Local Variability of CO2 Partial Pressure in a Mid-Latitude Mesotidal Estuarine System (Tagus Estuary, Portugal)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Meteorological Data
2.4. In Situ Measurements
2.5. Chemical Analysis
2.6. Estimated Parameters
3. Results
3.1. Hydrodynamics
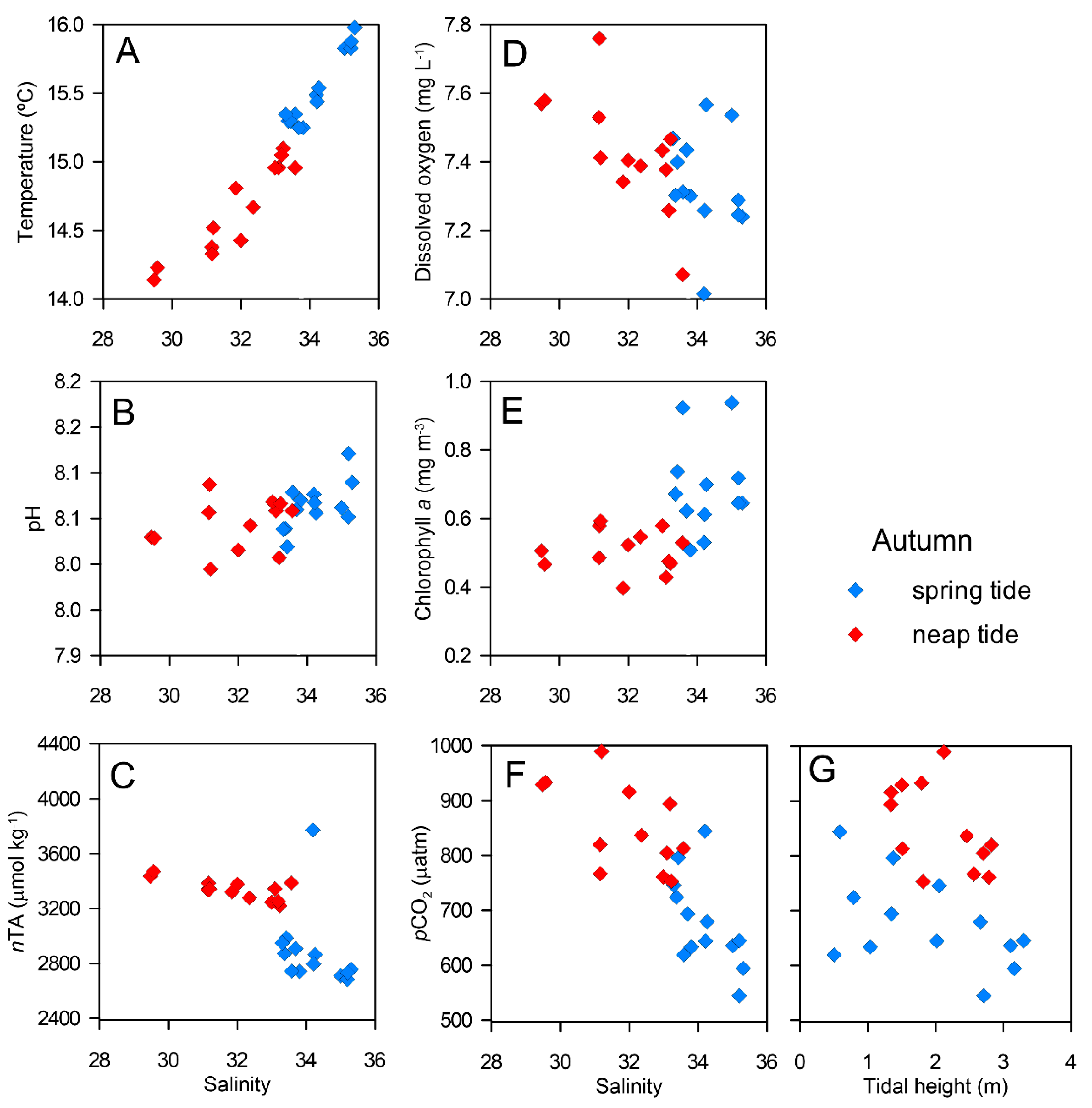
3.2. Tidal Variability
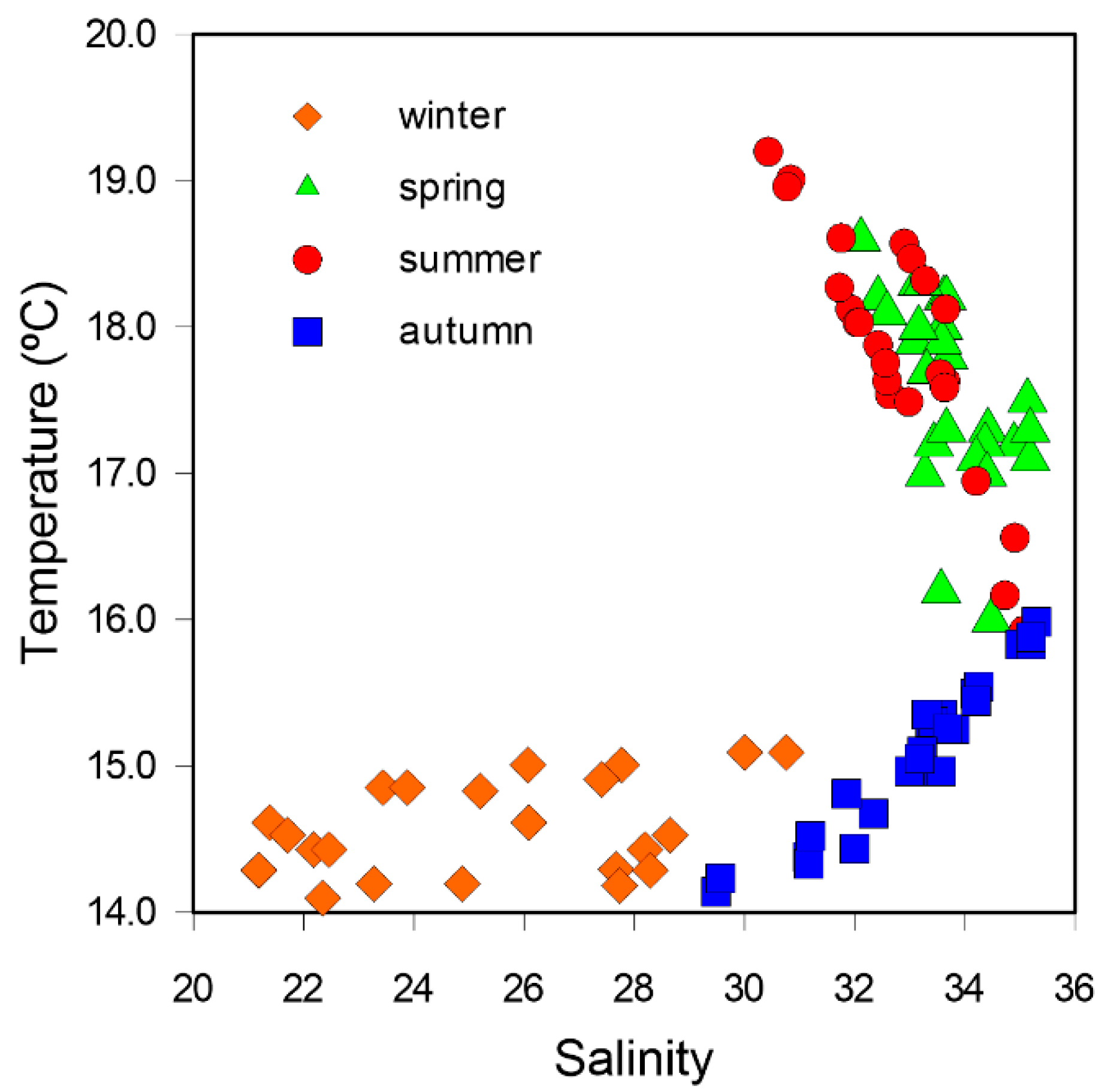
3.3. Seasonal Variability
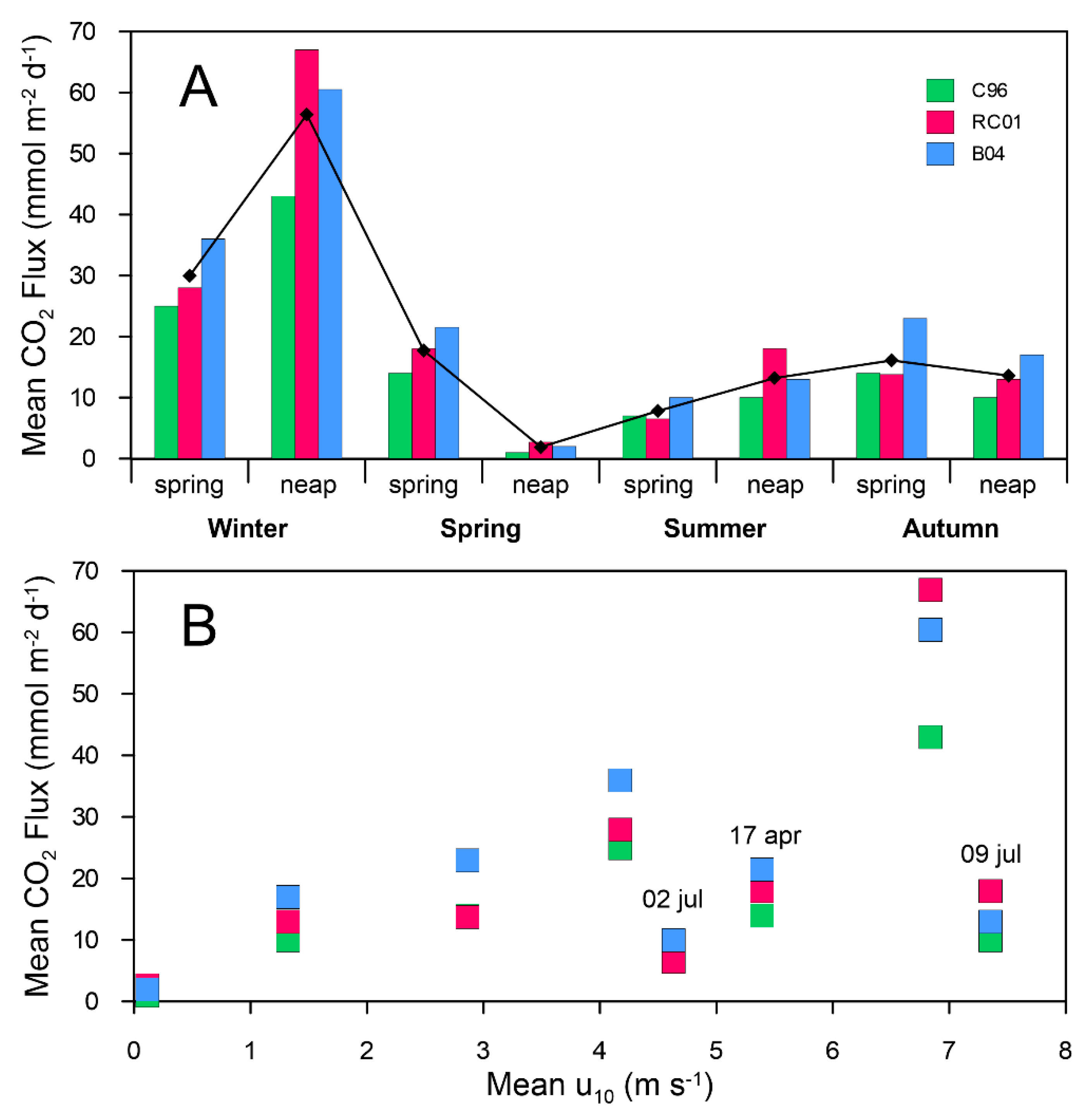
3.4. Air–Water CO2 Fluxes
4. Discussion
4.1. Tidal Variability
4.2. Seasonal Variability
4.3. Air–Water CO2 Exchange
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kemp, W.M.; Smith, E.M.; Marvin-DiPasquale, M.; Boynton, W.R. Organic carbon balance and net ecosystem metabolism in Chesapeake Bay. Mar. Ecol. Prog. Ser. 1997, 150, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Gattuso, J.P.; Frankignoulle, M.; Wollast, R. Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu. Rev. Ecol. Syst. 1998, 29, 405–434. [Google Scholar] [CrossRef]
- Gazeau, F.; Smith, S.V.; Gentili, B.; Frankignoulle, M.; Gattuso, J.P. The European coastal zone: Characterization and first assessment of ecosystem metabolism. Estuar. Coast. Shelf Sci. 2004, 60, 673–694. [Google Scholar] [CrossRef]
