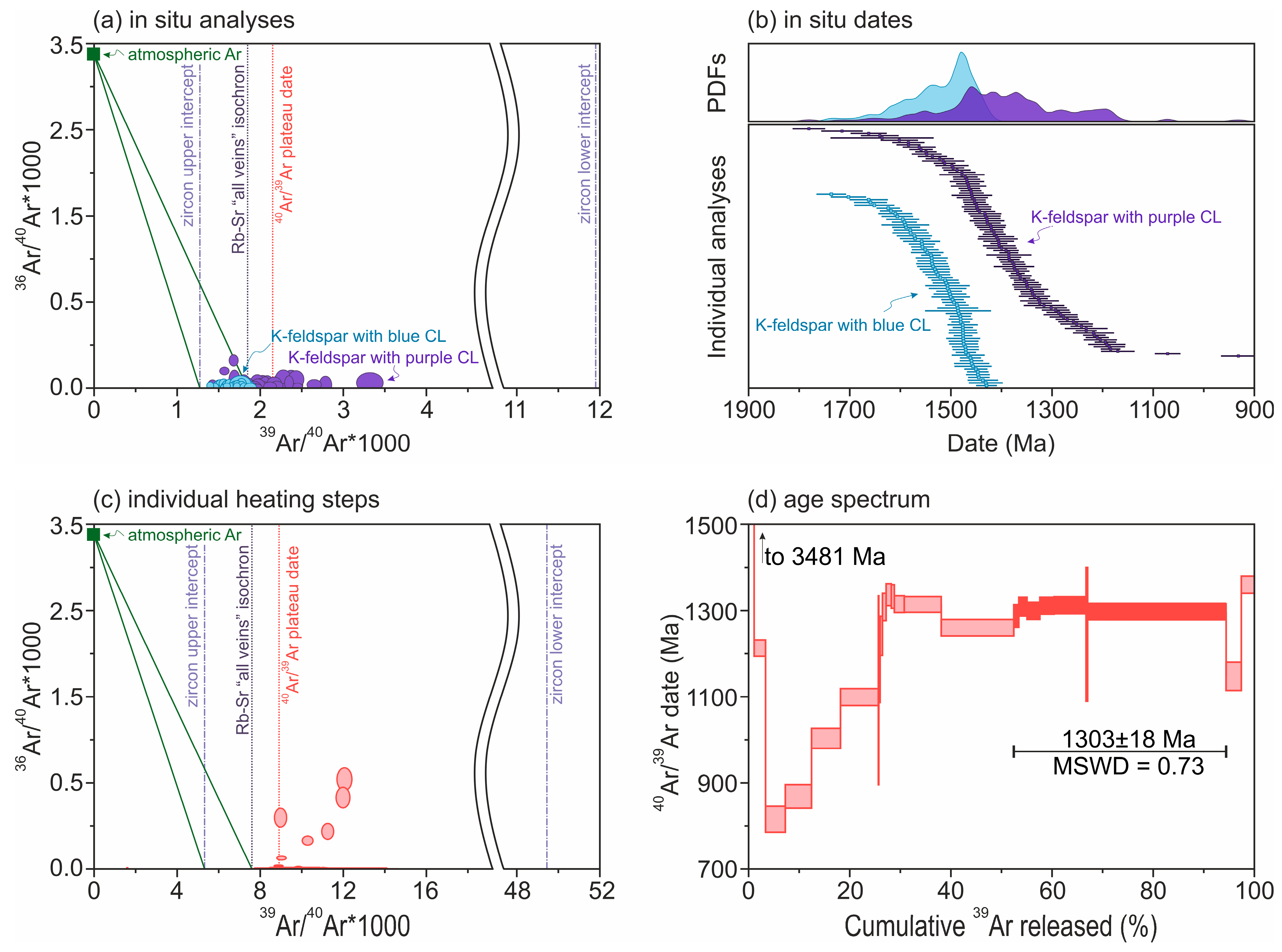
4.2.6. 40Ar/39Ar Results for Alkali Feldspar
4.2.7. Relationships Between Isotopic Disturbances in Different Minerals and Systems
5. Discussion
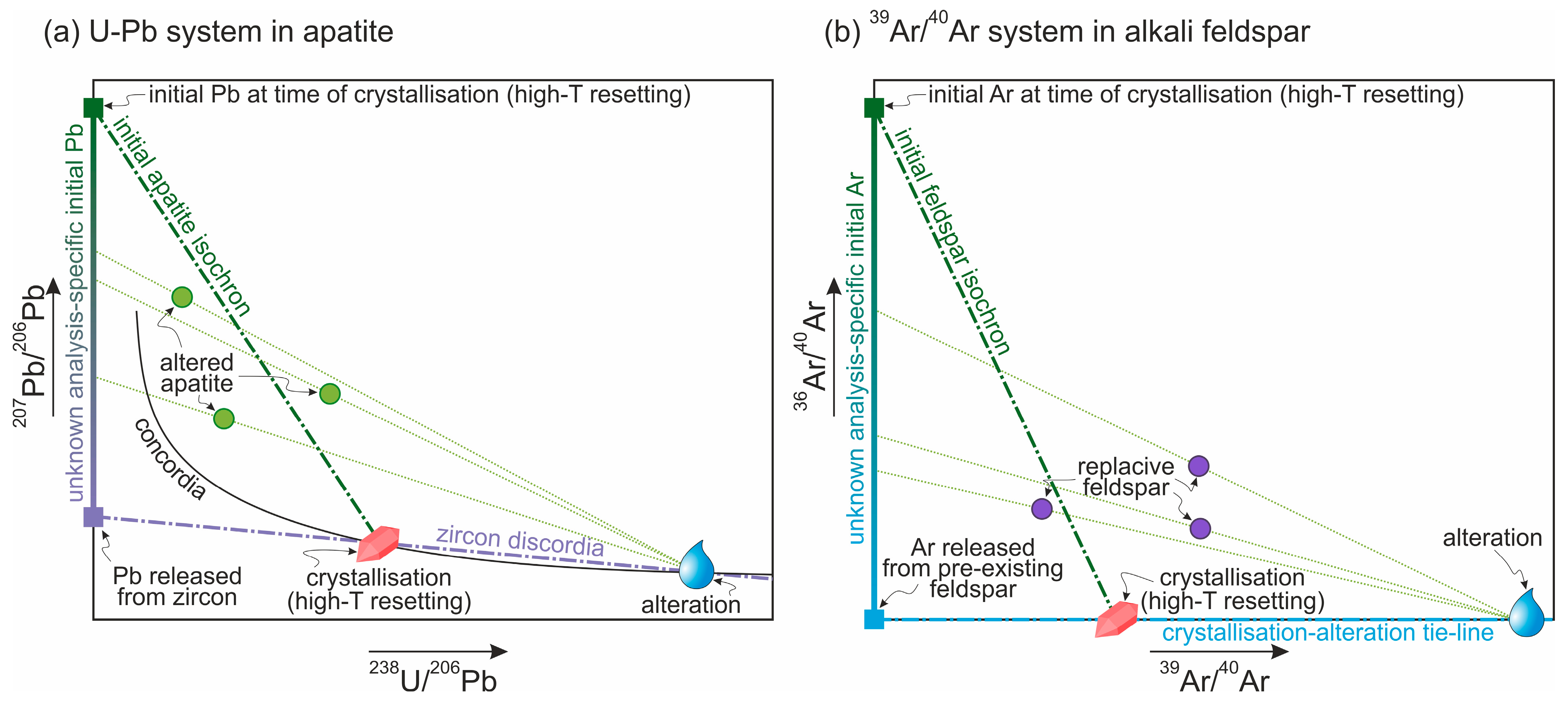
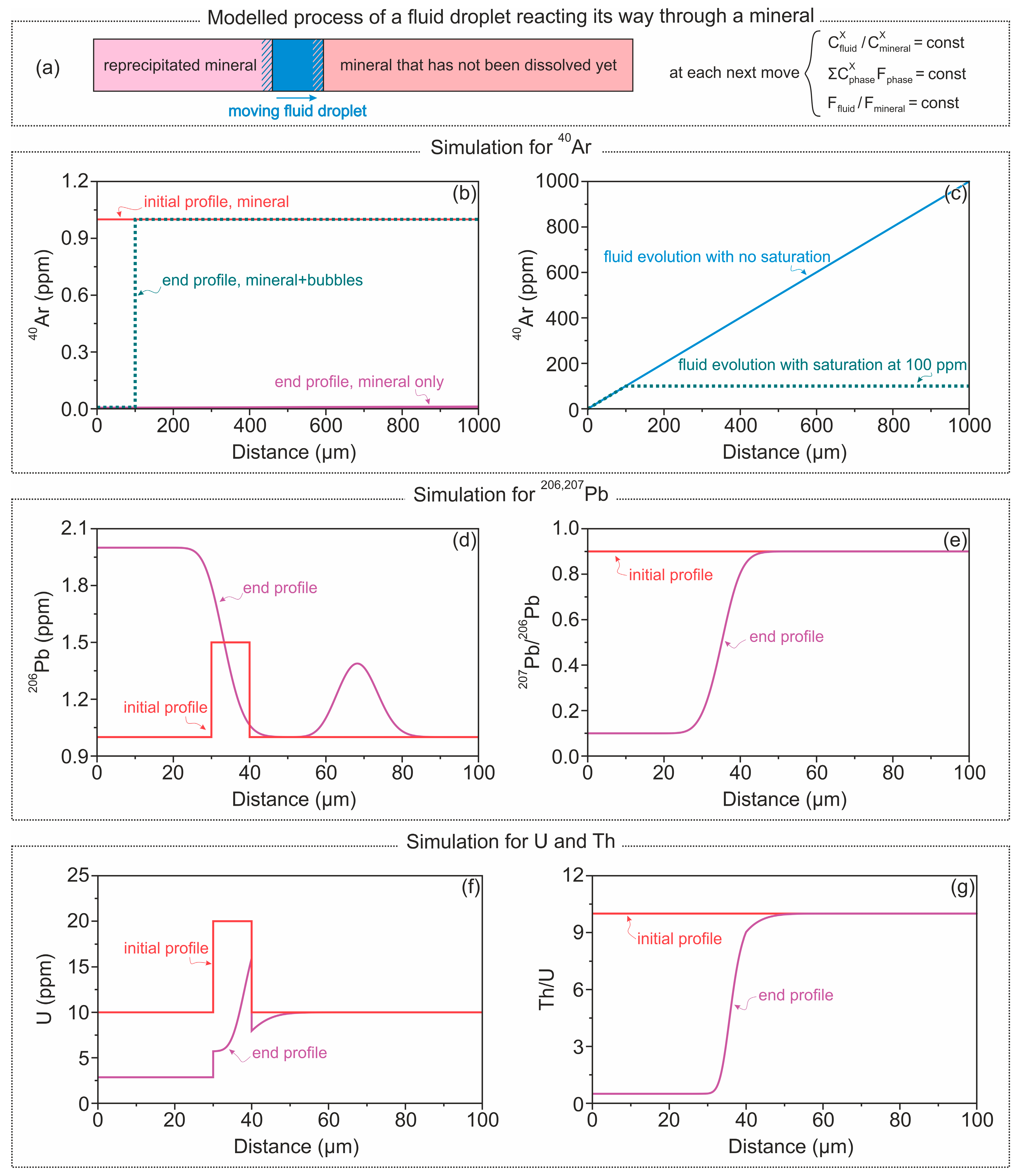
5.1. Fluid-Assisted Incorporation of Excess 40Ar and 206,207Pb
5.2. Imlications for 40Ar/39Ar Dating of Alkali Feldspar
5.3. Imlications for Noble Gas Geochronology of Groundwater
5.4. Imlications for U-Pb Dating of Apatite
5.5. Imlications for Fission Track Dating of Apatite
5.6. A Note on the Late Highly Porous Veins
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lovera, O.M.; Richter, F.M.; Harrison, T.M. The 40Ar/39Ar Thermochronometry for Slowly Cooled Samples Having a Distribution of Diffusion Domain Sizes. J. Geophys. Res. Solid Earth 1989, 94, 17917–17935. [Google Scholar] [CrossRef]
- Harrison, T.M.; Lovera, O.M. The Multi-Diffusion Domain Model: Past, Present and Future. Geol. Soc. Lond. Spec. Publ. 2014, 378, 91–106. [Google Scholar] [CrossRef]
- Flude, S.; Halton, A.M.; Kelley, S.P.; Sherlock, S.C.; Schwanethal, J.; Wilkinson, C.M. Observation of Centimetre-Scale Argon Diffusion in Alkali Feldspars: Implications for 40Ar/39Ar Thermochronology. Geol. Soc. Lond. Spec. Publ. 2014, 378, 265–275. [Google Scholar] [CrossRef]
- Chamberlain, K.R.; Bowring, S.A. Apatite–Feldspar U–Pb Thermochronometer: A Reliable, Mid-Range (450 °C), Diffusion-Controlled System. Chem. Geol. 2001, 172, 173–200. [Google Scholar] [CrossRef]
- Cochrane, R.; Spikings, R.A.; Chew, D.; Wotzlaw, J.-F.; Chiaradia, M.; Tyrrell, S.; Schaltegger, U.; Van der Lelij, R. High Temperature (>350 °C) Thermochronology and Mechanisms of Pb Loss in Apatite. Geochim. Et Cosmochim. Acta 2014, 127, 39–56. [Google Scholar] [CrossRef]
- Schoene, B.; Bowring, S.A. Determining Accurate Temperature–Time Paths from U–Pb Thermochronology: An Example from the Kaapvaal Craton, Southern Africa. Geochim. Et Cosmochim. Acta 2007, 71, 165–185. [Google Scholar] [CrossRef]
- Villa, I.M. From Nanometer to Megameter: Isotopes, Atomic-Scale Processes, and Continent-Scale Tectonic Models. Lithos 2006, 87, 155–173. [Google Scholar] [CrossRef]
- Chafe, A.N.; Villa, I.M.; Hanchar, J.M.; Wirth, R. A Re-Examination of Petrogenesis and 40Ar/39Ar Systematics in the Chain of Ponds K-Feldspar:“Diffusion Domain” Archetype versus Polyphase Hygrochronology. Contrib. Mineral. Petrol. 2014, 167, 1010. [Google Scholar] [CrossRef]
- Popov, D.V.; Spikings, R.A.; Scaillet, S.; O’Sullivan, G.; Chew, D.; Badenszki, E.; Daly, J.S.; Razakamanana, T.; Davies, J.H.F.L. Diffusion and Fluid Interaction in Itrongay Pegmatite (Madagascar): Evidence from in Situ 40Ar/39Ar Dating of Gem-Quality Alkali Feldspar and U-Pb Dating of Protogenetic Apatite Inclusions. Chem. Geol. 2020, 556, 119841. [Google Scholar] [CrossRef]
- Villa, I.M.; Hanchar, J.M. K-Feldspar Hygrochronology. Geochim. Et Cosmochim. Acta 2013, 101, 24–33. [Google Scholar] [CrossRef]
- Spikings, R.A.; Popov, D.V. Thermochronology of Alkali Feldspar and Muscovite at T > 150 °C Using the 40Ar/39Ar Method: A Review. Minerals 2021, 11, 1025. [Google Scholar] [CrossRef]
- Glorie, S.; Jepson, G.; Konopelko, D.; Mirkamalov, R.; Meeuws, F.; Gilbert, S.; Gillespie, J.; Collins, A.S.; Xiao, W.; Dewaele, S.; et al. Thermochronological and Geochemical Footprints of Post-Orogenic Fluid Alteration Recorded in Apatite: Implications for Mineralisation in the Uzbek Tian Shan. Gondwana Res. 2019, 71, 1–15. [Google Scholar] [CrossRef]
- Chew, D.M.; Spikings, R.A. Apatite U-Pb Thermochronology: A Review. Minerals 2021, 11, 1095. [Google Scholar] [CrossRef]
- Henrichs, I.A.; O’Sullivan, G.; Chew, D.M.; Mark, C.; Babechuk, M.G.; McKenna, C.; Emo, R. The Trace Element and U-Pb Systematics of Metamorphic Apatite. Chem. Geol. 2018, 483, 218–238. [Google Scholar] [CrossRef]
- Kirkland, C.L.; Yakymchuk, C.; Szilas, K.; Evans, N.; Hollis, J.; McDonald, B.; Gardiner, N.J. Apatite: A U-Pb Thermochronometer or Geochronometer? Lithos 2018, 318–319, 143–157. [Google Scholar] [CrossRef]
- Betts, P.G.; Giles, D.; Mark, G.; Lister, G.S.; Goleby, B.R.; Aillères, L. Synthesis of the Proterozoic Evolution of the Mt Isa Inlier. Aust. J. Earth Sci. 2006, 53, 187–211. [Google Scholar] [CrossRef]
- Blake, D.H. Geology of the Mount Isa Inlier and Environs, Queensland and Northern Territory; Bulletin; Australian Government Publishing Service: Canberra, 1987; ISBN 978-0-644-06177-3. [Google Scholar]
- Page, R.; Williams, I. Age of the Barramundi Orogeny in Northern Australia by Means of Ion Microprobe and Conventional U-Pb Zircon Studies. Precambrian Res. 1988, 40–41, 21–36. [Google Scholar] [CrossRef]
- Wyborn, L.A.I.; Page, R.W. The Proterozoic Kalkadoon and Ewen Batholiths, Mount Isa Inlier, Queensland-Source, Chemistry, Age, and Metamorphism. BMR J. Aust. Geol. Geophys. 1983, 8, 53–69. [Google Scholar]
- Connors, K.; Page, R.W. Relationships between Magmatism, Metamorphism and Deformation in the Western Mount Isa Inlier, Australia. Precambrian Res. 1995, 71, 131–153. [Google Scholar] [CrossRef]
- Wyborn, L.; Page, R.; Mcculloch, M. Petrology, Geochronology and Isotope Geochemistry of the Post-1820 Ma Granites of the Mount Isa Inlier: Mechanisms for the Generation of Proterozoic Anorogenic Granites. Precambrian Res. 1988, 40–41, 509–541. [Google Scholar] [CrossRef]
- Page, R.W.; Sun, S. Aspects of Geochronology and Crustal Evolution in the Eastern Fold Belt, Mt Isa Inlier. Aust. J. Earth Sci. 1998, 45, 343–361. [Google Scholar] [CrossRef]
