Geochemical Characterization of Trace MVT Mineralization in Paleozoic Sedimentary Rocks of Northeastern Wisconsin, USA
Abstract
:1. Introduction
1.1. Geologic Setting of the Study Area
1.2. Origin of the MVT Mineralization in Eastern Wisconsin
1.3. Relevance to Water Quality Issues
2. Materials and Methods
2.1. Sample Collection
2.2. Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM-EDS)
2.3. Whole-Rock Geochemistry and Data Analysis
3. Results
3.1. Field and Core Observations
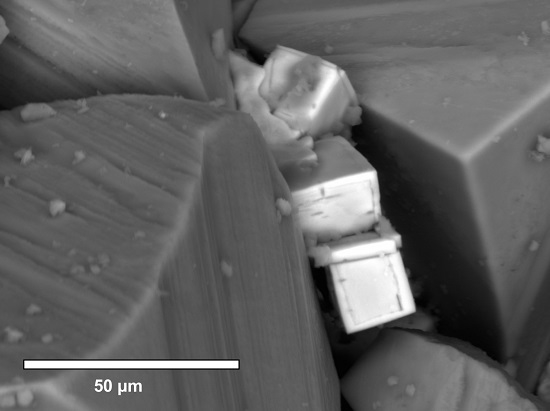
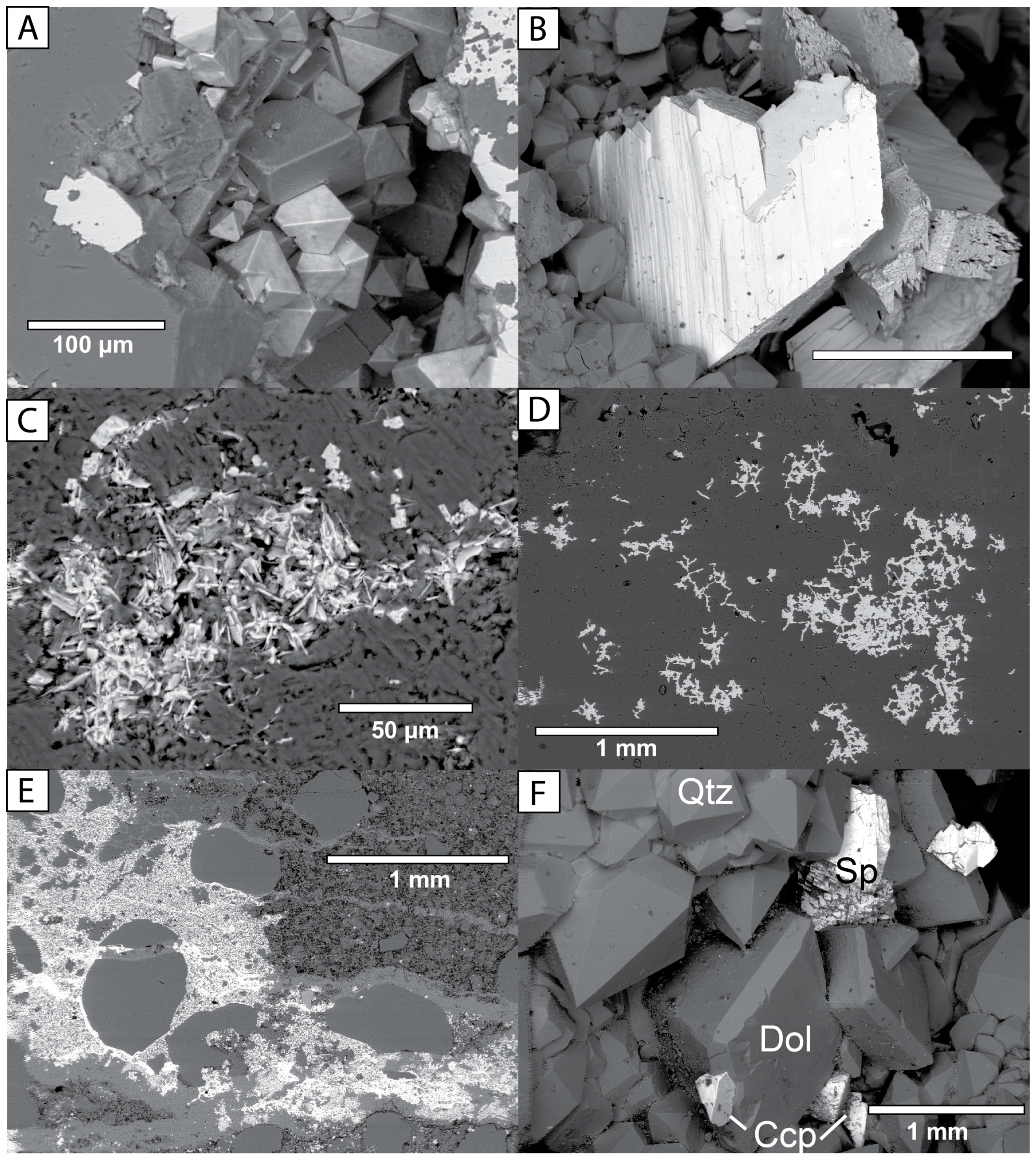
3.2. Petrographic Analysis Using SEM with EDS
3.3. Paragenetic Sequence of Mineralization
3.4. ICP-AES Analytical Results and Discussion
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MVT | Mississippi Valley-type |
| ICP-AES | Inductively Coupled Plasma—Atomic Emission Spectroscopy |
| SEM-EDS | Scanning Electron Microscopy—Energy Dispersive Spectroscopy |
| SCH | Sulfide Cement Horizon |
| PDC | Prairie du Chien Group |
References
- Sverjensky, D.A. Genesis of Mississippi Valley-type lead-zinc deposits. Annu. Rev. Earth Planet. Sci. 1986, 14, 177–199. [Google Scholar] [CrossRef]
- Garven, G.; Ge, S.; Person, M.A.; Sverjensky, D.A. Genesis of stratabound ore deposits in the midcontinent basins of North America. 1. The role of regional groundwater flow. Am. J. Sci. 1993, 293, 497–568. [Google Scholar] [CrossRef]
- Leach, D.L.; Taylor, R.D.; Fey, D.L.; Diehl, S.F.; Saltus, R.W. A Deposit Model for Mississippi Valley-Type Lead-Zinc Ores. Chapter A of Mineral Deposit Models for Resource Assessment; United States Geological Survey, Scientific Investigations Report; United States Geological Survey: Reston, VA, USA, 2010.
- Kyle, J.R. Geology of the pine point lead-zinc district. In Handbook of Strata-Bound and Stratiform Ore Deposits; Wold, K.H., Ed.; Elsevier: New York, NY, USA, 1981; Volume 9, pp. 643–741. [Google Scholar]
- Leach, D.L.; Sangster, D.F.; Kelley, K.D.; Large, R.R.; Garven, G.; Allen, C.R.; Gutzmer, J.; Walters, S. Sediment-Hosted Lead-Zinc Deposits: A Global Perspective. Econ. Geol. 2005, 561–607. [Google Scholar]