- Hopkinson, C.S.J.; Smith, E.M. Estuarine respiration: An overview of benthic, pelagic and whole system respiration. In Respiration in Aquatic Ecosystems; del Giorgio, P.A., Williams, P.J.L., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 123–147. [Google Scholar]
- Cai, W.-J. Estuarine and Coastal Ocean Carbon Paradox: CO2 Sinks or Sites of Terrestrial Carbon Incineration? Annu. Rev. Mar. Sci. 2011, 3, 123–145. [Google Scholar] [CrossRef]
- Laruelle, G.G.; Dürr, H.H.; Slomp, C.P.; Borges, A.V. Evaluation of sinks and sources of CO2 in the global coastal ocean using a spatially-explicit typology of estuaries and continental shelves. Geophys. Res. Lett. 2010, 37, 15. [Google Scholar] [CrossRef]
- Laruelle, G.G.; Dürr, H.H.; Lauerwald, R.; Hartmann, J.; Slomp, C.P.; Goossens, N.; Regnier, P.A.G. Global multi-scale segmentation of continental and coastal waters from the watersheds to the continental margins. Hydrol. Earth Syst. Sci. 2013, 17, 2029–2051. [Google Scholar] [CrossRef]
- Chen, C.T.A.; Huang, T.H.; Chen, Y.C.; Bai, Y.; He, X.; Kang, Y. Air-sea exchanges of CO2 in the world’s coastal seas. Biogeosciences 2013, 10, 6509–6544. [Google Scholar] [CrossRef]