- Foster, D.R.W.; Rubenach, M.J. Isograd Pattern and Regional Low-Pressure, High-Temperature Metamorphism of Pelitic, Mafic and Calc-Silicate Rocks along an East—West Section through the Mt Isa Inlier. Aust. J. Earth Sci. 2006, 53, 167–186. [Google Scholar] [CrossRef]
- Rubenach, M.; Foster, D.; Evins, P.; Blake, K.; Fanning, C. Age Constraints on the Tectonothermal Evolution of the Selwyn Zone, Eastern Fold Belt, Mount Isa Inlier. Precambrian Res. 2008, 163, 81–107. [Google Scholar] [CrossRef]
- Spikings, R.A.; Foster, D.A.; Kohn, B.P.; Lister, G.S. Post-Orogenic (<1500 Ma) Thermal History of the Proterozoic Eastern Fold Belt, Mount Isa Inlier, Australia. Precambrian Res. 2001, 109, 103–144. [Google Scholar] [CrossRef]
- Spikings, R.A.; Foster, D.A.; Kohn, B.P.; Lister, G.S. Post-Orogenic (<1500 Ma) Thermal History of the Palaeo-Mesoproterozoic, Mt. Isa Province, NE Australia. Tectonophysics 2002, 349, 327–365. [Google Scholar] [CrossRef]
- Li, J.; Pourteau, A.; Li, Z.; Jourdan, F.; Nordsvan, A.R.; Collins, W.J.; Volante, S. Heterogeneous Exhumation of the Mount Isa Orogen in NE Australia After 1.6 Ga Nuna Assembly: New High-Precision 40Ar/39Ar Thermochronological Constraints. Tectonics 2020, 39, e2020TC006129. [Google Scholar] [CrossRef]
- Spikings, R.A.; Foster, D.A.; Kohn, B.P. Phanerozoic Denudation History of the Mount Isa Inlier, Northern Australia: Response of a Proterozoic Mobile Belt to Intraplate Tectonics. Int. Geol. Rev. 1997, 39, 107–124. [Google Scholar] [CrossRef]
- Spikings, R.A.; Foster, D.A.; Kohn, B.P. Low-Temperature (<110 °C) Thermal History of the Mt Isa and Murphy Inliers, Northeast Australia: Evidence from Apatite Fission Track Thermochronology. Aust. J. Earth Sci. 2006, 53, 151–165. [Google Scholar] [CrossRef]
- Wyborn, L. Younger ca 1500 Ma Granites of the Williams and Naraku Batholiths, Cloncurry District, Eastern Mt Isa Inlier: Geochemistry, Origin, Metallogenic Significance and Exploration Indicators. Aust. J. Earth Sci. 1998, 45, 397–411. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the Visualisation and Processing of Mass Spectrometric Data. J. Anal. At. Spectrom. 2011, 26, 2508. [Google Scholar] [CrossRef]
- Jackson, S.E.; Pearson, N.J.; Griffin, W.L.; Belousova, E.A. The Application of Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry to in Situ U–Pb Zircon Geochronology. Chem. Geol. 2004, 211, 47–69. [Google Scholar] [CrossRef]
- Sláma, J.; Košler, J.; Condon, D.J.; Crowley, J.L.; Gerdes, A.; Hanchar, J.M.; Horstwood, M.S.A.; Morris, G.A.; Nasdala, L.; Norberg, N.; et al. Plešovice Zircon—A New Natural Reference Material for U–Pb and Hf Isotopic Microanalysis. Chem. Geol. 2008, 249, 1–35. [Google Scholar] [CrossRef]
- Jackson, S.E. LAMTRACE Data Reduction Software for LA-ICP-MS. In Laser Ablation–ICP–MS in the Earth Sciences CURRENT PRACTICES AND OUTSTANDING ISSUES; Mineralogical Association of Canada Short Course Series: Québec, QC, Canada, 2008; Volume 40, pp. 305–307. [Google Scholar]
- Ulianov, A.; Müntener, O.; Schaltegger, U.; Bussy, F. The Data Treatment Dependent Variability of U–Pb Zircon Ages Obtained Using Mono-Collector, Sector Field, Laser Ablation ICPMS. J. Anal. At. Spectrom. 2012, 27, 663. [Google Scholar] [CrossRef]
- Jaffey, A.H.; Flynn, K.F.; Glendenin, L.E.; Bentley, W.C.; Essling, A.M. Precision Measurement of Half-Lives and Specific Activities of 235U and 238U. Phys. Rev. C 1971, 4, 1889–1906. [Google Scholar] [CrossRef]
- Schoene, B.; Bowring, S.A. U–Pb Systematics of the McClure Mountain Syenite: Thermochronological Constraints on the Age of the 40Ar/39Ar Standard MMhb. Contrib. Mineral. Petrol. 2006, 151, 615–630. [Google Scholar] [CrossRef]
- Chew, D.M.; Petrus, J.A.; Kamber, B.S. U–Pb LA–ICPMS Dating Using Accessory Mineral Standards with Variable Common Pb. Chem. Geol. 2014, 363, 185–199. [Google Scholar] [CrossRef]
- Chew, D.M.; Sylvester, P.J.; Tubrett, M.N. U–Pb and Th–Pb Dating of Apatite by LA-ICPMS. Chem. Geol. 2011, 280, 200–216. [Google Scholar] [CrossRef]
- Stacey, J.S.; Kramers, J.D. Approximation of Terrestrial Lead Isotope Evolution by a Two-Stage Model. Earth Planet. Sci. Lett. 1975, 26, 207–221. [Google Scholar] [CrossRef]
- Condon, D.J.; Schoene, B.; McLean, N.M.; Bowring, S.A.; Parrish, R.R. Metrology and Traceability of U–Pb Isotope Dilution Geochronology (EARTHTIME Tracer Calibration Part I). Geochim. Et Cosmochim. Acta 2015, 164, 464–480. [Google Scholar] [CrossRef]
- McLean, N.M.; Condon, D.J.; Schoene, B.; Bowring, S.A. Evaluating Uncertainties in the Calibration of Isotopic Reference Materials and Multi-Element Isotopic Tracers (EARTHTIME Tracer Calibration Part II). Geochim. Et Cosmochim. Acta 2015, 164, 481–501. [Google Scholar] [CrossRef]
- Paul, A.N.; Spikings, R.A.; Gaynor, S.P. U-Pb ID-TIMS Reference Ages and Initial Pb Isotope Compositions for Durango and Wilberforce Apatites. Chem. Geol. 2021, 586, 120604. [Google Scholar] [CrossRef]
- Gerstenberger, H.; Haase, G. A Highly Effective Emitter Substance for Mass Spectrometric Pb Isotope Ratio Determinations. Chem. Geol. 1997, 136, 309–312. [Google Scholar] [CrossRef]
- Bowring, J.F.; McLean, N.M.; Bowring, S.A. Engineering Cyber Infrastructure for U-Pb Geochronology: Tripoli and U-Pb_Redux. Geochem. Geophys. Geosyst. 2011, 12, Q0AA19. [Google Scholar] [CrossRef]
- McLean, N.M.; Bowring, J.F.; Bowring, S.A. An Algorithm for U-Pb Isotope Dilution Data Reduction and Uncertainty Propagation. Geochem. Geophys. Geosystems 2011, 12, Q0AA18. [Google Scholar] [CrossRef]
