Non-Mineralized Fossil Wood
Abstract
:1. Introduction
2. Analytical Methods
3. Mummified Wood
3.1. Canadian Arctic Localities
3.2. Ellesmere Island—Late Paleocene/early Eocene, Pliocene
3.3. Axel Heiberg Island—Middle Eocene
3.4. Banks Island—Middle Miocene-Pliocene
3.5. Cornwallis Island—Miocene
3.6. Northwestern Canada
3.7. United States of America
3.8. Wilkes Formation, Washington, USA—Late Miocene
3.9. Two Creek Forest, Wisconsin USA—Late Pleistocene
3.10. Puget Sound, Washington—Late Pleistocene
3.11. European Locations
3.12. Ipolytarnóc Fossil Forest, Hungary—Late Miocene
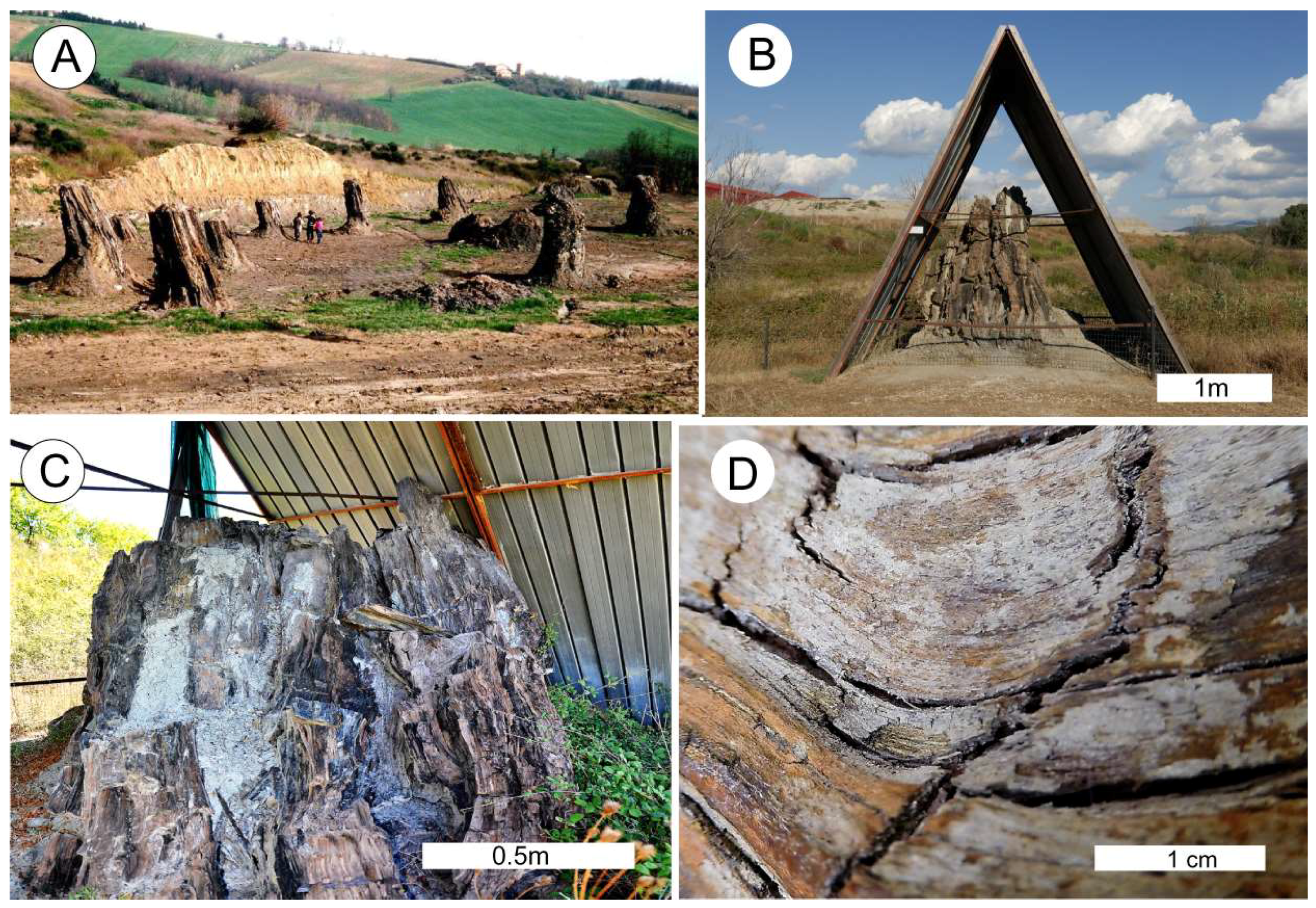
3.13. Dunarobba Fossil Forest, Italy—Pliocene
3.14. Fossano Fossil Forest, Northern Italy—Pliocene
3.15. Stura di Lanzo Fossil Forest, Northwest Italy—Pliocene
3.16. Other Locations
4. Charcoalified Wood
4.1. Evidence of Ancient Wildfires
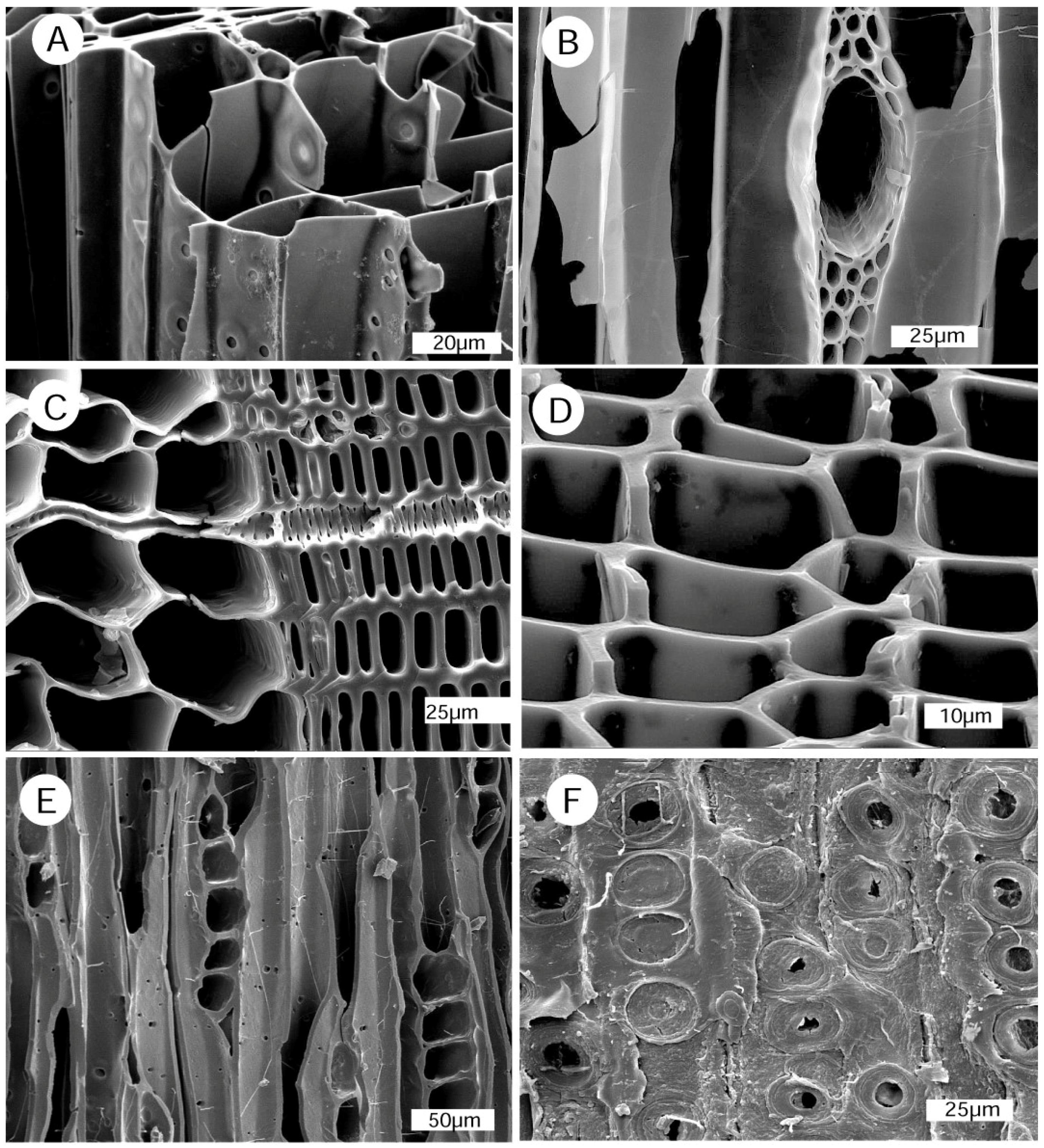
4.2. Anatomical Evidence
5. Coalified Wood
5.1. Coexistence of Mummifoed and Coalified Wood
5.2. Accessory Minerals in Mummified and Coalified Wood
Funding
Acknowledgments
Conflicts of interest
References
- Rocky Mountain Tree Ring Research. Available online: www.rmtrr.org/oldlist.htm (accessed on 18 May 2018).
- Grant, M.C. The trembling giant. Available online: www.discovermagazine.com/1993/oct/thetremblingian285 (accessed on 18 May 2018).
- Mustoe, G.E. Wood petrifaction: A new view of permineralization and replacement. Geosciences 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Mustoe, G.E. Mineralogy of non-silicified wood. Geosciences 2018, 8, 1–32. [Google Scholar] [CrossRef]
- Akahane, H.; Furuno, T.; Miyajima, H.; Yoshikawa, T.; Yamamoto, S. Rapid wood silicification in hot spring water: An explanation of silicification of wood during the Earth’s history. Sediment. Geol. 2004, 169, 219–228. [Google Scholar] [CrossRef]
- Hellawell, J.; Ballhaus, C.; Gee, C.T.; Mustoe, G.E.; Nagel, T.J.; Wirth, R.; Rethemeyer, J.; Tomaschek, F.; Geisler, T.; Greef, K.; Mansfeldt, T. Incipient silicification of recent conifer wood at a Yellowstone hot spring. Geochim. Cosmochim. Acta 2014, 149, 79–87. [Google Scholar] [CrossRef]
- Channing, A.; Edwards, D. Experimental taphonmy: Silicification of plants in Yellowstone hot-spring environments. Trans. R. Soc. Edinb. 2003, 94, 503–521. [Google Scholar] [CrossRef]
- Ericksen, K.E.L.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; Springer Verlag: Berlin, Germany, 1990; 407p. [Google Scholar]
- Passialis, C.N. Physico-chemical characteristic of waterlogged archaeological wood. Holzforschung 1997, 51, 111–113. [Google Scholar] [CrossRef]
- Fengel, D. Ageing and fossilization of wood and its components. Wood Sci. Technol. 1991, 25, 153–177. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sing, A. Micromorphological characteristics of wood biodegradation in wet environments: A review. IAWA J. 2000, 21, 135–155. [Google Scholar] [CrossRef]
- Klusek, M.; Pawelczyk, S. Stable carbon isotope analysis of subfossil wood from Australian Alps. Geochronometrica 2014, 41, 400–408. [Google Scholar]
- Barghoorn, E. Degradation of plant tissues in organic sediments. J. Sediment. Petrogrol. 1952, 2, 34–41. [Google Scholar]
- Blanchette, R.A.; Cease, K.R.; Abad, A.R.; Burnes, T.A. Ultrastructural characterization of wood from Tertiary fossil forests in the Canadian Arctic. Can. J. Bot. 1991, 69, 560–568. [Google Scholar] [CrossRef]
- Jahren, A.H.; Sternberg, L.S.L. Humidity evidence for the middle Eocene Arctic rain forest. Geology 2003, 31, 463–466. [Google Scholar] [CrossRef]
- Richter, S.L.; Johnson, A.H.; Dranoff, M.M.; LePage, B.A.; Williams, C.J. Oxygen isotope ratios in fossil wood cellulose: Isotopic composition of Eocene-to-Holocene-aged cellulose. Geochim. Cosochim. Acta 2008, 72, 2744–2753. [Google Scholar] [CrossRef]