- Chen, C.-T.A.; Borges, A.V. Reconciling opposing views on carbon cycling in the coastal ocean: Continental shelves as sinks and nearshore ecosystems as sources of atmospheric CO2. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 578–590. [Google Scholar] [CrossRef]
- Chen, D.; Zuo, T.; Chen, J.N. Variation of Air-Sea Heat Fluxes over the Kuroshio Area and Its Relationship with the Flood Season Precipitation in Qingdao. In Proceedings of the 2013 IEEE International Geoscience and Remote Sensing Symposium, Melbourne, Australia, 21–26 July 2013. [Google Scholar]
- Oliveira, A.P.; Cabeçadas, G.; Pilar-Fonseca, T. Iberia coastal ocean in the CO2 sink/source context: Portugal case study. J. Coast. Res. 2012, 28, 184–195. [Google Scholar] [CrossRef]
- Frankignoulle, M.; Abril, G.; Borges, A.; Bourge, I.; Canon, C.; DeLille, B.; Libert, E.; Theate, J.M. Carbon dioxide emission from European estuaries. Science 1998, 282, 434–436. [Google Scholar] [CrossRef]
- Abril, G.; Etcheber, H.; Borges, A.V.; Frankignoulle, M. Excess atmospheric carbon dioxide transported by rivers into the Scheldt estuary. Comptes Rendus de l’Académie des Sci. Ser. IIA Earth Planet. Sci. 2000, 330, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Dinauer, A.; Mucci, A. Spatial variability in surface-water pCO2 and gas exchange in the world’s largest semi-enclosed estuarine system: St. Lawrence Estuary (Canada). Biogeosciences 2017, 14, 3221–3237. [Google Scholar] [CrossRef]