- Hitzman, M.W.; Beaty, D.W. The Irish Zn-Pb-(Ba) Orefield; Society of Economic Geologists Special Publication: Littleton, CO, USA, 1996; Volume 4, pp. 112–143. [Google Scholar]
- Leach, D.L.; Viets, J.G.; Kozlowski, A.; Kibitlewski, S. Geology, Geochemistry, and Genesis of the Silesia-Cracow Zinc-Lead District, Southern Poland. Society Economic Geolog. Special Publication 1996, 4, 144–170. [Google Scholar]
- Heyl, A.V., Jr.; Agnew, A.F.; Lyons, E.J.; Behre, C.H., Jr. The Geology of the Upper Mississippi Valley Zinc-Lead District; United States Geological Survey Professional Paper; United States Geological Survey: Reston, VA, USA, 1959; Volume 309, p. 310.
- McLimans, R.K. Geological, Fluid Inclusion, and Stable Isotope Studies of the Upper Mississippi Valley Zinc-Lead District, Southwest Wisconsin. Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 1977. [Google Scholar]
- Bethke, C.M. Hydrologic constraints on the genesis of the Upper Mississippi Valley mineral district from Illinois basin brines. Econ. Geol. 1986, 81, 233–249. [Google Scholar] [CrossRef]
- Rowan, E. Thermal and Hydrogeologic History of a Sedimentary Basin: Case Studies in the Illinois Basin, USA, and the Albigeois District, France. Ph.D. Thesis, Université Pierre et Marie Curie (Paris VI), Paris, France, 1998. [Google Scholar]
- Catacosinos, P.A.; Daniels, P.A., Jr.; Harrison, W.B., III. Structure, stratigraphy, and petroleum geology of the Michigan basin. In Interior Cratonic Basins; Leighton, M., Kolata, D., Oltz, D., Eidel, J., Eds.; American Association of Petroleum Geologists Memoir: Tulsa, OK, USA, 1990; pp. 561–601. [Google Scholar]
- Luczaj, J.; Masarik, K. Groundwater quantity and quality issues in a water-rich region: Examples from Wisconsin, USA. Resources 2015, 4, 323–357. [Google Scholar] [CrossRef]
- Bagrowski, B.P. Occurrence of millerite at Milwaukee, Wisconsin. Am. Mineral. 1940, 25, 556–559. [Google Scholar]
- Garvin, P.L.; Ludvigson, G.A.; Ripley, E.M. Sulfur isotope reconnaissance of minor metal sulfide deposits fringing the Upper Mississippi Valley zinc-lead district. Econ. Geol. 1987, 82, 1386–1394. [Google Scholar] [CrossRef]
- Evans, T.; Wisconsin Geological & Natural History Survey, Madison, WI, USA. Personal communication, 21 June 2011.