- Schmitz, M.D.; Schoene, B. Derivation of Isotope Ratios, Errors, and Error Correlations for U-Pb Geochronology Using 205Pb-235 U-(233U)-spiked Isotope Dilution Thermal Ionization Mass Spectrometric Data. Geochem. Geophys. Geosystems 2007, 8, Q08006. [Google Scholar] [CrossRef]
- Chiaradia, M.; Fontboté, L. Separate Lead Isotope Analyses of Leachate and Residue Rock Fractions: Implications for Metal Source Tracing in Ore Deposit Studies. Miner. Depos. 2003, 38, 185–195. [Google Scholar] [CrossRef]
- Baker, J.; Peate, D.; Waight, T.; Meyzen, C. Pb Isotopic Analysis of Standards and Samples Using a 207Pb–204Pb Double Spike and Thallium to Correct for Mass Bias with a Double-Focusing MC-ICP-MS. Chem. Geol. 2004, 211, 275–303. [Google Scholar] [CrossRef]
- Hogmalm, K.J.; Zack, T.; Karlsson, A.K.-O.; Sjöqvist, A.S.L.; Garbe-Schönberg, D. In Situ Rb–Sr and K–Ca Dating by LA-ICP-MS/MS: An Evaluation of N2O and SF6 as Reaction Gases. J. Anal. At. Spectrom. 2017, 32, 305–313. [Google Scholar] [CrossRef]
- Jegal, Y.; Zimmermann, C.; Reisberg, L.; Yeghicheyan, D.; Cloquet, C.; Peiffert, C.; Gerardin, M.; Deloule, E.; Mercadier, J. Characterisation of Reference Materials for In Situ RB-SR Dating by LA-ICP-MS/MS. Geostand. Geoanalytical Res. 2022, 46, 645–671. [Google Scholar] [CrossRef]
- Woodhead, J.D.; Hergt, J.M. Strontium, Neodymium and Lead Isotope Analyses of NIST Glass Certified Reference Materials: SRM 610, 612, 614. Geostand. Newsl. 2001, 25, 261–266. [Google Scholar] [CrossRef]
- Kutzschbach, M.; Glodny, J. LA-ICP-MS/MS-Based Rb–Sr Isotope Mapping for Geochronology. J. Anal. At. Spectrom. 2024, 39, 455–477. [Google Scholar] [CrossRef]
- Wu, F.-Y.; Yang, Y.-H.; Li, Q.-L.; Mitchell, R.H.; Dawson, J.B.; Brandl, G.; Yuhara, M. In Situ Determination of U–Pb Ages and Sr–Nd–Hf Isotopic Constraints on the Petrogenesis of the Phalaborwa Carbonatite Complex, South Africa. Lithos 2011, 127, 309–322. [Google Scholar] [CrossRef]
- Dahlgren, S.; Corfu, F.; Heaman, L. Datering av plutoner og pegmatitter i Larvik pluton-kompleks, sydlige Oslo Graben, ved hjelp av U-Pb isotoper i zirkon og baddeleyitt. Nor. Bergverksmus. Skr. 1998, 14, 32–39. [Google Scholar]
- Fisher, C.M.; Longerich, H.P.; Jackson, S.E.; Hanchar, J.M. Data Acquisition and Calculation of U–Pb Isotopic Analyses Using Laser Ablation (Single Collector) Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom. 2010, 25, 1905. [Google Scholar] [CrossRef]
- Popov, D.V. Short Communication: On the Potential Use of Materials with Heterogeneously Distributed Parent and Daughter Isotopes as Primary Standards for Non-U–Pb Geochronological Applications of Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS). Geochronology 2022, 4, 399–407. [Google Scholar] [CrossRef]
- Villa, I.M.; De Bièvre, P.; Holden, N.E.; Renne, P.R. IUPAC-IUGS Recommendation on the Half Life of 87Rb. Geochim. Et Cosmochim. Acta 2015, 164, 382–385. [Google Scholar] [CrossRef]
- Redaa, A.; Farkaš, J.; Gilbert, S.; Collins, A.S.; Wade, B.; Löhr, S.; Zack, T.; Garbe-Schönberg, D. Assessment of Elemental Fractionation and Matrix Effects during in Situ Rb–Sr Dating of Phlogopite by LA-ICP-MS/MS: Implications for the Accuracy and Precision of Mineral Ages. J. Anal. At. Spectrom. 2021, 36, 322–344. [Google Scholar] [CrossRef]
- Glorie, S.; Gilbert, S.E.; Hand, M.; Lloyd, J.C. Calibration Methods for Laser Ablation Rb–Sr Geochronology: Comparisons and Recommendation Based on NIST Glass and Natural Reference Materials. Geochronology 2024, 6, 21–36. [Google Scholar] [CrossRef]
- Kuiper, K.F.; Deino, A.; Hilgen, F.J.; Krijgsman, W.; Renne, P.R.; Wijbrans, J.R. Synchronizing Rock Clocks of Earth History. Science 2008, 320, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.A.P. ArArCALC—Software for 40Ar/39Ar Age Calculations. Comput. Geosci. 2002, 28, 605–619. [Google Scholar] [CrossRef]
- Steiger, R.H.; Jäger, E. Subcommission on Geochronology: Convention on the Use of Decay Constants in Geo- and Cosmochronology. Earth Planet. Sci. Lett. 1977, 36, 359–362. [Google Scholar] [CrossRef]
- Igneous Rocks: A Classification and Glossary of Terms, 2nd ed.; Le Maitre, R.W., Streckeisen, A., Zanettin, B., Le Bas, M.J., Bonin, B., Bateman, P., Eds.; Cambridge University Press: Cambridge, UK, 2002; ISBN 978-0-511-53558-1. [Google Scholar]
- Metamorphic Rocks: A Classification and Glossary of Terms; Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Metamorphic Rocks; Fettes, D., Desmons, J., International Union of Geological Sciences, Eds.; Cambridge Univ. Press: Cambridge, UK, 2011; ISBN 978-0-521-33618-5. [Google Scholar]
- Corfu, F.; Hanchar, J.M.; Hoskin, P.W.O.; Kinny, P. Atlas of Zircon Textures. Rev. Mineral. Geochem. 2003, 53, 469–500. [Google Scholar] [CrossRef]
- Geisler, T.; Schaltegger, U.; Tomaschek, F. Re-Equilibration of Zircon in Aqueous Fluids and Melts. Elements 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Geisler, T.; Pidgeon, R.T.; Kurtz, R.; van Bronswijk, W.; Schleicher, H. Experimental Hydrothermal Alteration of Partially Metamict Zircon. Am. Mineral. 2003, 88, 1496–1513. [Google Scholar] [CrossRef]
- Chakoumakos, B.C.; Murakami, T.; Lumpkin, G.R.; Ewing, R.C. Alpha-Decay-Induced Fracturing in Zircon: The Transition from the Crystalline to the Metamict State. Science 1987, 236, 1556–1559. [Google Scholar] [CrossRef]
- Nasdala, L.; Kronz, A.; Hanchar, J.M.; Tichomirowa, M.; Davis, D.W.; Hofmeister, W. Effects of Natural Radiation Damage on Back-Scattered Electron Images of Single Crystals of Minerals. Am. Mineral. 2006, 91, 1739–1746. [Google Scholar] [CrossRef]