- Jahren, A.H.; Sternberg, L.S.L. Annual patterns within tree rings of the Arctic middle Eocene. (ca. 45 Ma): Isotopic evidence of precipitation, relative humidity, and deciduousness. Geology 2008, 36, 99–102. [Google Scholar] [CrossRef]
- Eberle, J.J.; Greenwood, D.R. Life at the top of the greenhouse Eocene world—A review of the Eocene floara and vertebrate fauna from Canada’s High Arctic. Geol. Soc. Am. Bull. 2012, 124, 3–23. [Google Scholar] [CrossRef]
- Taggart, R.E.; Cross, A.T. Global greenhouse to icehouse and back again: The origin and future of the Boreal Forest biome. Glob. Planet. Chang. 2009, 65, 115–121. [Google Scholar] [CrossRef]
- Greely, A.W. Report of Sergeant D.L. Brainard on a petrified forests discovered May 20, 1883, near Cape Baird 81°30’ N, 64° W. In Three Years of Arctic Service, Volume 2, Appendix 14; Charles Scribner’s Sons: New York City, NY, USA, 1886; pp. 419–420. [Google Scholar]
- Francis, J.E. A 50-million year old fossil forest from Strathcona Fjord, Ellesmere Island, Arctic Canada. Arctic 1988, 41, 314–318. [Google Scholar] [CrossRef]
- Williams, C.J.; Johnson, A.H.; LePage, B.; Vann, D.R.; Sweda, T. Reconstruction of Tertiary forests. II. Structure, biomass, and productivity of the Eocene floodplain forests in the Canadian Arctic. Paleobiology 2003, 29, 271–292. [Google Scholar] [CrossRef]
- Dolezych, M.; Estrada, S. A fossil wood of Taxodium vandenburghi in Palaeogene sediment of Ellesmere Island (Nunavut, Canada). Z. Dt. Ges. Geowiss. 2012, 163, 283–292. [Google Scholar]
- Mitchell, W.T.; Rybcyznski, N.; Schröeder-Adama, C.; Hamilton, P.N.; Smith, R.; Douglas, M. Stratigraphic and paleoenvironemtnal reconstruction of amid-Pliocene fossil site in the High Arctic (Ellesmere Island, Nunavut): Evidence of an ancient peatland with beaver activity. Arctic 2016, 68, 185–204. [Google Scholar] [CrossRef]
- Davies, N.S.; Gosse, J.C.; Rybczynski, N. Cross-bedded woody debris from a Pliocene forester rivers stem in the High Arctic Beaufort Formation, Meighen Island, Canada. J. Sediment. Res. 2014, 84, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Jahren, A.H. The Arctic forest of the middle Eocene. Annu. Rev. Earth Planet. Sci. 2007, 35, 509–540. [Google Scholar] [CrossRef]
- Basinger, J.F.; McIver, E.E.; LePage, B.A. The fossil forests of Axel Heiberg Island. Musk-Ox 1988, 36, 50–55. [Google Scholar]
- Basinger, J.F. The fossil forests of the Buchanan Lake Formation (early Tertiary), Axel Heiberg Island, Canadian Arctic Archipelago: Preliminary floristics and paleoclimate. In Tertiary Fossil Forests of the Geodetic Hills, Axel Heiberg Island, Arctic Archipelago; Christie, R.L., McMillan, N.J., Eds.; Geological Survey of Canada Bulletin 403: Ottawa, ON, Canada, 1991; pp. 39–65. [Google Scholar]
- Francis, J.E. Polar fossil forests. Geol. Today 1990, 6, 92–95. [Google Scholar] [CrossRef]
- Francis, J.E. The dynamics of polar fossil forests: Tertiary fossil forests of Axel Heiberg Island, Canadian Arctic Archipelago. In Tertiary Fossil Forests of the Geodetic Hills, Axel Heiberg Island, Arctic Archipelago; Christie, R.L., McMillan, N.J., Eds.; Geological Survey of Canada: Ottawa, ON, Canada, 1991; pp. 29–38. [Google Scholar]
- Christie, R.L.; McMillan, N.J. Tertiary Fossil Forests of the Geodetic Hills, Axel Heiberg Island, Arctic Archipelago; Geological Survey of Canada Bull. 403: Ottawa, ON, Canada, 1991; pp. 1–227. [Google Scholar]
- Greenwood, D.R.; Basinger, J.F. Stratigraphy and floristics 0f peat-coal layers in Eocene swamp forests from Axel Heiberg Island, Canadian Arctic Archipelago. Can. J. Earth Sci. 1993, 30, 1914–1923. [Google Scholar] [CrossRef]