- Braunschweig, F.; Martins, F.; Chambel, P.; Neves, R. A methodology to estimate renewal time scales in estuaries: The Tagus Estuary case. Ocean Dyn. 2003, 53, 137–145. [Google Scholar] [CrossRef]
- Oliveira, A.; Mateus, M.; Cabecadas, G.; Neves, R. Water-air CO2 fluxes in the Tagus estuary plume (Portugal) during two distinct winter episodes. Carbon Balance Manag. 2015, 10, 2. [Google Scholar] [CrossRef]
- Gama, C.; Dias, J.; Ferreira, O.; Taborda, R. Analysis of storm surge in Portugal, between June 1986 and May 1988. In Proceedings of the Littoral 94, Lisboa, Portugal, 26–29 September 1994; pp. 381–387. [Google Scholar]
- Sebastião, P.; Guedes Soares, C.; Alvarez, E. 44 years hindcast of sea level in the Atlantic Coast of Europe. Coast. Eng. 2008, 55, 843–848. [Google Scholar] [CrossRef]
- Johnson, H.K. Simple expressions for correcting wind speed data for elevation. Coast. Eng. 1999, 36, 263–269. [Google Scholar] [CrossRef]
- Conway, T.J.; Lang, P.M.; Masarie, K.A. Atmospheric Carbon Dioxide Dry Air Mole Fractions from the NOAA ESRL Carbon Cycle Cooperative Global Air Sampling Network, 1968–2006. Available online: ftp://ftp.cmdl.noaa.gov/ccg/co2/flask/event/ (accessed on 15 April 2008).