- Smye, A.J.; Marsh, J.H.; Vermeesch, P.; Garber, J.M.; Stockli, D.F. Applications and Limitations of U-Pb Thermochronology to Middle and Lower Crustal Thermal Histories. Chem. Geol. 2018, 494, 1–18. [Google Scholar] [CrossRef]
- Holder, R.M.; Hacker, B.R. Fluid-Driven Resetting of Titanite Following Ultrahigh-Temperature Metamorphism in Southern Madagascar. Chem. Geol. 2019, 504, 38–52. [Google Scholar] [CrossRef]
- Li, W.; Costa, F.; Nagashima, K. Apatite Crystals Reveal Melt Volatile Budgets and Magma Storage Depths at Merapi Volcano, Indonesia. J. Petrol. 2021, 62, egaa100. [Google Scholar] [CrossRef]
- Tepper, J.H.; Kuehner, S.M. Complex Zoning in Apatite from the Idaho Batholith; a Record of Magma Mixing and Intracrystalline Trace Element Diffusion. Am. Mineral. 1999, 84, 581–595. [Google Scholar] [CrossRef]
- Dempster, T.J.; Jolivet, M.; Tubrett, M.N.; Braithwaite, C.J.R. Magmatic Zoning in Apatite: A Monitor of Porosity and Permeability Change in Granites. Contrib. Mineral. Petrol. 2003, 145, 568–577. [Google Scholar] [CrossRef]
- Rønsbo, J.G. Apatite in the Ilímaussaq Alkaline Complex: Occurrence, Zonation and Compositional Variation. Lithos 2008, 106, 71–82. [Google Scholar] [CrossRef]
- Knutson, C.; Peacor, D.R.; Kelly, W.C. Luminescence, Color and Fission Track Zoning in Apatite Crystals of the Panasqueira Tin-Tungsten Deposit, Beira-Baixa, Portugal. Am. Mineral. 1985, 70, 829–837. [Google Scholar]
- Blanc, P.; Roger, G.; Couto, H. Recherche de signatures magmatique et hydrothermale dans des apatites du nord du Portugal: Étude par cathodoluminescence, microscopie électronique à balayage et microsonde électronique. Bull. De La Société Géologique De Fr. 1994, 265, 331–341. [Google Scholar]
- Kempe, U.; Götze, J. Cathodoluminescence (CL) Behaviour and Crystal Chemistry of Apatite from Rare-Metal Deposits. Mineral. Mag. 2002, 66, 151–172. [Google Scholar] [CrossRef]
- Götze, J.; Schertl, H.-P.; Neuser, R.D.; Kempe, U.; Hanchar, J.M. Optical Microscope-Cathodoluminescence (OM–CL) Imaging as a Powerful Tool to Reveal Internal Textures of Minerals. Mineral. Petrol. 2013, 107, 373–392. [Google Scholar] [CrossRef]
- Bouzari, F.; Hart, C.J.R.; Bissig, T.; Barker, S. Hydrothermal Alteration Revealed by Apatite Luminescence and Chemistry: A Potential Indicator Mineral for Exploring Covered Porphyry Copper Deposits. Econ. Geol. 2016, 111, 1397–1410. [Google Scholar] [CrossRef]
- Harlov, D.E. Formation of Monazite and Xenotime Inclusions in Fluorapatite Megacrysts, Gloserheia Granite Pegmatite, Froland, Bamble Sector, Southern Norway. Mineral. Petrol. 2011, 102, 77–86. [Google Scholar] [CrossRef]
- Henrichs, I.A.; Chew, D.M.; O’Sullivan, G.J.; Mark, C.; McKenna, C.; Guyett, P. Trace Element (Mn-Sr-Y-Th-REE) and U-Pb Isotope Systematics of Metapelitic Apatite During Progressive Greenschist- to Amphibolite-Facies Barrovian Metamorphism. Geochem. Geophys. Geosystems 2019, 20, 4103–4129. [Google Scholar] [CrossRef]
- Bea, F.; Montero, P. Behavior of Accessory Phases and Redistribution of Zr, REE, Y, Th, and U during Metamorphism and Partial Melting of Metapelites in the Lower Crust: An Example from the Kinzigite Formation of Ivrea-Verbano, NW Italy. Geochim. Et Cosmochim. Acta 1999, 63, 1133–1153. [Google Scholar] [CrossRef]
- Bingen, B.; Demaiffe, D.; Hertogen, J. Redistribution of Rare Earth Elements, Thorium, and Uranium over Accessory Minerals in the Course of Amphibolite to Granulite Facies Metamorphism: The Role of Apatite and Monazite in Orthogneisses from Southwestern Norway. Geochim. Et Cosmochim. Acta 1996, 60, 1341–1354. [Google Scholar] [CrossRef]
- Wenzel, T.; Ramseyer, K. Mineralogical and Mineral-Chemical Changes in a Fractionation-Dominated Diorite-Monzodiorite-Monzonite Sequence; Evidence from Cathodoluminescence. Eur. J. Mineral. 1992, 6, 1391–1399. [Google Scholar] [CrossRef]
- Słaby, E.; Götze, J. Feldspar Crystallization under Magma-Mixing Conditions Shown by Cathodoluminescence and Geochemical Modelling—a Case Study from the Karkonosze Pluton (SW Poland). Mineral. Mag. 2004, 68, 561–577. [Google Scholar] [CrossRef]
- O’Sullivan, G.; Chew, D.; Kenny, G.; Henrichs, I.; Mulligan, D. The Trace Element Composition of Apatite and Its Application to Detrital Provenance Studies. Earth-Sci. Rev. 2020, 201, 103044. [Google Scholar] [CrossRef]
- Cox, R.A.; Dempster, T.J.; Bell, B.R.; Rogers, G. Crystallization of the Shap Granite: Evidence from Zoned K-Feldspar Megacrysts. J. Geol. Soc. 1996, 153, 625–635. [Google Scholar] [CrossRef]
- Lee, M.R.; Waldron, K.A.; Parsons, I. Exsolution and Alteration Microtextures in Alkali Feldspar Phenocrysts from the Shap Granite. Mineral. Mag. 1995, 59, 63–78. [Google Scholar] [CrossRef]
- Fitz Gerald, J.D.; Parsons, I.; Cayzer, N. Nanotunnels and Pull-Aparts: Defects of Exsolution Lamellae in Alkali Feldspars. Am. Mineral. 2006, 91, 772–783. [Google Scholar] [CrossRef]
- Parsons, I.; Fitz Gerald, J.D.; Heizler, M.T.; Heizler, L.L.; Ivanic, T.; Lee, M.R. Eight-Phase Alkali Feldspars: Low-Temperature Cryptoperthite, Peristerite and Multiple Replacement Reactions in the Klokken Intrusion. Contrib Miner. Pet. 2013, 165, 931–960. [Google Scholar] [CrossRef]
- Parsons, I.; Fitz Gerald, J.D.; Lee, M.R. Routine Characterization and Interpretation of Complex Alkali Feldspar Intergrowths. Am. Mineral. 2015, 100, 1277–1303. [Google Scholar] [CrossRef]