- Greenwood, D.R.; Basinger, J.F. The paleoecology of high-latitude Eocene swamp forests from Axel Heiberg Island, Canadian High Arctic. Rev. Palaeobot. Palynol. 1994, 81, 83–97. [Google Scholar] [CrossRef]
- Irving, E.; Wynne, P.J. The paleoaltitude of the Eocene fossil forests of the Geodetic Hills, Axel Heiberg Island, Arctic Archiplago. In Tertiary Fossil Forests of the Geodetic Hills, Axel Heiberg Island, Arctic Archipelago; Christie, R.L., McMillan, N.J., Eds.; Geological Survey of Canada Bull. 403: Ottawa, ON, Canada, 1991; pp. 209–211. [Google Scholar]
- Basinger, J.F.; Greenwood, D.G.; Sweda, T. Early Tertiary vegetation of Arctic Canada and its relevance to paleoclimatic interpretation. In Cenozoic Plants and Climates of the High Arctic v. 27; Boulter, M.C., Fisher, H.C., Eds.; NATO ASI Series I; Springer-Verlag: Berlin, Germany, 1994; pp. 175–198. [Google Scholar]
- Kumagai, H.; Sweda, T.; Hayashi, K.; Kojima, S.; Basinger, J.F.; Shibuya, M.; Fukaoa, Y. Growth ring analysis of early Tertiary conifer woods from the Canadian High Arctic and its paleoclimatic interpretation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1995, 116, 247–262. [Google Scholar] [CrossRef]
- Jahren, A.H.; Byrne, M.C.; Graham, H.V.; Sternberg, L.S.L.; Summons, R.E. The environmental water of the middle Eocene Arctic: Evidence from δD, δ180 and δ13C within specific compounds. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 271, 96–103. [Google Scholar] [CrossRef]
- Jahren, A.H.; LePage, B.A.; Werts, S.P. Methanogenesis in Eocene Arctic soils inferred from (EҰ13C of tree fossil carbonates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 214, 347–358. [Google Scholar] [CrossRef]
- Williams, C.J.; Mendell, E.K.; Murphy, J.; Court, W.M.; Johnson, A.H.; Richter, S.L. Paleoenvironmental reconstruction of a Miocene forest from the western Canadian Arctic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 261, 160–176. [Google Scholar] [CrossRef]
- LePage, B.A.; Basinger, J.F. Early Tertiary Larix from the Buchanan Lake Formation, Canadian Arctic, and a consideration of the phytogeography of the genus. Geol. Surv. Can. Bull. 1991, 403, 67–82. [Google Scholar]
- LePage, B.A.; Basinger, J.F. A new species of Larix (Pinaceae) from the early Tertiary of Axel Heiberg Island, Arctic Canada. Rev. Palaeobot. Palynol. 1991, 70, 89–111. [Google Scholar] [CrossRef]
- Harrington, G.J.; Eberle, J.; LePage, B.A.; Dawson, M.; Hutchison, J.H. Arctic plant diversity in the Early Eocene greenhouse. Proc. R. Soc. B Biol. Sci. 2010, 279, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, P.E.; Hugget, W.H.; Anderson, K.B. Comparison of vitrified and unvitrified Eocene woody tissue by TMAH thermochemolysis—Implications for the early stages of formation of virtrinite. Geochem. Trans. 2006, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fyles, J.G.; Hills, L.V.; Matthews, J.V., Jr.; Barendregt, R.; Baker, J.; Irving, E.; Jette, H. Ballast Brook and Beaufort Formations (Late Tertiary) on northern Banks Island, Arctic Canada. Quat. Int. 1994, 22/23, 141–171. [Google Scholar] [CrossRef]
- Mendell, E. Using fossil trees to estimate paleoclimate of Banks Island, Arctic Canada and the effects of modern climate change on the Arctic. In Proceedings of the 19th Annual Keck Symposium in Geology, Amherst, MA, USA, 20–23 April 2006; pp. 14–19. Available online: http:/keck.wooster.edu/publications (accessed on 18 April 2018).
- Hills, L.V. Beaufort Formation, Northwestern Banks Island, District of Franklin; Paper 69-1A; Geological Survey of Canada Bull. 403: Ottawa, ON, Canada, 1969; pp. 204–207. [Google Scholar]
- Hills, L.V. The stratigraphy, sedimentology, and paleobotany of the Beaufort Formation, Arctic Archipelago, Canada. In Proceedings of the Workshop on Circum-Arctic Late Tertiary/Early Pleistocene Stratigraphy and Environments, Denver, CO, USA, 15–17 October 1987; pp. 1–3. [Google Scholar]