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean CO2 Measurements; North Pacific Marine Science Organization: Sidney, BC, Canada, 2007; p. 191. [Google Scholar]
- Carrit, D.E.; Carpenter, J.H. Comparison and evaluation of currently employed modifications of the Winkler method for determining oxygen in seawater. A NASCO Report. J. Mar. Res. 1966, 24, 286–318. [Google Scholar]
- Martins, F.; Leitão, P.; Silva, A.; Neves, R. 3D modelling in the Sado estuary using a new generic vertical discretization approach. Oceanol. Acta 2001, 24, 51–62. [Google Scholar] [CrossRef]
- Vaz, N.; Mateus, M.; Dias, J.M. Semidiurnal and spring-neap variations in the Tagus Estuary: Application of a process-oriented hydro-biogeochemical model. J. Coast. Res. 2011, 64, 1619–1623. [Google Scholar]
- Mateus, M.; Vaz, N.; Neves, R. A process-oriented model of pelagic biogeochemistry for marine systems. Part II: Application to a mesotidal estuary. J. Mar. Syst. 2012, 94, 90–101. [Google Scholar] [CrossRef]
- Hunter, K.A. The temperature dependence of pH in surface seawater. Deep Sea Res. Part I Oceanogr. Res. Pap. 1998, 45, 1919–1930. [Google Scholar] [CrossRef]
- Millero, F.J.; Graham, T.B.; Huang, F.; Bustos-Serrano, H.; Pierrot, D. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar. Chem. 2006, 100, 80–94. [Google Scholar] [CrossRef]
- Weiss, R.F. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Carini, S.; Weston, N.; Hopkinson, C.; Tucker, J.; Giblin, A.; Vallino, J. Gas exchange rates in the Parker River estuary, Massachusetts. Biol. Bull. 1996, 191, 333–334. [Google Scholar] [CrossRef]
- Raymond, P.A.; Cole, J.J. Gas exchange in rivers and estuaries: Choosing a gas transfer velocity. Estuaries 2001, 24, 312–317. [Google Scholar] [CrossRef]
- Borges, A.V.; Delille, B.; Schiettecatte, L.S.; Gazeau, F.; Abril, G.; Frankignoulle, M. Gas transfer velocities of CO2 in three European estuaries (Randers Fjord, Scheldt, and Thames). Limnol. Oceanogr. 2004, 49, 1630–1641. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Dobbins, W.E. Mechanism of reaeration in natural streams. Trans. Am. Soc. Civ. Eng. 1958, 123, 641–684. [Google Scholar]
- Hastie, T.J.; Pregibon, D. Generalized linear models. In Statistical Models in S; Chambers, J.M., Hastie, T.J., Eds.; Chapman & Hall/CRC: Boca Raton, FL, USA, 1993. [Google Scholar]
- Fortunato, A.; Oliveira, A.; Baptista, A.M. On the effect of tidal flats on the hydrodynamics of the Tagus estuary. Oceanol. Acta 1999, 22, 31–44. [Google Scholar] [CrossRef]
- De la Paz, M.; Gómez-Parra, A.; Forja, J. Inorganic carbon dynamic and air–water CO2 exchange in the Guadalquivir Estuary (SW Iberian Peninsula). J. Mar. Syst. 2007, 68, 265–277. [Google Scholar] [CrossRef]
- Hammond, D.E.; Giordani, P.; Berelson, W.M.; Poletti, R. Diagenesis of carbon and nutrients and benthic exchange in sediments of the Northern Adriatic Sea. Mar. Chem. 1999, 66, 53–79. [Google Scholar] [CrossRef]