- Rout, S.S.; Blum-Oeste, M.; Wörner, G. Long-Term Temperature Cycling in a Shallow Magma Reservoir: Insights from Sanidine Megacrysts at Taápaca Volcano, Central Andes. J. Petrol. 2021, 62, egab010. [Google Scholar] [CrossRef]
- Iovine, R.S.; Fedele, L.; Mazzeo, F.C.; Arienzo, I.; Cavallo, A.; Wörner, G.; Orsi, G.; Civetta, L.; D’Antonio, M. Timescales of Magmatic Processes Prior to the ∼4.7 Ka Agnano-Monte Spina Eruption (Campi Flegrei Caldera, Southern Italy) Based on Diffusion Chronometry from Sanidine Phenocrysts. Bull. Volcanol. 2017, 79, 18. [Google Scholar] [CrossRef]
- Shcherbakov, V.D.; Plechov, P.Y.; Izbekov, P.E.; Shipman, J.S. Plagioclase Zoning as an Indicator of Magma Processes at Bezymianny Volcano, Kamchatka. Contrib. Mineral. Petrol. 2011, 162, 83–99. [Google Scholar] [CrossRef]
- Ginibre, C.; Kronz, A.; Wörner, G. High-Resolution Quantitative Imaging of Plagioclase Composition Using Accumulated Backscattered Electron Images: New Constraints on Oscillatory Zoning. Contrib. Mineral. Petrol. 2002, 142, 436–448. [Google Scholar] [CrossRef]
- Pryer, L.L.; Robin, P.-Y.F. Retrograde Metamorphic Reactions in Deforming Granites and the Origin of Flame Perthite. J. Metamorph. Geol. 1995, 13, 645–658. [Google Scholar] [CrossRef]
- Vernon, R.H. Flame Perthite in Metapelitic Gneisses at Cooma, SE Australia. Am. Mineral. 1999, 84, 1760–1765. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Sengupta, P. Modelling of Dissolution–Reprecipitation Ion-Exchange Reactions for the Development of Flame Perthite in a Suite of Sheared Alkaline Rocks: An Example from Chimakurthy, Eastern Ghats, India. Mineral. Mag. 2014, 78, 1301–1323. [Google Scholar] [CrossRef]
- Parsons, I.; Lee, M.R. Minerals Are Not Just Chemical Compounds. Can. Mineral. 2005, 43, 1959–1992. [Google Scholar] [CrossRef]
- York, D. Least Squares Fitting of a Straight Line with Correlated Errors. Earth Planet. Sci. Lett. 1968, 5, 320–324. [Google Scholar] [CrossRef]
- York, D.; Evensen, N.M.; Martínez, M.L.; De Basabe Delgado, J. Unified Equations for the Slope, Intercept, and Standard Errors of the Best Straight Line. Am. J. Phys. 2004, 72, 367–375. [Google Scholar] [CrossRef]
- Wendt, I.; Carl, C. The Statistical Distribution of the Mean Squared Weighted Deviation. Chem. Geol. Isot. Geosci. Sect. 1991, 86, 275–285. [Google Scholar] [CrossRef]
- Ludwig, K.R. User’s Manual for Isoplot Version 3.75–4.15: A Geochronological Toolkit for Microsoft Excel; Special Publication; Berkeley Geochronological Center: Berkeley, CA, USA, 2000. [Google Scholar]
- Mezger, K.; Krogstad, E.J. Interpretation of Discordant U-Pb Zircon Ages: An Evaluation. J. Metamorph. Geol. 1997, 15, 127–140. [Google Scholar] [CrossRef]
- Pidgeon, R.T. Recrystallisation of Oscillatory Zoned Zircon: Some Geochronological and Petrological Implications. Contrib. Mineral. Petrol. 1992, 110, 463–472. [Google Scholar] [CrossRef]
- Sinha, A.K.; Wayne, D.M.; Hewitt, D.A. The Hydrothermal Stability of Zircon: Preliminary Experimental and Isotopic Studies. Geochim. Et Cosmochim. Acta 1992, 56, 3551–3560. [Google Scholar] [CrossRef]
- Miles, A.J.; Woodcock, N.H. A Combined Geochronological Approach to Investigating Long Lived Granite Magmatism, the Shap Granite, UK. Lithos 2018, 304–307, 245–257. [Google Scholar] [CrossRef]
- Farina, F.; Weber, G.; Hartung, E.; Rubatto, D.; Forni, F.; Luisier, C.; Caricchi, L. Magma Flux Variations Triggering Shallow-Level Emplacement of the Takidani Pluton (Japan): Insights into the Volcanic-Plutonic Connection. Earth Planet. Sci. Lett. 2024, 635, 118688. [Google Scholar] [CrossRef]
- Hartung, E.; Caricchi, L.; Floess, D.; Wallis, S.; Harayama, S. Establishing Genetic Relationships between the Takidani Pluton and Two Large Silicic Eruptions in the Northern Japan Alps. J. Petrol. 2021, 62, egab085. [Google Scholar] [CrossRef]
- Tyrrell, S.; Haughton, P.D.W.; Daly, J.S.; Kokfelt, T.F.; Gagnevin, D. The Use of the Common Pb Isotope Composition of Detrital K-Feldspar Grains as a Provenance Tool and Its Application to Upper Carboniferous Paleodrainage, Northern England. J. Sediment. Res. 2006, 76, 324–345. [Google Scholar] [CrossRef]
- Moxey, M.; Belousova, E. A New Duo for Mineral Exploration: Apatite and Zircon in Granitoids from Mt Isa, Australia. In Proceedings of the Goldschmidt Abstracts, Virtual, 4–9 July 2021. [Google Scholar]
- Popov, D.; Spikings, R.; Ovtcharova, M.; O’Sullivan, G.; Chew, D.; Badenszki, E.; Daly, S.; Davies, J.; Chiaradia, M.; Ulianov, A. Multi-Method Approach to Understanding the Migration Mechanisms of Pb in Apatite and Ar in Alkali Feldspar from Proterozoic Granitic Batholiths from the Mt. Isa Inlier (Australia). In Proceedings of the Goldschmidt Abstracts, Virtual, 4–9 July 2021. [Google Scholar]
- Clarke, D.B.; Harlov, D.E.; Brenan, J.M.; Jähkel, A.; Cichy, S.B.; Wilke, F.D.H.; Yang, X. Assimilation of Xenocrystic Apatite in Peraluminous Granitic Magmas. Am. Mineral. 2023, 108, 1421–1435. [Google Scholar] [CrossRef]
- Baker, D.R. The Escape of Pegmatite Dikes from Granitic Plutons; Constraints from New Models of Viscosity and Dike Propagation. Can. Mineral. 1998, 36, 255–263. [Google Scholar]
- Scaillet, S.; Cuney, M.; Le Carlier De Veslud, C.; Cheilletz, A.; Royer, J.J. Cooling Pattern and Mineralization History of the Saint Sylvestre and Western Marche Leucogranite Pluton, French Massif Central: II. Thermal Modelling and Implications for the Mechanisms of Uranium Mineralization. Geochim. Et Cosmochim. Acta 1996, 60, 4673–4688. [Google Scholar] [CrossRef]