- Hills, L.V.; Ogilvie, R.T. Picea banksii n. sp., Beaufort Formation (Tertiary), northwestern Banks Island, Arctic Canada. Can. J. Bot. 1970, 48, 457–464. [Google Scholar] [CrossRef]
- Hills, L.V.; Klovan, J.E.; Sweet, A.R. Juglans eocinera n. sp., Beaufort Formation (Tertiary), southwestern Banks Island, Arctic Canada. Can. J. Bot. 1974, 52, 65–90. [Google Scholar] [CrossRef]
- Hook, B.A. Paleoclimatology of the Paleocene/Eocene Using Kimberlite-Hosted Mummified Wood from the Canadian Subarctic. Ph.D. Thesis, Department of Earth Sciences, University of Toronto, Toronto, ON, Canada, 2014. 165p. [Google Scholar]
- Wolfe, A.P.; Csank, A.Z.; Reyes, A.V.; McKellar, R.C.; Tappert, R. Pristine Early Eocene wood buried deeply in kimberlite from northern Canada. PLoS ONE 2012, 17, e45537. [Google Scholar] [CrossRef] [PubMed]
- Hook, B.A.; Halfar, J.; Gedalof, Z.; Bollman, J.; Schulze, D.J. Stable isotope paleoclimatology from the earliest Eocene using kimberlite-hosted mummified wood from the Canadian Subarctic. Biogeosciences 2015, 12, 5899–5914. [Google Scholar] [CrossRef]
- Hansel, A.K.; Johnson, W.H. Wedron and Mason Groups: Lithostratigraphic Reclassification of Deposits of the Wisconsin Episode, Lake Michigan Lobe Area; Illinois State Geological Survey Bulletin 104: Champaign, IL, USA, 1996; 116p. [Google Scholar]
- Mossa, J.; Autin, W.J. Quaternary Geomorphology and Stratigraphy of the Florida Parishes, Southeastern Louisiana: Friends of the Pleistocene, Field Trip Guide Book; South-Central Cell: Baton Rouge, LA, USA, 1986; pp. 76–94. [Google Scholar]
- Yancey, T.E.; Mustoe, G.E.; Leopold, E.B.; Heizler, M.T. Mudflow disturbance in latest Miocene forests in Lewis County, Washington. PALAIOS 2013, 28, 343–358. [Google Scholar] [CrossRef]
- Mustoe, G.E.; Leopold, E.B. Paleobotanical evidence for the post-Miocene uplift of the Cascade Range. Can. J. Earth Sci. 2014, 51, 809–824. [Google Scholar] [CrossRef]
- Mickelson, D.M.; Hooyer, T.S.; Soch, B.J.; Winguth, C. Late Glacial ice advances and vegetation changes in east-central Wisonsin. In Late Glacial History of East-Cenral Wisconson; Survey Open-File Report 2007-1; Hooyer, T.S., Ed.; Wisconsin Geological and Natural History: Madison, WI, USA, 2007; pp. 72–87. [Google Scholar]
- Black, R.F. Glacial Geology of the Two Creeks Forest Bed, Valderan Type Locality, and Northern Kettle Moraine State Forest (Information Circular 13); Geological and Natural History Survey, University of Wisconsin: Madison, WI, USA, 1970; pp. 21–30. [Google Scholar]
- Goldthwait, J.W. The abandoned shorelines of eastern Wisconsin. Wis. Geol. Nat. Hist. Surv. Bull. 1907, 17, 1–134. [Google Scholar]
- Wilson, L.R. The Two Creeks forest bed, Manitowoc County, Wisconsin. Trans. Wis. Acad. Sci. Arts Lett. 1932, 27, 31–46. [Google Scholar]
- Wilson, L.R. Fossil studies of the Two Creeks forest bed, Manitowoc County, Wisconsin. Bull. Torrey Bot. Club 1936, 63, 317–325. [Google Scholar] [CrossRef]
- Broecker, W.S.; Farrand, W.R. Radiocarbon age of the Two Creeks Forest bed, Wisconsin. Geol. Soc. Am. Bull. 1963, 74, 647–649. [Google Scholar] [CrossRef]
- Kaiser, K.F. Two Creeks interstade dated through dendrochronology and AMS. Quat. Res. 1994, 32, 288–298. [Google Scholar] [CrossRef]
- Panyushkina, I.P.; Leavitt, S.W. Ancient boreal forests under the environmental instability of the glacial to post-glacial transition in the Great Lakes region (14,000–11,000 years BP). Can. J. For. Res. 2013, 3, 1032–1039. [Google Scholar] [CrossRef]
- Panyshukina, I.P.; Leavitt, S.W.; Mode, W.N. A 1400-Year Bølling-Allerød Tree-Ring Record from the U.S. Great Lakes Region. Tree-Ring Res. 2017, 73, 102–112. [Google Scholar] [CrossRef]