- Cai, W.J.; Zhao, P.S.; Wang, Y.C. pH and pCO(2) microelectrode measurements and the diffusive behavior of carbon dioxide species in coastal marine sediments. Mar. Chem. 2000, 70, 133–148. [Google Scholar] [CrossRef]
- Forja, J.M.; Ortega, T.; DelValls, T.A.; Gómez-Parra, A. Benthic fluxes of inorganic carbon in shallow coastal ecosystems of the Iberian Peninsula. Mar. Chem. 2004, 85, 141–156. [Google Scholar] [CrossRef]
- Canário, J.P.; Vale, C.D.; Caetano, M.D.N. Distribution of monomethylmercury and mercury in surface sediments of the Tagus Estuary (Portugal). Mar. Pollut. Bull. 2005, 50, 1142–1145. [Google Scholar] [CrossRef]
- Brotas, V.; Catarino, F. Microphytobenthos primary production of Tagus estuary intertidal flats (Portugal). Netherland J. Aquat. Ecol. 1995, 29, 333–339. [Google Scholar] [CrossRef]
- Tobias, C.; Giblin, A.; McClelland, J.; Tucker, J.; Peterson, B. Sediment DIN fluxes and preferential recycling of benthic microalgal nitrogen in a shallow macrotidal estuary. Mar. Ecol. Prog. Ser. 2003, 257, 25–36. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Brotas, V. Seasonal variation in denitrification and dissolved nitrogen fluxes in intertidal sediments of the Tagus estuary, Portugal. Mar. Ecol. Prog. Ser. 2000, 202, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Olafsson, J.; Goddard, J.G.; Chipman, D.W.; Sutherland, S.C. Seasonal variation of CO2 and nutrients in the high-latitude surface oceans—A comparative study. Glob. Biogeochem. Cycles 1993, 7, 843–878. [Google Scholar] [CrossRef]
- Borges, A.V.; Schiettecatte, L.S.; Abril, G.; Delille, B.; Gazeau, E. Carbon dioxide in European coastal waters. Estuar. Coast. Shelf Sci. 2006, 70, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Ribas, M.; Hernández-Ayón, J.M.; Camacho-Ibar, V.F.; Cabello-Pasini, A.; Mejia-Trejo, A.; Durazo, R.; Galindo-Bect, S.; Souza, A.J.; Forja, J.M.; Siqueiros-Valencia, A. Effects of upwelling, tides and biological processes on the inorganic carbon system of a coastal lagoon in Baja California. Estuar. Coast. Shelf Sci. 2011, 95, 367–376. [Google Scholar] [CrossRef]
- Ribas-Ribas, M.; Anfuso, E.; Gómez-Parra, A.; Forja, J.M. Tidal and seasonal carbon and nutrient dynamics of the Guadalquivir estuary and the Bay of Cádiz (SW Iberian Peninsula). Biogeosciences 2013, 10, 4481–4491. [Google Scholar] [CrossRef] [Green Version]
- Abril, G.; Borges, A.V. Carbon dioxide and methane emissions from estuaries. In Greenhouse Gases Emissions from Natural Environments and Hydroelectric Reservoirs: Fluxes and Processes; Tremblay, A., Varfalvy, L., Roehm, M., Garneau, M., Eds.; Springer-Verlag: New York, NY, USA, 2004; pp. 187–207. [Google Scholar]
- Borges, A.; Vanderborght, J.-P.; Schiettecatte, L.-S.; Gazeau, F.; Ferrón-Smith, S.; Delille, B.; Frankignoulle, M. Variability of the gas transfer velocity of CO2 in a macrotidal estuary (the Scheldt). Estuaries 2004, 27, 593–603. [Google Scholar] [CrossRef]