- Badiozamani, K. The dorag dolomitization model—Application to the middle Ordovician of Wisconsin. J. Sediment. Petrol. 1973, 43, 965–984. [Google Scholar]
- Deininger, R.W. Limestone-dolostone transition in the Ordovician Platteville Formation in Wisconsin. J. Sediment. Petrol. 1964, 34, 281–288. [Google Scholar]
- Morrow, D.W. Dolomite—Part 2: Dolomitization models and ancient dolostones. Geosci. Can. 1982, 9, 95–107. [Google Scholar]
- Muchez, P.; Viaene, W. Dolomitization caused by water circulation near the mixing zone: And example from the Lower Viséan of the Campine Basin (northern Belgium). In Dolomites: A Volume in Honor of Dolomieu; Purser, B., Tucker, M., Zenger, D., Eds.; Special Publication of the International Association of Sedimentologists: Ghent, Belgium, 1994; pp. 155–166. [Google Scholar]
- Dixon, R.J. A hydrogeological assessment of mixed water dolomitization in a confined coastal aquifer; Cambrian-Ordovician deposits, Illinois Basin. Master’s Thesis, University of New Hampshire, Durham, NH, USA, 2000. [Google Scholar]
- Luczaj, J.A. Epigenetic Dolomitization and Sulfide Mineralization in Paleozoic rocks of eastern Wisconsin: Implications for fluid flow out of the Michigan Basin, U.S.A. Ph.D. Thesis, Johns Hopkins University, Baltimore, MD, USA, 2000. [Google Scholar]
- Luczaj, J.A. Evidence against the Dorag (Mixing-Zone) model for dolomitization along the Wisconsin arch—A case for hydrothermal diagenesis. AAPG Bull. 2006, 90, 1719–1738. [Google Scholar] [CrossRef]
- Luczaj, J.A. Preliminary Geologic Map of the Buried Bedrock Surface, Brown County, Wisconsin. Wisconsin Geological and Natural History Survey Open File Report. 2011, WOFR2011-02, 1:100000 Scale Map Sheet. Available online: https://wgnhs.uwex.edu/pubs/download_wofr201102/ (accessed on 1 February 2016).
- Luczaj, J.A. Geology of the Niagara escarpment in Wisconsin. Geosci. Wis. 2013, 22, 1–34. [Google Scholar]
- Brannon, J.C.; Podosek, F.A.; McLimans, R.K. Alleghenian age of the Upper Mississippi Valley zinc-lead deposit determined by Rb-Sr dating of sphalerite. Nature 1992, 356, 509–511. [Google Scholar] [CrossRef]
- Wisconsin Geological & Natural History Survey (WGNHS). Bedrock Stratigraphic Units in Wisconsin; Educational Series; Wisconsin Geological & Natural History Survey: Madison, WI, USA, 2011. [Google Scholar]
- McLaughlin, P.I.; Mikulic, D.G.; Kluessendorf, J. Age and correlation of Silurian rocks in Sheboygan, Wisconsin, using integrated stable carbon isotope stratigraphy and facies analysis. Geosci. Wis. 2013, 21, 15–38. [Google Scholar]
- Gotkowitz, M.B.; Schreiber, M.S.; Simo, J.A. Effects of water use on arsenic release to well water in a confined aquifer. Ground Water 2004, 42, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, K.; Sahai, N. Arsenic occurrence, mobility, and retardation in sandstone and dolomite formations of the Fox River Valley, Eastern Wisconsin. Environ. Sci. Technol. 2004, 38, 5087–5094. [Google Scholar] [CrossRef] [PubMed]
- Burkel, R.S.; Stoll, R.C. Naturally occurring arsenic in sandstone aquifer water supply wells of Northeastern Wisconsin. Groundw. Monit. Remediat. 1999, 19, 114–121. [Google Scholar] [CrossRef]
- Schreiber, M.E.; Simo, J.A.; Freiberg, P.G. Stratigraphic and geochemical controls on naturally occurring arsenic in groundwater, Eastern Wisconsin, USA. Hydrogeol. J. 2000, 8, 161–176. [Google Scholar] [CrossRef]
- Riewe, T.; Weissbach, A.; Heinen, L.; Stoll, R. Naturally occurring arsenic in well water in Wisconsin. Water Well J. 2000, 49, 24–29. [Google Scholar]
- Johnson, D.M.; Riewe, T. Arsenic and Northeastern Wisconsin. Water Well J. 2006, 60, 26–31. [Google Scholar]
- Knobeloch, L.M.; Zierold, K.M.; Anderson, H.A. Association of arsenic-contaminated drinking-water with prevalence of skin cancer in Wisconsin’s Fox River valley. J. Health Popul. Nutr. 2006, 24, 206–213. [Google Scholar] [PubMed]