- Black, R.F.; Geology of the Ice Age Natural Science Reserve of Wisconsin. National Park Service Scientific Monograph No. 2, Chapter 2: Two Creeks Forest Bed 1974. Available online: https://www.nps.gov/parkhistory/online_books/science/2/chap2.htm (accessed on 21 April 2018).
- Panyushkina, I.P.; Leavitt, S.W.; Schneider, A.F.; Thompson, T.A.; Lange, T. Environment and paleoecology of a 12 ka mid-North American Younger Dryas forest chronicled in tree rings. Quat. Res. 2008, 70, 433–441. [Google Scholar] [CrossRef]
- Panyushkina, I.P.; Leavitt, S.W. Tapping ancient tree-ring archives in the U.S. Great Lakes region. Eos Trans. Am. Geophys. Union 2010, 91, 489–490. [Google Scholar] [CrossRef]
- Easterbrook, D.J. Stratigraphy and chronology of early to late Pleistocene glacial and interglacial sediments in the Puget Lowland, Washingon. In Geologic Field Trips in the Pacific Northwest, Proceedings of the 1994 Geological Society of America Annual Meeting, Seattle, WA, USA, 24–27 October 1994; Swanson, D.A., Haugerud, R.A., Eds.; Dept. of Geological Sciences, Univ. of Washington: Seattle, WA, USA, 1994; Volume 1, pp. IJ1–IJ38. [Google Scholar]
- Hansen, H.P.; Mackin, J.H. A pre-Wisconsin forest succession in the Puget Lowland, Washington. Am. J. Sci. 1949, 247, 833–855. [Google Scholar] [CrossRef]
- Easterbrook, D.J.; Crandell, D.R.; Leopold, E.B. Pre-Olympia stratigraphy and chronology in the central Puget Lowland, Washington. Geol. Soc. Am. Bull. 1967, 78, 13–20. [Google Scholar] [CrossRef]
- Stoffel, K.L. Stratigraphy of pre-Vashon Quaternary sediments applied to the evolution of a proposed major tectonic structure in Island County, Washington. In Washington Division of Geology and Earth Resources, Open File Report OFR 80-0; Washington Division of Geology and Earth Resources: Olympia, WA, USA, 1980; 161p. [Google Scholar]
- Lechien, V.; Rodriguez, C.; Onenga, M.; Hiligsmann, S.; Rulmont, A.; Thonart, P. Physiochemical and biochemical characterization of non-biodegradable cellulose in Miocene gymnosperm wood from the Entre-Sambre-et-Meuse, southern Belgium. Org. Chem. 2006, 37, 1465–1476. [Google Scholar]
- Simpson, I.M.; West, R.G. On the stratigraphy and palaeobotany of a late-Pleistocene organic deposit at Chelford, Cheshire. New Phytol. 1958, 57, 239–250. [Google Scholar] [CrossRef]
- Klusik, M.A.; Pawelczyk, S.R. Stable carbon isotope analysis of subfossil wood from the Austrian Alps. Geochronometria 2014, 41, 400–408. [Google Scholar]
- Dorofeyev, P.I. Oligocene flora of the Dunayevsky Crag on the Tym River in western Siberia. Dokl. Akademii Nauk SSSR 1960, 132, 659–661. [Google Scholar]
- Gnibidenko, Z.N.; Semakov, N.N. Paleomagnetism of boundary Oligocene-Miocene deposits in the Kompasski Bor tract on the Tym River (western Siberia). Izvestia, Phys. Solid Earth 2009, 45, 70–79. [Google Scholar] [CrossRef]
- Kázmér, M. The Miocene Bükkábrány Fossil Forest in Hungary—Field observations and project outline. In 125th Anniversary of the Department of Palaeontology at Budapest University, a Jubilee Volume; Galácz, A, Ed.. Hantkeniana 2008, 6, 220–244. [Google Scholar]
- Erdei, B.; Dolezych, M.; Hably, L. The buried Miocene forest at Bükkábrány, Hungary. Rev. Palaeobot. Palynol. 2009, 15, 69–79. [Google Scholar] [CrossRef]
- Gryce, V.; Sakala, J. Identification of fossil trunks from Bükkábrány newly installed in the visitor centre of the Ipolytarnóc Fossils Nature Reserve (Novhrad-Nóográd Geopark) in northern Hungary. Acta Univ. Agric. Silvic. Mendel. Brun. 2010, 58, 117–122. [Google Scholar] [CrossRef]
- Dolezych, M.; Van der Burgh, J. Xylotomische Untersuchungen an inkholten Hölzern aus dem Braunkohlentagebau Berzdorf (Oberlausitz, Deutschland). Feddes Repert. 2009, 115, 397–437. [Google Scholar] [CrossRef]