- Zappa, C.J.; McGillis, W.R.; Raymond, P.A.; Edson, J.B.; Hintsa, E.J.; Zemmelink, H.J.; Dacey, J.W.H.; Ho, D.T. Environmental turbulent mixing controls on air-water gas exchange in marine and aquatic systems. Geophys. Res. Lett. 2007, 34, 10. [Google Scholar] [CrossRef]
- Zappa, C.J.; Raymond, P.A.; Terray, E.A.; McGillis, W.R. Variation in surface turbulence and the gas transfer velocity over a tidal cycle in a macro-tidal estuary. Estuaries 2003, 26, 1401–1415. [Google Scholar] [CrossRef]
- Vieira, V.; Jurus, P.; Clementi, E.; Mateus, M. The FuGas 2.3 Framework for Atmosphere–Ocean Coupling: Comparing Algorithms for the Estimation of Solubilities and Gas Fluxes. Atmosphere 2018, 9, 310. [Google Scholar] [CrossRef]
- Bigdeli, A.; Hara, T.; Loose, B.; Nguyen, A.T. Wave Attenuation and Gas Exchange Velocity in Marginal Sea Ice Zone. J. Geophys. Res. Oceans 2018, 123, 2293–2304. [Google Scholar] [CrossRef]
- Shuiqing, L.; Dongliang, Z. Gas transfer velocity in the presence of wave breaking. Tellus B Chem. Phys. Meteorol. 2016, 68, 27034. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liao, Q.; Fillingham, J.H.; Bootsma, H.A. On the coefficients of small eddy and surface divergence models for the air-water gas transfer velocity. J. Geophys. Res. Oceans 2015, 120, 2129–2146. [Google Scholar] [CrossRef] [Green Version]
- Kremer, J.N.; Reischauer, A.; D’Avanzo, C. Estuary-specific variation in the air-water gas exchange coefficient for oxygen. Estuaries 2003, 26, 829–836. [Google Scholar] [CrossRef]
- Cai, W.J.; Pomeroy, L.R.; Moran, M.A.; Wang, Y.C. Oxygen and carbon dioxide mass balance for the estuarine-intertidal marsh complex of five rivers in the southeastern US. Limnol. Oceanogr. 1999, 44, 639–649. [Google Scholar] [CrossRef]
- Borges, A.V.; Frankignoulle, M. Daily and seasonal variations of the partial pressure of CO2 in surface seawater along Belgian and southern Dutch coastal areas. J. Mar. Syst. 1999, 19, 251–266. [Google Scholar] [CrossRef]
- Yates, K.K.; Dufore, C.; Smiley, N.; Jackson, C.; Halley, R.B. Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay. Mar. Chem. 2007, 104, 110–124. [Google Scholar] [CrossRef]
- Laruelle, G.G.; Cai, W.-J.; Hu, X.; Gruber, N.; Mackenzie, F.T.; Regnier, P. Continental shelves as a variable but increasing global sink for atmospheric carbon dioxide. Nat. Commun. 2018, 9, 454. [Google Scholar] [CrossRef]
- Orr, J.C.; Epitalon, J.-M.; Dickson, A.G.; Gattuso, J.-P. Routine uncertainty propagation for the marine carbon dioxide system. Mar. Chem. 2018, 207, 84–107. [Google Scholar] [CrossRef]




| Season | Sampling Dates | Tide | # of Samples | Tidal Ampl. (m) | Q 1 (m3∙s−1) | u10 (m∙s−1) |
|---|---|---|---|---|---|---|
| Winter | 13 Feb. 2007 | neap | 12 | 1.3 | 94 | 0.15–7.84 |
| 22 Feb. 2007 | spring | 11 | 3.2 | 370 | ||
| Spring 2 | 17 Apr. 2007 | spring | 13 | 3.9 | 15 | 0.00–6.55 |