- Mudrey, M.G.; Brown, B.A.; Greenberg, J.K. Bedrock Geologic Map of Wisconsin. 1:1,000,000 Scale Map Sheet; Wisconsin Geological & Natural History Survey: Madison, WI, USA, 1982. [Google Scholar]
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar] [PubMed]
- Pelczar, J.S. Groundwater Chemistry of Wells Exhibiting Natural Arsenic Contamination in East-Central Wisconsin. Master’s Thesis, University of Wisconsin—Green Bay, Green Bay, WI, USA, 1996. [Google Scholar]
- Schreiber, M.E.; Gotkowitz, M.B.; Simo, J.A.; Freiberg, P.G. Mechanisms of arsenic release to ground water from naturally occurring sources, eastern Wisconsin. In Arsenic in Ground Water; Welch, A., Stollenwerk, K., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2003; pp. 259–280. [Google Scholar]
- Gotkowitz, M.B.; Simo, J.A.; Schreiber, M. Geologic and Geochemical Controls on Arsenic in Groundwater in Northeastern Wisconsin; Wisconsin Geological and Natural History Survey Open-File Report; Wisconsin Geological and Natural History Survey: Madison, WI, USA, 2003; p. 60. [Google Scholar]
- Wisconsin Department of Natural Resources. DNR Groundwater Retrieval Network. Available online: http://prodoasext.dnr.wi.gov/inter1/grn$.startup (accessed on 11 May 2016).
- Mudrey, M.G., Jr.; Bradbury, K.R. Evaluation of NURE Hydrogeochemical Data for Use in Wisconsin Groundwater Studies; Wisconsin Geological & Natural History Survey Open File Report 93-2; Wisconsin Geological and Natural History Survey: Madison, WI, USA, 1992; p. 61. [Google Scholar]
- Smith, S.M. National Geochemical Database: Reformatted Data from the National Uranium Resource Evaluation (NURE) Hydrogeochemical and Stream Sediment Reconnaissance (HSSR) Program: U.S. Geological Survey Open-File Report. 1997; pp. 97–492. Available online: http://pubs.usgs.gov/of/1997/ofr-97-0492/ (accessed on 13 May 2016). [Google Scholar]
- Dickoff, M.E. Modeling Flow and Arsenic Contamination during Aquifer Storage and Recovery Pilot Tests in Green Bay, WI. Master’s Thesis, University of Wisconsin, Madison, WI, USA, 2010. [Google Scholar]
- Johnson, D.; Wisconsin Department of Natural Resources, Madison, WI, USA. Written communication, 28 August 2008.
- Hamby, A.; Luczaj, J. Detection and evaluation of an inadvertent cross-connection of a water supply pipeline to a deep well using time-series geochemical and stable isotopic indicators. In Proceedings of the American Water Resources Association, Wisconsin Section Meeting, Wisconsin Dells, WI, USA, 10–11 March 2016.
- Drever, J.E. The Geochemistry of Natural Waters, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1997; p. 436. [Google Scholar]
- Wisconsin Department of Natural Resources. Drinking Water Quality Data. Available online: http://dnr.wi.gov/topic/drinkingwater/qualitydata.html (accessed on 10 May 2016).
- Güler, C.; Thyne, G.D.; McCray, J.E.; Turner, A.K. Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol. J. 2002, 10, 455–474. [Google Scholar] [CrossRef]
- Ponzio, K.; (private mineral dealer). Written communication and samples provided, 14 November 2015.
- Wisconsin Geological & Natural History Survey. Minerals of Wisconsin Database. Available online: https://wgnhs.uwex.edu/wisconsin-geology/minerals-wisconsin/ (accessed on 17 March 2016).
- Heyl, A.V.; Milton, C.; Axelrod, J.M. Nickel minerals from near Linden, Iowa County, Wisconsin. Am. Mineral. 1959, 44, 995–1009. [Google Scholar]
- Kerr, P.F. Cattierite and vaesite: New co-ni minerals from the Belgian Congo. Am. Mineral. 1945, 30, 483–497. [Google Scholar]
- Luczaj, J.A.; Millen, T.; Martin, J. A lead-isotopic study of Galena from Eastern Wisconsin: Evidence for lead sources in Precambrian basement rocks. In Proceedings of the Geological Society of America, North-Central/South Central Meeting in Lawrence Kansas, Lawrence, KS, USA, 11–13 April 2007.
- Luczaj, J.A.; Millen, T.; Martin, J. A lead-isotopic study of galena from Eastern Wisconsin: Evidence for lead sources in Precambrian basement rocks. Econ. Geol. in preparation.