- Hámor-Vidó, M.; Hofmann, T.; Albert, L. In Situ preservation and paleonvironmental assessment of Taxodiacea fossil trees in the Bükkalja Lignite Formation, Bükkábrány open cast mine, Hungary. Int. J. Coal Geol. 2010, 81, 203–210. [Google Scholar] [CrossRef]
- Baldanza, A.; Sabatino, G.; Triscari, M.; De Angeles, M.C. The Dunarobba Fossil Forest (Umbria, Italy): Mineralogical transformations evidences as possible decay effects. Per. Mineral. 2009, 78, 51–60. [Google Scholar]
- Vassio, E.; Martinetto, E.; Dolezych, M.; Van der Burg, J. Wood anatomy of the Glyptostrobus europaeus “whole-plant” from a Pliocene fossil forest in Italy. Rev. Palaeobot. Palynol. 2008, 151, 81–89. [Google Scholar] [CrossRef]
- Staccioli, G.; Menchi, G.; Matteoli, U.; Seraglia, R.; Traldi, P. Chemotaxonomic onservations on some pliocenic woods from Arno Basin and fossil forest of Dunarobba (Italy). Flora Mediterr. 1996, 6, 113–117. [Google Scholar]
- Staccioli, G.; Bartolini, G. New biomarkers of the extinct species Taxodioxylon gypsaceum. Wood. Sci. Technol. 1997, 31, 311–315. [Google Scholar] [CrossRef]
- Staccioli, G.; Fratini, F.; Meli, A.; Lazzeri, S. Mineralization processes in some samples from the fossil forest of Dunarobba (Umbria, Central Italy). Wood. Sci. Technol. 2001, 35, 353–362. [Google Scholar] [CrossRef]
- Boyce, C.K.; Hazen, R.M.; Knoll, A.H. Nondestructive, in-situ, cellular scale mapping of elemental abundances including organic carbon in permineralized fossils. Proc. Nat. Acad. Sci. USA 2001, 98, 5970–5974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, A.C.; Collinson, M.E. Non-destructive multiple approaches to interpret the preservation of plant fossils: Implications for calcium-rich permineralizations. J. Geol. Sci. Lond. 2003, 160, 857–862. [Google Scholar] [CrossRef]
- Martinetto, E.; Bertini, A.; Basilica, G.; Baldanza, A.; Bizzarri, R.; Cherin, M.; Gentili, S.; Pontini, M.R. The plant record of the Dunarobba and Pietrafitta sites in the Plio-Pleistocene palaeoenvironmental context of central Italy. Alp. Mediter. Quat. 2014, 27, 29–72. [Google Scholar]
- Macaluso, L.; Martinetto, E.; Vigna, B.; Bertini, A.; Cilia, A.; Teodordis, V.; Kvaček, S. Palaeofloral and stratigraphic context of a new fossil forest from the Pliocene of NW Italy. Rev. Palaeobot. Palynol. 2018, 248, 15–33. [Google Scholar] [CrossRef]
- Ambrosetti, P.; Basilici, G.; Ciangherotti, A.D.; Codipietro, G.; Corona, E.; Esu, D.; Girotta, O.; Lo Manco, A.; Meneghina, M.; Paganelli, A.; et al. La Foresta Fossile di Dunarobba (Terni, Unbria, Italia Centrale): Contest lithostratigraphico, sedimentologico, palinologico, dendrochronologico e paleomalacologico. Ital. J. Quat. Sci. 1995, 8, 465–508. [Google Scholar]
- Martinetto, E.; Sardia, G.; Varrone, D. Magnetostratigraphy of the Stura di Lanzo fossil forest succession (Piedmont, Italy). Riv. Ital. Paleontol. Stratigr. 2007, 113, 109–125. [Google Scholar]
- Ferrero, E. Foresta fossile: Fruizione didattica. In La Foresta Fossile del Torrente Stura di Lanzo. I Quaderni de La Mandria, Ente Parco Regionale La Mandria, Torino; Martinetto, E., Farina, T., Eds.; Ente di Gestione del Parco Regionale La Mandria e dei Parchi e delle Riserve Naturali delle Valli di Lanzo: Venaria, Italy, 2005; Volume 1, pp. 44–45. [Google Scholar]
- Godone, F.; Baaaldo, M.; Maraga, F. Una foresta nel’alveo del F. Stura di Lanzo (TO). Rilevamentu topografici, cartografia e GIS. Atti 8° Conferenza Nationale ASITA, Roma, 14–17 dic. 2004, Artesanpa sas. Varese 2004, 2, 1219–1224. [Google Scholar]
- Chiariglione, A.; Farina, T.; Ferrero, O.; Forna, M.G.; Gattiglio, M.; Lucchesi, S.; Martinetto, E. La Foresta Fossile dello Stura de Lanzo, Quaderni de la Mandria, Parco La Mandria 2005, 48p. Available online: https://www.researchgate.net/profile/Edoardo_Martinetto (accessed on 19 May 2018).