| 24 Apr. 2007 | neap | 13 | 1.4 | 24 | ||
| Summer 2 | 2 Jul. 2007 | spring | 13 | 3.0 | 26 | 2.05–9.06 |
| 9 Jul. 2007 | neap | 13 | 2.1 | 109 | ||
| Autumn | 26 Nov. 2007 | spring | 13 | 3.5 | 18 | 0.00–5.37 |
| 3 Dec. 2007 | neap | 13 | 1.7 | 17 |
| Season | Sampling Dates | S | T (°C) | Chl a (mg∙m−3) | DO (mg∙L−1) | pH | nTA (µmol∙kg−1) | pCO2 (µatm) |
|---|---|---|---|---|---|---|---|---|
| Winter | 13 Feb. 2007 | 21.2–30.8 | 14.1–15.1 | 0.4–1.1 | 7.44–8.78 | 7.88–8.04 | 2762–3985 | 644–890 |
| 22 Feb. 2007 | ||||||||
| Mean (SE) 1 | 25.2 (3.0) | 14.6 (0.3) | 0.7 (0.2) | 8.2 (0.3) | 7.98 (0.03) | 3397 (394) | 756 (58) | |
| Spring | 17 Apr. 2007 | 32.1–35.2 | 16.0–18.6 | 0.5–10.2 | 7.13–9.06 | 8.05–8.18 | 2506–3570 | 422–752 |
| 24 Apr. 2007 | ||||||||
| Mean (SE) 1 | 33.7 (0.8) | 17.6 (0.6) | 3.7 (2.9) | 8.1 (0.7) | 8.10 (0.03) | 2756 (248) | 568 (67) | |
| Summer | 2 Jul. 2007 | 30.4–35.2 | 15.8–19.2 | 1.3–9.7 | 6.86–7.49 | 8.08–8.19 | 2433–2808 | 402–557 |
| 9 Jul. 2007 | ||||||||
| Mean (SE) 1 | 33.0 (1.4) | 17.7 (1.0) | 3.0 (1.6) | 7.2 (0.2) | 8.13 (0.03) | 2597 (105) | 481 (39) | |
| Autumn | 26 Nov. 2007 | 29.5–35.3 | 14.1–16.0 | 0.4–0.9 | 7.02–7.76 | 7.99–8.12 | 2671–4111 | 545–990 |
| 3 Dec. 2007 | ||||||||
| Mean (SE) 1 | 33.1 (1.6) | 15.1 (0.5) | 0.6 (0.1) | 7.4 (0.2) | 8.05 (0.03) | 3317 (452) | 762 (119) | |
| Year mean (SE) 2 | 31.4 (3.9) | 16.3 (1.6) | 2.0 (2.2) | 7.7 (0.6) | 8.1 (0.1) | 3009 (470) | 638 (143) | |
| Model | % Deviance Explained | Residual Deviance | F-Value | Probability of F | Final Model | |
|---|---|---|---|---|---|---|
| Estimate | Std. Error | |||||
| NULL | 2,002,703 | 7.50148 1 | 0.32882 | |||
| DO | 3.3 | 1,936,673 | 10.085 | <0.001 | 0.05030 | 0.02701 |
| T | 61.7 | 767,122 | 178.632 | <0.0001 | −0.08383 | 0.01139 |
| Chl a | 69.3 | 615,443 | 23.167 | <0.0001 | −0.04135 | 0.00921 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.P.; Pilar-Fonseca, T.; Cabeçadas, G.; Mateus, M. Local Variability of CO2 Partial Pressure in a Mid-Latitude Mesotidal Estuarine System (Tagus Estuary, Portugal). Geosciences 2018, 8, 460. https://doi.org/10.3390/geosciences8120460
Oliveira AP, Pilar-Fonseca T, Cabeçadas G, Mateus M. Local Variability of CO2 Partial Pressure in a Mid-Latitude Mesotidal Estuarine System (Tagus Estuary, Portugal). Geosciences. 2018; 8(12):460. https://doi.org/10.3390/geosciences8120460
Chicago/Turabian StyleOliveira, Ana Paula, Tereza Pilar-Fonseca, Graça Cabeçadas, and Marcos Mateus. 2018. "Local Variability of CO2 Partial Pressure in a Mid-Latitude Mesotidal Estuarine System (Tagus Estuary, Portugal)" Geosciences 8, no. 12: 460. https://doi.org/10.3390/geosciences8120460
APA StyleOliveira, A. P., Pilar-Fonseca, T., Cabeçadas, G., & Mateus, M. (2018). Local Variability of CO2 Partial Pressure in a Mid-Latitude Mesotidal Estuarine System (Tagus Estuary, Portugal). Geosciences, 8(12), 460. https://doi.org/10.3390/geosciences8120460