- Krohelski, J.T. Hydrogeology and Ground-Water Use and Quality, Brown County, Wisconsin; Wisconsin Geological and Natural History Survey: Madison, WI, USA, 1986; Volume 57, pp. 1–42. [Google Scholar]
- Luczaj, J.A.; Hart, D.J. Drawdown in the Northeast Groundwater Management Area (Brown, Outagamie, and Calumet Counties, WI), Wisconsin Geological and Natural History Survey—Open File Report, 2009, WOFR2009-04, 60 pages. Available online: http://wgnhs.uwex.edu/pubs/wofr200904/ (accessed on 2 February 2016).
- Baeten, J.B. Spatial Distribution and Source Identification of Dissolved Strontium in Eastern Wisconsin’s Cambrian-Ordovician Aquifers. Master’s Thesis, University of Wisconsin-Green Bay, Green Bay, WI, USA, 2015. [Google Scholar]
- Tuttle, M.L.W.; Breit, G.N.; Goldhaber, M.B. Weathering of the New Albany Shale, Kentucky: II. Redistribution of minor and trace elements. Appl. Geochem. 2009, 24, 1565–1578. [Google Scholar] [CrossRef]
- CH2M Hill. Green Bay ASR Phase IIA Mineralogical and Water Quality Testing and Results; Technical Memorandum Prepared for Green Bay (Wisconsin) Water Utility August 24, 2000; CH2M Hill: St. Louis, MO, USA, 2000; p. 23. [Google Scholar]
- Simo, J.A.; Freiberg, P.G.; Freiberg, K.S. Geologic Constraints on Arsenic in Groundwater with Application to Groundwater Modeling; Groundwater Research Report WRC GRR 96-01; University of Wisconsin Water Resources Center: Madison, WI, USA, 1996. [Google Scholar]









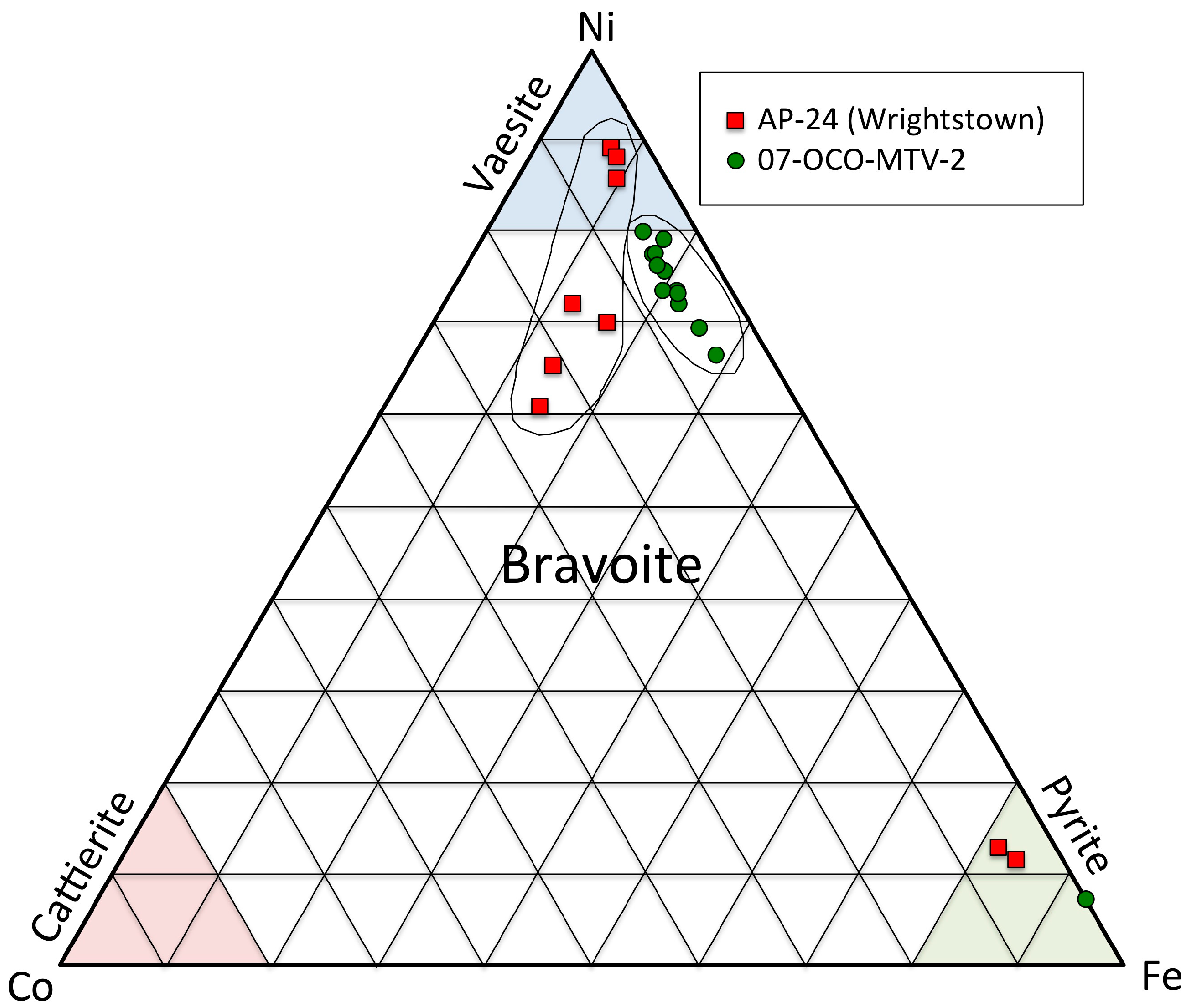


| Sample; Crystal | Ni | Co | As | Fe | C | O | Mg | Si | S | Cl | Ca |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AP-24; 510-515 ft; 1a | 30.69 | 9.91 | - | 6.22 | 18.19 | 11.49 | - | - | 20.44 | - | 3.05 |
| AP-24; 510-515 ft; 1b | 28.70 | 6.32 | - | 4.62 | 16.30 | 14.16 | - | - | 26.13 | 1.61 | 2.15 |