- Ferrero, E.; Magagna, A. Natural hazards and geological heritage in Earth science education projects. In Geoethics: The Role and Responsibility of Geoscientists; Peppoloni, S., Di Capua, G., Eds.; Geological Society of London Special Publications: London, UK, 2015; p. 419. [Google Scholar]
- Ferrero, E.; Gimigliano, D.; Pogliano, A. Educazione ambientale in un’area protetta del territorio piemontese. Il progetto ‘La foresta ritrovata’. In Proceedings of the Atti 3rd World Environmental Education Congress, Turin, Italy, 2–6 October 2005; pp. 170–177. [Google Scholar]
- Quan, C.; Fu, Q.Y.; Shu, G.L.; Li, Y.S.; Lu, L.; Liu, X.Y.; Jin, J.H. First Oligocene mummified plant Lagerstätte at low latitudes of East Asia. Sci. China Earth Sci. 2016, 59, 445–560. [Google Scholar] [CrossRef]
- Yamakawa, C.; Momohara, A.; Saito, T.; Nunotani, T. Composition and paleoenvironment of wetland forests dominated by Glyptostrobus and Metasequoia in the latest Pliocene (2.6Ma) in central Japan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 467, 191–210. [Google Scholar] [CrossRef]
- Scott, A.C. The pre-Quaternary history of fire. Palaeogeogr. Palaoclimatol. Palaeoecol. 2000, 154, 281–329. [Google Scholar] [CrossRef]
- Scott, A.C.; Cripps, J.A.; Collinson, M.E.; Nicjols, G.J. The taphonomy of charcoal in a Recent heathland fire and some implications for the interpretation of fossil charcoal deposits. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 164, 1–31. [Google Scholar] [CrossRef]
- Scott, A.C. The preservation of woods in volcanic pyroclastic flows and surges. In Proceedings of the Geological Society of America 2001 Annual Meeting, Boston, MA, USA, 5–8 November; p. 68.
- Scott, A.C. Charcoal recognition, taphonomy, and uses in paleoenvironmenetal analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 291, 11–39. [Google Scholar] [CrossRef]
- Scott, A.C.; Damblon, F. Charcoal: Taphonomy and significance in geology, botany, and archaeology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 291, 1–10. [Google Scholar] [CrossRef]
- Sander, O.M.; Gee, C.T. Fossil charcoal: Techniques and applications. Rev. Palaeobot. Palynol. 1990, 63, 269–279. [Google Scholar] [CrossRef]
- Glasspool, I.J.; Scott, A.C. Phanerozoic concentrations of atmospheric oxygen reconstructed from sedimentary charcoal. Nat. Geosci. 2010, 3, 627–630. [Google Scholar] [CrossRef]
- Moore, P.R.; Wallace, R. Petrified wood from the Miocene volcanic sequence of Coromandel Peninsula, northern New Zealand. J. R. Soc. N. Z. 2000, 30, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Hudspeth, V.A.; Scott, A.C.; Wilson, C.J.N.; Collinson, M.E. Charring of woods by volcanic processes: An example from the Taupo ignimbrite, New Zealand. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 291, 40–51. [Google Scholar] [CrossRef]
- Glasspool, I.J.; Edwards, D.; Axe, L. Charcoal in the Silurian as evidence for the earliest wildfiew. Geology 2004, 32, 381–383. [Google Scholar] [CrossRef]
- Glasspool, I.J.; Edwards, D.; Axe, L. Charcoal in the Early Devonian: A wildfire-derived Konservate-Lagerstätte. Rev. Paleobot. Palynol. 2006, 142, 131–136. [Google Scholar] [CrossRef]
- Prestianni, C.; Decombeix, A.L.; Thorez, J.; Fokan, D.; Gerrienne, P. Famennian charcoal of Belgium. Palaeoecology 2010, 291, 60–71. [Google Scholar] [CrossRef]
- Berner, R.Z.; Canfield, D.E. A new model for atmospheric oxygen over Phanerozoic time. Am. J. Sci. 1989, 289, 333–361. [Google Scholar] [CrossRef] [PubMed]
- Berner, R.A.; Beerling, D.J.; Dudley, R.; Robinson, J.M.; Wildman, R.A., Jr. Phanerozoic atmospheric oxygen. Annu. Rev. Earth Planet. Sci. 2003, 31, 105–134. [Google Scholar] [CrossRef]
- Berner, R.A. The carbon and sulfur cycles and atmospheric oxygen from Middle Permian to late Triassic. Geochim. Cosmochim. Acta 2005, 69, 3211–3217. [Google Scholar] [CrossRef]
- Jones, T.P.; Chaloner, W.H. Fossil charcoal, its recognition and paleoatmospheric significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 97, 39–50. [Google Scholar] [CrossRef]
- Tanner, L.H.; Wang, X.; Morabito, A.C. Fossil charcoal from the Middle Jurassic of the Ordos Basin, China and its paleoatmospheric implications. Geosci. Front. 2012, 3, 493–502. [Google Scholar] [CrossRef]