- Annen, C.; Blundy, J.D.; Leuthold, J.; Sparks, R.S.J. Construction and Evolution of Igneous Bodies: Towards an Integrated Perspective of Crustal Magmatism. Lithos 2015, 230, 206–221. [Google Scholar] [CrossRef]
- Popov, D.V.; Spikings, R.A. Numerical Modelling of Radiogenic Ingrowth and Diffusion of Pb in Apatite Inclusions with Variable Shape and U-Th Zonation. Minerals 2021, 11, 364. [Google Scholar] [CrossRef]
- Cherniak, D.J. Diffusion in Accessory Minerals: Zircon, Titanite, Apatite, Monazite and Xenotime. Rev. Mineral. Geochem. 2010, 72, 827–869. [Google Scholar] [CrossRef]
- Perez, W.A.; Dunn, T. Diffusivity of Strontium, Neodymium, and Lead in Natural Rhyolite Melt at 1.0 GPa. Geochim. Et Cosmochim. Acta 1996, 60, 1387–1397. [Google Scholar] [CrossRef]
- Prowatke, S.; Klemme, S. Trace Element Partitioning between Apatite and Silicate Melts. Geochim. Et Cosmochim. Acta 2006, 70, 4513–4527. [Google Scholar] [CrossRef]
- Cherniak, D.J. Cation Diffusion in Feldspars. Rev. Mineral. Geochem. 2010, 72, 691–733. [Google Scholar] [CrossRef]
- Dodson, M.H. Closure Temperature in Cooling Geochronological and Petrological Systems. Contrib. Mineral. Petrol. 1973, 40, 259–274. [Google Scholar] [CrossRef]
- Brent Dalrymple, G.; Lanphere, M.A. 40Ar/39Ar Age Spectra of Some Undisturbed Terrestrial Samples. Geochim. Et Cosmochim. Acta 1974, 38, 715–738. [Google Scholar] [CrossRef]
- Lovera, O.M.; Grove, M.; Harrison, T.M.; Mahon, K.I. Systematic Analysis of K-Feldspar 40Ar/39Ar Step Heating Results: I. Significance of Activation Energy Determinations. Geochim. Et Cosmochim. Acta 1997, 61, 3171–3192. [Google Scholar] [CrossRef]
- Gentner, W.; Kley, W. Argonbestimmungen an Kaliummineralien—IV die frage der argonverluste in kalifeldspäten und glimmermineralien. Geochim. Et Cosmochim. Acta 1957, 12, 323–329. [Google Scholar] [CrossRef]
- Mussett, A.E. Diffusion Measurements and the Potassium-Argon Method of Dating. Geophys. J. Int. 1969, 18, 257–303. [Google Scholar] [CrossRef]
- Pushkarev, Y.D.; Ivanenko, V.V.; Pupyrev, Y.G. Peculiarities of Ar migration in minerals and the method of age spectra. In Mass spectrometry and isotope geology; Ryabchikov, I.D., Chernyshev, I.V., Eds.; Nauka: Moscow, Russia, 1983. [Google Scholar]
- Arnaud, N.O.; Kelley, S.P. Argon Behaviour in Gem-Quality Orthoclase from Madagascar: Experiments and Some Consequences for 40Ar/39Ar Geochronology. Geochim. Et Cosmochim. Acta 1997, 61, 3227–3255. [Google Scholar] [CrossRef]
- Bai, X.-J.; Li, Y.-L.; Hu, R.-G.; Liu, X.; Tang, B.; Gu, X.-P.; Qiu, H.-N. High-Precision Microcline 40Ar/39Ar Dating by Combined Techniques. Chem. Geol. 2024, 655, 122086. [Google Scholar] [CrossRef]
- Kelley, S. Excess Argon in K–Ar and Ar–Ar Geochronology. Chem. Geol. 2002, 188, 1–22. [Google Scholar] [CrossRef]
- Scaillet, S. Excess 40Ar Transport Scale and Mechanism in High-Pressure Phengites: A Case Study from an Eclogitized Metabasite of the Dora-Maira Nappe, Western Alps. Geochim. Et Cosmochim. Acta 1996, 60, 1075–1090. [Google Scholar] [CrossRef]
- Scaillet, S. K-Ar (40Ar/39Ar) Geochronology of Ultrahigh Pressure Rocks. In When Continents Collide: Geodynamics and Geochemistry of Ultrahigh-Pressure Rocks; Hacker, B.R., Liou, J.G., Eds.; Petrology and Structural Geology; Springer: Dordrecht, The Netherlands, 1998; ISBN 978-90-481-4028-2. [Google Scholar]
- Warren, C.J.; Kelley, S.P.; Sherlock, S.C.; McDonald, C.S. Metamorphic Rocks Seek Meaningful Cooling Rate: Interpreting 40Ar/39Ar Ages in an Exhumed Ultra-High Pressure Terrane. Lithos 2012, 155, 30–48. [Google Scholar] [CrossRef]
- Kirkland, C.L.; Spaggiari, C.V.; Johnson, T.E.; Smithies, R.H.; Danišík, M.; Evans, N.; Wingate, M.T.D.; Clark, C.; Spencer, C.; Mikucki, E.; et al. Grain Size Matters: Implications for Element and Isotopic Mobility in Titanite. Precambrian Res. 2016, 278, 283–302. [Google Scholar] [CrossRef]
- Janots, E.; Berger, A.; Gnos, E.; Whitehouse, M.; Lewin, E.; Pettke, T. Constraints on Fluid Evolution during Metamorphism from U–Th–Pb Systematics in Alpine Hydrothermal Monazite. Chem. Geol. 2012, 326–327, 61–71. [Google Scholar] [CrossRef]
- Jenkin, G.R.; Rogers, G.; Fallick, A.E.; Farrow, C.M. Rb-Sr Closure Temperatures in Bi-Mineralic Rocks: A Mode Effect and Test for Different Diffusion Models. Chem. Geol. 1995, 122, 227–240. [Google Scholar] [CrossRef]
- Baxter, E.F. Quantification of the Factors Controlling the Presence of Excess 40Ar or 4He. Earth Planet. Sci. Lett. 2003, 216, 619–634. [Google Scholar] [CrossRef]
- Dohmen, R.; Chakraborty, S. Mechanism and Kinetics of Element and Isotopic Exchange Mediated by a Fluid Phase. Am. Mineral. 2004, 88, 1251–1270. [Google Scholar] [CrossRef]
- Putnis, A. Mineral Replacement Reactions. Rev. Mineral. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Schiano, P.; Provost, A.; Clocchiatti, R.; Faure, F. Transcrystalline Melt Migration and Earth’s Mantle. Science 2006, 314, 970–974. [Google Scholar] [CrossRef]
- Crovetto, R.; Fernández-Prini, R.; Japas, M.L. Solubilities of Inert Gases and Methane in H₂O and in D₂O in the Temperature Range of 300 to 600 K. J. Chem. Phys. 1982, 76, 1077–1086. [Google Scholar] [CrossRef]
- Wartho, J.-A.; Kelley, S.P.; Brooker, R.A.; Carroll, M.R.; Villa, I.M.; Lee, M.R. Direct Measurement of Ar Diffusion Profiles in a Gem-Quality Madagascar K-Feldspar Using the Ultra-Violet Laser Ablation Microprobe (UVLAMP). Earth Planet. Sci. Lett. 1999, 170, 141–153. [Google Scholar] [CrossRef]
- Popov, D.V.; Spikings, R.A. Diffusion vs. Fluid Alteration in Alkali Feldspar 40Ar/39Ar Thermochronology: Does Cross-Correlation of Log(r/R₀) and Age Spectra Validate Thermal Histories? Chem. Geol. 2020, 539, 119506. [Google Scholar] [CrossRef]