| AP-24; 510-515 ft; 1c | 27.55 | 1.59 | - | 2.80 | 16.78 | 13.07 | - | - | 37.46 | - | 0.76 |
| AP-24; 510-515 ft; 1d | 32.10 | 1.42 | - | 2.77 | 21.33 | 7.13 | - | - | 33.72 | - | 1.54 |
| AP-24; 510-515 ft; 2 | 35.31 | 1.49 | - | 2.75 | - | 10.98 | - | - | 49.48 | - | - |
| AP-24; 510-515 ft; 3 | 24.55 | 9.98 | - | 5.67 | 17.97 | 14.04 | 1.46 | 0.82 | 20.80 | 2.12 | 2.59 |
| AP-24; 510-515 ft; 4 | 17.75 | 3.41 | - | 4.01 | 27.59 | 16.35 | 2.37 | - | 26.36 | - | 2.15 |
| AP-24; 520-525 ft; A1 | 1.84 | 0.75 | 2.27 | 13.08 | 43.34 | 24.47 | 3.06 | 0.45 | 7.89 | 0.66 | 2.18 |
| AP-24; 520-525 ft; A2 | 3.16 | 1.36 | 4.09 | 19.41 | 35.64 | 20.57 | 3.44 | 0.50 | 7.36 | 0.59 | 3.90 |
| 07-OCO-MTV-2; 1 | 20.25 | 1.41 | - | 4.21 | 19.68 | 23.93 | 1.77 | - | 27.06 | - | 1.68 |
| 07-OCO-MTV-2; 2a-1 | 18.99 | 0.90 | - | 3.95 | 25.00 | 25.00 | - | - | 25.36 | - | 0.80 |
| 07-OCO-MTV-2; 2a-2 | 15.76 | 1.20 | - | 5.55 | 26.04 | 28.08 | 1.06 | - | 21.20 | - | 1.11 |
| 07-OCO-MTV-2; 2b | 19.05 | 1.29 | - | 3.99 | 22.83 | 26.25 | - | - | 26.58 | - | - |
| 07-OCO-MTV-2; 2c | 17.12 | 1.26 | - | 4.71 | 20.19 | 32.61 | - | - | 23.21 | - | 0.90 |
| 07-OCO-MTV-2; 2d | 2.47 | - | - | 29.70 | - | 31.84 | 2.08 | - | 32.45 | - | 1.47 |
| 07-OCO-MTV-2; 3 | 18.66 | 1.23 | - | 4.21 | 25.53 | 26.01 | 1.48 | - | 21.06 | - | 1.83 |
| 07-OCO-MTV-2; 4 | 17.66 | 1.61 | - | 4.57 | 32.29 | 20.54 | 1.74 | - | 19.23 | - | 2.35 |
| 07-OCO-MTV-2; 5 | 20.80 | 1.50 | - | 4.99 | 32.73 | 20.87 | 1.63 | - | 15.48 | - | 2.00 |
| 07-OCO-MTV-2; 6 | 22.24 | 1.65 | - | 4.97 | 32.35 | 20.34 | 1.64 | - | 14.48 | - | 2.33 |
| 07-OCO-MTV-2; 7 | 21.19 | 1.38 | - | 3.75 | 26.49 | 20.64 | - | - | 26.54 | - | - |
| 07-OCO-MTV-2; 8 | 15.00 | 1.24 | - | 4.37 | 29.88 | 27.63 | - | - | 20.90 | - | 0.98 |
| 07-OCO-MTV-2; 9 | 15.27 | 1.13 | - | 4.25 | 20.90 | 30.33 | 2.89 | 0.65 | 22.91 | - | 1.67 |