- Belcher, C.M.; Yearsle, J.M.; Hadden, R.M.; McElwaine, J.C.; Rein, G. Baseline intrinsic flammability of Earth’s exosystems estimated from paleoatmospheric oxygen over the past 350 million years. PNAS 2010, 107, 22448–22543. [Google Scholar] [CrossRef] [PubMed]
- Uhl, D.; Dolezych, M.; Böhme, M. Taxodioxylon-like charcoal from the Late Miocene of western Bulgaraia. Acta Palaeontol. 2014, 54, 101–111. [Google Scholar]
- McPartland, L.C.; Collinson, M.E.; Scott, A.C.; Steart, D.C.; Grassineau, N.V.; Gibbins, S.J. Ferns and fires: Experimental charring of ferns compared to wood and implications for paleobiology, paleoecology, coal petrology, and isotope geochemistry. PALAIOS 2007, 22, 528–538. [Google Scholar] [CrossRef]
- Byers, B.A.; Ash, S.R.; Chaney, D.; DeSoto, L. First known fire scar on a fossil free trunk provides evidence of Late Triassic wildfire. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 411, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Stout, S.A.; Brown, J.J.; Packman, W. Molecular aspects of the peatification and early coalification of angiosperm and gymnosperm woods. Geochim. Cosmochim. Acta 1988, 52, 405–414. [Google Scholar] [CrossRef]
- Rodgers, R.E.; Ramurthy, M.; Rodvelt, G.; Mullin, M. Coalbed Methane, Principles and Practices, 3rd ed.; Oktibbeha Pub. Co.: Starkville, MS, USA, 2007; pp. 97–98. [Google Scholar]
- Ravazzi, C.; Van der Burgh, J. Coniferous woods in the early Pleistocene brown coals of the Leffe Basin (Lombardy, Italy). Riv. It. Paleont. Strat. 1995, 100. [Google Scholar]
- Dolezych, M. Taxodiaceous woods in Lusatia (central Europe), including curiosities in their nomenclature and taxonomy, with a focus on Taxodioxylon. Jpn. J. Hist. Bot. 2011, 19, 25–46. [Google Scholar]
- Dolezych, M. A remarkable extinct wood from Lusatia (central Europe)—Juniperoxylon schneiderianum sp. Nov. with affinity to Cupressospernum saxonicum MAI. Palaeontogr. Abt. B Palaeobot.-Paleopjhytol. 2016, 295, 5–31. [Google Scholar]
- Nestler, K.; Deitrich, D.; Witke, K.; Röβler, R.; Marx, G. Thermogravimetric and Raman spectroscopic investigations on different coals in comparison to dispersed anthracite found in permineralized tree fern Psaronius sp. J. Mol. Struct. 2003, 661–662, 357–362. [Google Scholar] [CrossRef]
- Hedges, J.I.; Cowie, G.L.; Ertel, J.R.; Barbour, R.J.; Hatcher, P.G. Degradation of carbohydrates and lignins in buried woods. Geochim. Cosmochim. Acta 1985, 49, 700–711. [Google Scholar] [CrossRef]
- Bestland, E. Volcanic stratigraphy of the Oligocene Colestin Formation in the Siskiyou Pass area of southern Oregon. Or. Geol. 1987, 49, 79–86. [Google Scholar]
- Williams, C.J.; Trostle, K.D.; Sunderlin, D. Fossil wood in coal-forming environments of the Late Paleocene –Early Eocene Chickaloon Formation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 295, 363–375. [Google Scholar] [CrossRef]
- Sweeney, I.J.; Chin, K.; Hower, J.C.; Budd, D.A.; Wolfe, D.G. Fossil wood from the middle Cretaceous Moreno Hill Formation: Unique expressions of wood mineralization and implications for the processes of wood preservation. Int. J. Coal Geol. 2009, 79, 1–17. [Google Scholar] [CrossRef]
- Havelcová, M.; Sýkorová, I.; Bectel, A.; Mach, K.; Trejtnarová, H.; Žaloudková, M.; Matusová, P.; Blažek, J.; Boudová, J.; Sakala, J. “Stump Horizon” Bílina Mine (Most Basin, Czech Republic)-GC-MS, optical and electron microscopy in identification of wood of biological origin. Int. J. Coal Geol. 2013, 107, 62–77. [Google Scholar] [CrossRef]
























© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustoe, G.E. Non-Mineralized Fossil Wood. Geosciences 2018, 8, 223. https://doi.org/10.3390/geosciences8060223
Mustoe GE. Non-Mineralized Fossil Wood. Geosciences. 2018; 8(6):223. https://doi.org/10.3390/geosciences8060223
Chicago/Turabian StyleMustoe, George E. 2018. "Non-Mineralized Fossil Wood" Geosciences 8, no. 6: 223. https://doi.org/10.3390/geosciences8060223
APA StyleMustoe, G. E. (2018). Non-Mineralized Fossil Wood. Geosciences, 8(6), 223. https://doi.org/10.3390/geosciences8060223