- Parsons, I.; Brown, W.L.; Smith, J.V. 40Ar/39Ar Thermochronology Using Alkali Feldspars: Real Thermal History or Mathematical Mirage of Microtexture? Contrib. Mineral. Petrol. 1999, 136, 92–110. [Google Scholar] [CrossRef]
- Ballentine, C.J.; Burnard, P.G. Production, Release and Transport of Noble Gases in the Continental Crust. Rev. Mineral. Geochem. 2002, 47, 481–538. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman Spectroscopy for Fluid Inclusion Analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Holland, G.; Lollar, B.S.; Li, L.; Lacrampe-Couloume, G.; Slater, G.F.; Ballentine, C.J. Deep Fracture Fluids Isolated in the Crust since the Precambrian Era. Nature 2013, 497, 357–360. [Google Scholar] [CrossRef]
- Warr, O.; Sherwood Lollar, B.; Fellowes, J.; Sutcliffe, C.N.; McDermott, J.M.; Holland, G.; Mabry, J.C.; Ballentine, C.J. Tracing Ancient Hydrogeological Fracture Network Age and Compartmentalisation Using Noble Gases. Geochim. Et Cosmochim. Acta 2018, 222, 340–362. [Google Scholar] [CrossRef]
- Kietäväinen, R.; Ahonen, L.; Kukkonen, I.T.; Niedermann, S.; Wiersberg, T. Noble Gas Residence Times of Saline Waters within Crystalline Bedrock, Outokumpu Deep Drill Hole, Finland. Geochim. Et Cosmochim. Acta 2014, 145, 159–174. [Google Scholar] [CrossRef]
- Tolstikhin, I.; Lehmann, B.E.; Loosli, H.H.; Gautschi, A. Helium and Argon Isotopes in Rocks, Minerals, and Related Ground Waters: A Case Study in Northern Switzerland. Geochim. Et Cosmochim. Acta 1996, 60, 1497–1514. [Google Scholar] [CrossRef]
- Baxter, E.F. Diffusion of Noble Gases in Minerals. Rev. Mineral. Geochem. 2010, 72, 509–557. [Google Scholar] [CrossRef]
- Cassata, W.S.; Renne, P.R. Systematic Variations of Argon Diffusion in Feldspars and Implications for Thermochronometry. Geochim. Et Cosmochim. Acta 2013, 112, 251–287. [Google Scholar] [CrossRef]
- Freeze, G.A.; Stein, E.; Brady, P.V.; Lopez, C.; Sassani, D.; Travis, K.; Gibb, F.; Beswick, J. Deep Borehole Disposal Safety Case. Energies 2019, 12, 2141. [Google Scholar] [CrossRef]
- Baltzer, R.; Grimsich, J.; Voss, J. Deep Isolation-Development of the Safety Case for Disposal of Radioactive Wastes in Horizontal Boreholes—20028; WM Symposia: Tempe, AZ, USA, 2020. [Google Scholar]
- Popov, D.V.; Nekrylov, N.A.; Plechov, P.Y. The Petrology of the Upper Albian Tuffites from the Bakhchysarai District, Southwestern Crimea. Mosc. Univ. Geol. Bull. 2016, 71, 194–204. [Google Scholar] [CrossRef]
- Popov, D.V.; Nekrylov, N.; Plechov, P.Y.; Shcherbakov, V.D.; Portnyagin, M.V.; Serova, M.S. Composition and Conditions of Formation of the Parental Melts of Jurassic Dolerites of Southwestern Crimea: Evidence from Melt Inclusions in Olivine Phenocrysts. Petrology 2017, 25, 272–303. [Google Scholar] [CrossRef]
- Popov, D.V.; Brovchenko, V.D.; Nekrylov, N.A.; Plechov, P.Y.; Spikings, R.A.; Tyutyunnik, O.A.; Krigman, L.V.; Anosova, M.O.; Kostitsyn, Y.A.; Soloviev, A.V. Removing a Mask of Alteration: Geochemistry and Age of the Karadag Volcanic Sequence in SE Crimea. Lithos 2019, 324–325, 371–384. [Google Scholar] [CrossRef]
- Ketcham, R.A. Fission-Track Annealing: From Geologic Observations to Thermal History Modeling. In Fission-Track Thermochronology and its Application to Geology; Malusà, M.G., Fitzgerald, P.G., Eds.; Springer Textbooks in Earth Sciences, Geography and Environment; Springer International Publishing: Cham, Switzerland, 2019; pp. 49–75. ISBN 978-3-319-89419-5. [Google Scholar]
- Donelick, R.A.; O’Sullivan, P.B.; Ketcham, R.A. Apatite Fission-Track Analysis. Rev. Mineral. Geochem. 2005, 58, 49–94. [Google Scholar] [CrossRef]
- Tagami, T.; O’Sullivan, P.B. Fundamentals of Fission-Track Thermochronology. Rev. Mineral. Geochem. 2005, 58, 19–47. [Google Scholar] [CrossRef]
- Milesi, G.; Soliva, R.; Monié, P.; Münch, P.; Bellanger, M.; Bruguier, O.; Bonno, M.; Taillefer, A.; Mayolle, S. Mapping a Geothermal Anomaly Using Apatite (U-Th)/He Thermochronology in the Têt Fault Damage Zone, Eastern Pyrenees, France. Terra Nova 2019, 31, 569–576. [Google Scholar] [CrossRef]





| Type of Work | Technique(s) and Key Details |
|---|---|
| Petrological characterisation | Classical microscopy, optical cathodoluminescence (CL) and backscattered electron (BSE) imaging, qualitative analysis and phase identification by energy-dispersive X-ray spectroscopy (EDS), and semi-quantitative element mapping by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) with analytical data processing in Iolite 2.5 [31]. |
| In situ U-Pb for zircon | LA-ICP-MS. GJ-1 [32] and Plešovice [33] zircon standards were used. Analytical data were processed in Excel following [34,35] and using 235,238U decay constants from [36]. |
| In situ U-Pb for apatite | Laser ablation multiple collector inductively coupled plasma mass spectrometry (LA-MC-ICP-MS). McClure Mountain [37,38] Emerald Lake and Durango [39] apatite standards were used. Analytical data were reduced in Excel following [34,35,38]. 235,238U decay constants were taken from [36], while common Pb composition was estimated following [40]. |
| Bulk grain U-Pb for apatite | Isotope dilution thermal ionisation mass spectrometry (ID-TIMS). 205Pb-233U-235U tracer solution ET535 [41,42] was used. Procedures for chemical separation and analysis followed [43,44]. Analytical data were processed in Tripoli and YourLab software [45,46,47] and in Excel using 235,238U decay constants from [36] and model common Pb compositions from [40]. |