| Mineral Group | Minerals Observed 1 |
|---|---|
| Carbonates | Dolomite, calcite, strontianite, malachite (rare) |
| Sulfates | Barite, celestine, Sr-barite, gypsum; anhydrite2 (minor) |
| Sulfides (and related) | Pyrite, marcasite, sphalerite, chalcopyrite, galena, bravoite, vaesite, nickelian-pyrite, As-bearing-pyrite, erythrite? |
| Phosphates | Apatite/fluorapatite |
| Silicates | Quartz, K-feldspar, illite?, amethyst3, smoky quartz3 |
| Halides | Fluorite |
| Fe (%) | As | Cd | Co | Cr | Cu | Mo | Ni | Pb | V | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum 1 | 0.06 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Minimum detected value | 0.06 | 0.40 | 0.001 | 0.40 | 1.0 | 0.9 | 0.05 | 0.7 | 0.38 | 1.0 | 0.6 |
| Median | 0.57 | 4.00 | ND | 1.66 | 4.0 | 5.0 | 0.10 | 3.0 | 4.00 | 6.0 | 3.0 |
| Maximum | 28.4 | 499 | 4.20 | 133 | 152 | 1620 | 25.0 | 1110 | 584 | 353 | 3720 |
| Interquartile range | 0.89 | 9.00 | 0.01 | 2.58 | 4.9 | 9.5 | 0.90 | 8.0 | 7.41 | 6.0 | 6.0 |
| Rock Unit | Fe (%) | As | Co | Cr | Cu | Mo | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Silurian (33) | 0.16 (0.21) | 4.00 (6.00) | 1.00 (0.50) | 2.0 (2.0) | 2.0 (2.0) | ND (0.00) | ND (1.0) | 4.00 (3.60) | 1.0 (2.0) | ND (3.0) |
| Maquoketa-Neda (11) | 8.52 (13.31) | 132 (151) | 13.00 (24.50) | 7.0 (7.0) | 48.0 (7.0) | 2.00 (3.34) | 24.0 (25.1) | 50.00 (55.15) | 22.0 (64.0) | 14.0 (11.0) |
| Sinnipee Group (111) | 0.59 (0.25) | 2.00 (5.00) | 1.00 (1.20) | 3.0 (3.0) | 3.0 (2.8) | ND (0.29) | 2.7 (3.3) | 3.60 (3.85) | 5.0 (3.0) | 2.0 (4.5) |
| Ancell Group (18) | 1.24 (1.07) | 5.71 (4.16) | 4.89 (6.99) | 8.2 (13.8) | 13.5 (12.9) | 0.25 (0.79) | 11.8 (31.6) | 6.14 (8.86) | 8.1 (14.4) | 7.8 (8.4) |
| PDC Group (75) | 0.40 (0.17) | 3.00 (6.00) | 1.70 (1.25) | 3.0 (3.0) | 6.0 (8.0) | ND (0.24) | 2.0 (3.0) | 2.00 (2.95) | 9.0 (5.9) | 3.0 (6.5) |
| Cambrian (19) | 0.67 (0.68) | 1.02 (7.35) | 3.17 (8.69) | 12.0 (4.6) | 6.9 (13.4) | 1.17 (1.11) | 5.2 (8.0) | 1.56 (5.07) | 6.0 (7.2) | 2.4 (1.4) |
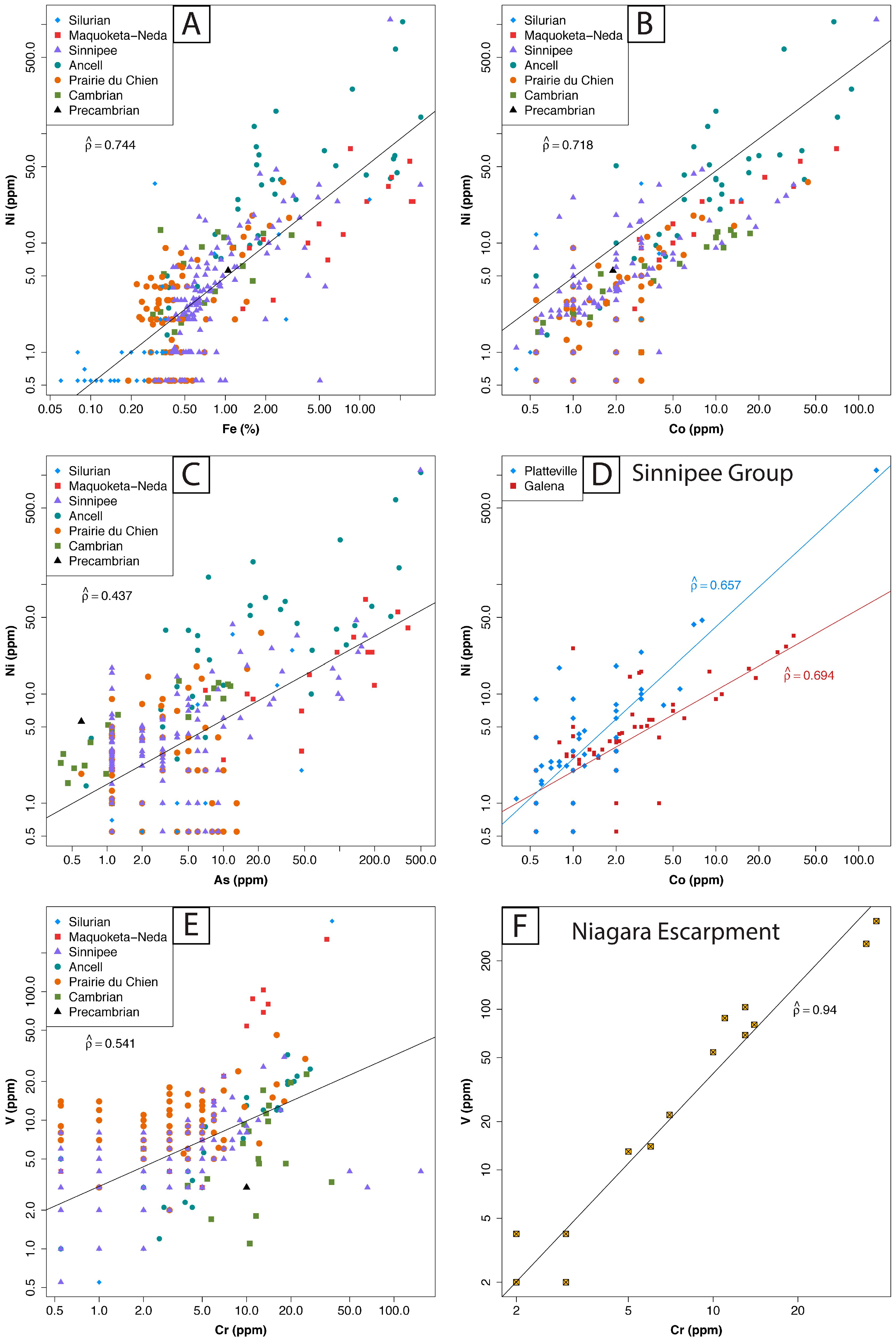
| Log10-Transformed Variables | Spearman’s All Samples | Spearman’s Enriched Samples | Spearman’s Matrix Samples |
|---|---|---|---|
| Cr vs. V (O-S subset) 1 | 0.940 | - | - |
| Ni vs. Fe | 0.744 | 0.587 | 0.642 |
| Ni vs. Co | 0.718 | 0.690 | 0.654 |
| Pb vs. Fe | 0.619 | 0.676 | 0.470 |
| Co vs. Fe | 0.612 | 0.449 | 0.529 |
| K vs. B | 0.594 | 0.694 | 0.578 |
| Co vs. As | 0.588 | 0.554 | 0.495 |
| Cr vs. V | 0.541 | 0.769 | 0.503 |
| As vs. Fe | 0.522 | 0.579 | 0.313 |
| Cu vs. Fe | 0.499 | 0.379 | 0.392 |
| Ni vs. As | 0.437 | 0.540 | 0.201 |
| Zn vs. Fe 2 | 0.326 | 0.156 | 0.354 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luczaj, J.A.; McIntire, M.J.; Olson Hunt, M.J. Geochemical Characterization of Trace MVT Mineralization in Paleozoic Sedimentary Rocks of Northeastern Wisconsin, USA. Geosciences 2016, 6, 29. https://doi.org/10.3390/geosciences6020029
Luczaj JA, McIntire MJ, Olson Hunt MJ. Geochemical Characterization of Trace MVT Mineralization in Paleozoic Sedimentary Rocks of Northeastern Wisconsin, USA. Geosciences. 2016; 6(2):29. https://doi.org/10.3390/geosciences6020029
Chicago/Turabian StyleLuczaj, John A., Michael J. McIntire, and Megan J. Olson Hunt. 2016. "Geochemical Characterization of Trace MVT Mineralization in Paleozoic Sedimentary Rocks of Northeastern Wisconsin, USA" Geosciences 6, no. 2: 29. https://doi.org/10.3390/geosciences6020029
APA StyleLuczaj, J. A., McIntire, M. J., & Olson Hunt, M. J. (2016). Geochemical Characterization of Trace MVT Mineralization in Paleozoic Sedimentary Rocks of Northeastern Wisconsin, USA. Geosciences, 6(2), 29. https://doi.org/10.3390/geosciences6020029