| Pb isotopes for alkali feldspar | Multiple collector inductively coupled plasma mass spectrometry (MC-ICP-MS). Chemical work followed [48], and SRM981 [49] was used for corrections. |
| In situ Rb-Sr for alkali feldspar | Reaction cell laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS/MS). MicaMg-NP [50,51], NIST 610 [52], SagaB biotite and alkali feldspar [53,54] and Phalaborwa biotite [53,55] were used as standards. Background and drift correction followed [56], while elemental fractionation correction followed [57], and all calculations were performed in Excel with 87Rb decay constant from [58]. Some chemical data were simultaneously acquired and processed using HDIP software. See Supplementary Archive for a discussion of our analytical approach in the context of prior studies [53,59,60]. |
| In situ, bulk grain 40Ar/39Ar for alkali feldspar | Noble gas mass spectrometry. Fish Canyon Tuff sanidine was used as the neutron flux monitor with age as reported in [61]. Analytical data were reduced in ArArCALC [62] using 40K decay constants and 40Ar/36Ar ratio in air from [63]. |
| Sample | Mineral | System | Type of Data | Result |
|---|---|---|---|---|
| Williams | zircon | U-Pb | upper intercept date | 1508 ± 8 Ma |
| zircon | U-Pb | lower intercept date | 320 ± 9 Ma | |
| zircon | U-Pb | estimated 207Pb/206Pb in released Pb | 0.104 | |
| K-feldspar | Pb isotopes | 207Pb/206Pb, % lower than S&K75 * | 0.742 ± 0.001, 22.2 | |
| apatite | U-Pb | 207Pb-corrected dates using S&K75 * | 1664 ± 37 to 1347 ± 86 Ma | |
| fluorite | U-Pb | location in the Tera-Wasserburg space | below the concordia | |
| Kalkadoon | zircon | U-Pb | upper intercept date | 1832 ± 8 Ma |
| zircon | U-Pb | lower intercept date | 315 ± 7 Ma | |
| zircon | U-Pb | estimated 207Pb/206Pb in released Pb | 0.123 | |
| K-feldspar | Pb isotopes | 207Pb/206Pb, % lower than S&K75 * | 0.9607 ± 0.0001, 2.5 | |
| apatite | U-Pb | 207Pb-corrected dates using S&K75 * | 1965 ± 69 to 1833 ± 42 Ma | |
| K-feldspar | Rb-Sr | isochron date, group with blue CL | 1841 ± 148 Ma | |
| K-feldspar | Rb-Sr | isochron date, 1st group with purple CL | 1468 ± 65 Ma | |
| K-feldspar | Rb-Sr | isochron date, 2nd group with purple CL | 1429 ± 42 Ma | |
| K-feldspar | 40Ar/39Ar | in situ dates for regions with blue CL | 1736 ± 29 to 1429 ± 21 Ma | |
| K-feldspar | 40Ar/39Ar | in situ dates for regions with purple CL | 1781 ± 32 and 931 ± 34 Ma | |
| K-feldspar | 40Ar/39Ar | step-heating plateau date | 1303 ± 18 Ma, | |
| Sybella S | zircon | U-Pb | upper intercept date | 1658 ± 7 Ma |
| zircon | U-Pb | lower intercept date | 315 ± 12 Ma | |
| zircon | U-Pb | estimated 207Pb/206Pb in released Pb | 0.112 | |
| K-feldspar | Pb isotopes | 207Pb/206Pb, % lower than S&K75 * | 0.95267 ± 0.00005, 1.6 | |
| apatite | U-Pb | 207Pb-corrected dates using S&K75 * | 2376 ± 67 to 1397 ± 23 Ma | |
| fluorite | U-Pb | location in the Tera-Wasserburg space | in line with apatite results | |
| Sybella N | zircon | U-Pb | upper intercept date | 1667 ± 15 Ma |
| zircon | U-Pb | lower intercept date | −117 ± 590 Ma | |
| zircon | U-Pb | estimated 207Pb/206Pb in released Pb | 0.099 | |
| K-feldspar | Pb isotopes | 207Pb/206Pb, % lower than S&K75 * | 0.96662 ± 0.00004, 0.2 | |
| apatite | U-Pb | 207Pb-corrected dates using S&K75 * | 1619 ± 88 to 1433 ± 24 Ma | |
| Selected data from the literature | amphibole | 40Ar/39Ar | date range from [25,26,27] | 1843–1398 Ma |
| biotite | 40Ar/39Ar | date range from [25,26,27] | 1490–1363 Ma | |
| K-feldspar | 40Ar/39Ar | date range from [25,26,27] | 1534–1122 Ma | |
| apatite | fission track | date range from [25,26,28,29] | 390–225 Ma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, D.; Spikings, R.; Paul, A.N.; Ovtcharova, M.; Chiaradia, M.; Kutzschbach, M.; Ulianov, A.; O’Sullivan, G.; Chew, D.; Kouzmanov, K.; et al. Excess 40Ar in Alkali Feldspar and 206,207Pb in Apatite Caused by Fluid-Induced Recrystallisation in a Semi-Closed Environment in Proterozoic (Meta)Granites of the Mt Isa Inlier, NE Australia. Geosciences 2024, 14, 358. https://doi.org/10.3390/geosciences14120358
Popov D, Spikings R, Paul AN, Ovtcharova M, Chiaradia M, Kutzschbach M, Ulianov A, O’Sullivan G, Chew D, Kouzmanov K, et al. Excess 40Ar in Alkali Feldspar and 206,207Pb in Apatite Caused by Fluid-Induced Recrystallisation in a Semi-Closed Environment in Proterozoic (Meta)Granites of the Mt Isa Inlier, NE Australia. Geosciences. 2024; 14(12):358. https://doi.org/10.3390/geosciences14120358
Chicago/Turabian StylePopov, Daniil, Richard Spikings, André Navin Paul, Maria Ovtcharova, Massimo Chiaradia, Martin Kutzschbach, Alexey Ulianov, Gary O’Sullivan, David Chew, Kalin Kouzmanov, and et al. 2024. "Excess 40Ar in Alkali Feldspar and 206,207Pb in Apatite Caused by Fluid-Induced Recrystallisation in a Semi-Closed Environment in Proterozoic (Meta)Granites of the Mt Isa Inlier, NE Australia" Geosciences 14, no. 12: 358. https://doi.org/10.3390/geosciences14120358
APA StylePopov, D., Spikings, R., Paul, A. N., Ovtcharova, M., Chiaradia, M., Kutzschbach, M., Ulianov, A., O’Sullivan, G., Chew, D., Kouzmanov, K., Badenszki, E., Daly, J. S., & Davies, J. H. F. L. (2024). Excess 40Ar in Alkali Feldspar and 206,207Pb in Apatite Caused by Fluid-Induced Recrystallisation in a Semi-Closed Environment in Proterozoic (Meta)Granites of the Mt Isa Inlier, NE Australia. Geosciences, 14(12), 358. https://doi.org/10.3390/geosciences14120358







